The Municipal Sewage Discharge May Impact the Dissemination of Antibiotic-Resistant Escherichia coli in an Urban Coastal Beach
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Sample Collection
2.3. Isolation and Identification of E. coli
2.4. Antibiotic Susceptibility Assay
2.5. Antibiotic-Resistant GENE Detection
2.6. Detection of the Integrase Gene
2.7. Amplification and Sequencing of the Gene Cassette Region
2.8. Statistical Analyses
3. Results
3.1. Prevalence of E. coli in Seawater and Sand
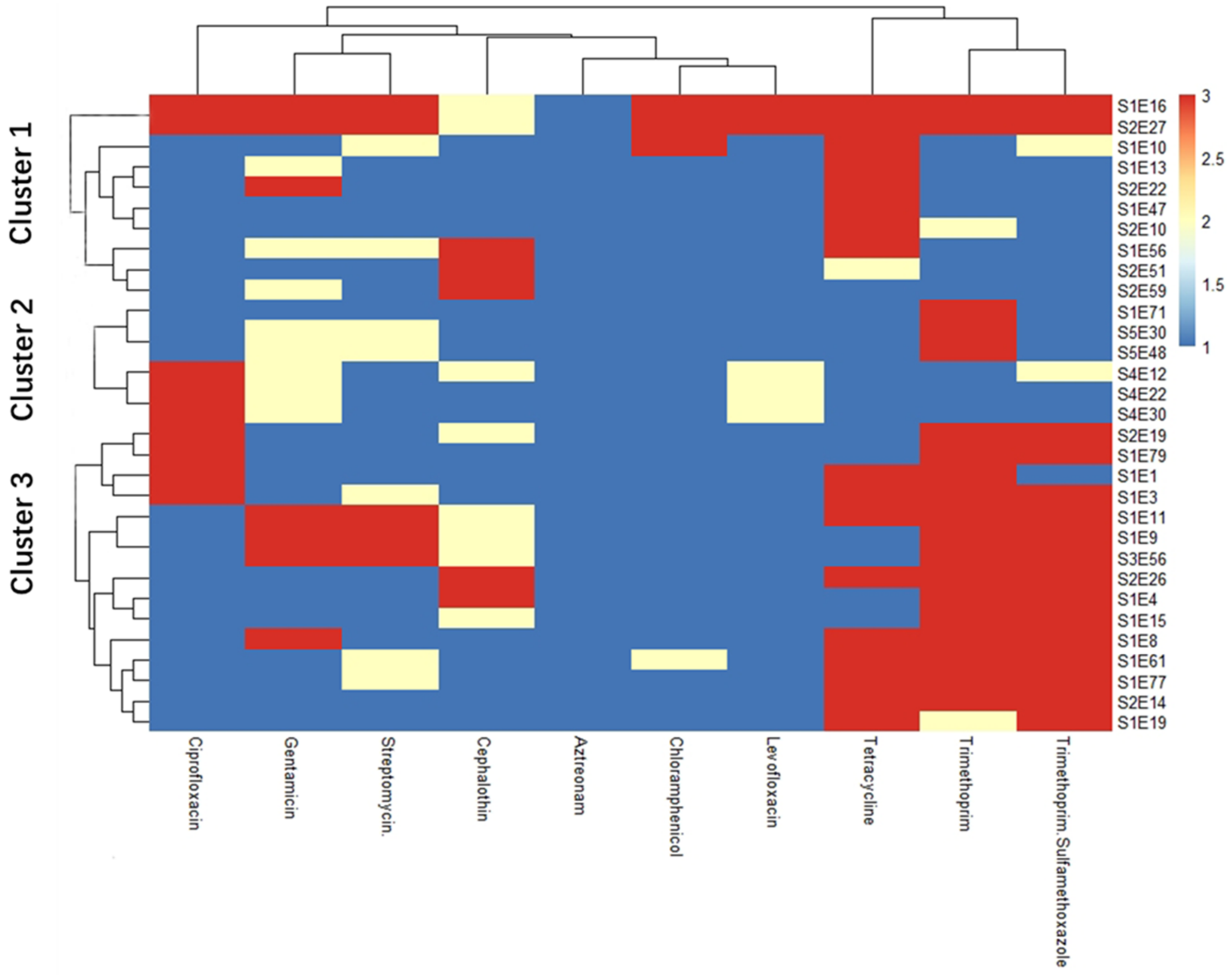
3.2. Cluster Analysis between Isolates’ Antibiogram Profiles
3.3. Correlation between Phenotypic Resistance Profile and Relative Genotypic Resistance Profile
3.4. Occurrence of Class 1 Integrons in the MAR Strain
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marti, E.; Variatza, E.; Balcazar, J.L. The role of aquatic ecosystems as reservoirs of antibiotic resistance. Trends Microbiol. 2014, 22, 36–41. [Google Scholar] [CrossRef] [PubMed]
- The Lancet Infectious Diseases. Time for global political action on antimicrobial resistance. Lancet Infect. Dis. 2016, 16, 1085. [Google Scholar] [CrossRef]
- O’neill, J.I.M. Antimicrobial resistance: Tackling a crisis for the health and wealth of nations. Rev. Antimicrob. Resist. 2014, 20, 1–16. [Google Scholar]
- Xiao, Y.; Li, L. China’s national plan to combat antimicrobial resistance. Lancet Infect. Dis. 2016, 16, 1216–1218. [Google Scholar] [CrossRef]
- Satoru, S.; Amy, P.; Marko, V.; Zhang, T. Editorial: Antibiotic resistance in aquatic systems. Front. Microbiol. 2017, 8, 14. [Google Scholar]
- Hatosy, S.M.; Martiny, A.C. The ocean as a global reservoir of antibiotic resistance genes. Appl. Environ. Microb. 2015, 81, 7593–7599. [Google Scholar] [CrossRef]
- Pérez, D.M.G.; Pérez, J.I.; Nieto, M.G. Carbamazepine behaviour and effects in an urban wastewater MBR working with high sludge and hydraulic retention time. J. Environ. Sci. Health Part A 2016, 51, 855–860. [Google Scholar] [CrossRef]
- González-Pérez, D.; Pérez, J.; Gómez, M. Behaviour of the main nonsteroidal anti-inflammatory drugs in a membrane bioreactor treating urban wastewater at high hydraulic-and sludge-retention time. J. Hazard. Mater. 2017, 336, 128–138. [Google Scholar] [CrossRef]
- Cornejo, J.; González-Pérez, D.M.; Pérez, J.I.; Gómez, M.A. Ibuprofen removal by a microfiltration membrane bioreactor during the startup phase. J. Environ. Sci. Health Part A 2019, 55, 374–384. [Google Scholar] [CrossRef]
- Mançano, S.M.C.N.; Campana, E.H.; Felix, T.P.; Barrueto, L.R.L.; Pereira, P.S.; Picão, R.C. Frequency and diversity of Stenotrophomonas spp. carrying blaKPC in recreational coastal waters. Water Res. 2020, 185, 116210. [Google Scholar] [CrossRef]
- Furlan, J.P.R.; Ramos, M.S.; dos Santos, L.D.R.; Gallo, I.F.L.; Lopes, R.; Stehling, E.G. Appearance of mcr-9, blaKPC, cfr and other clinically relevant antimicrobial resistance genes in recreation waters and sands from urban beaches, Brazil. Mar. Pollut. Bull. 2021, 167, 112334. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Guo, Y.; Zhu, D.; Wang, F.; Jiang, X.; Fu, Y.; Xiaolong, Z. CHINET surveillance of bacterial resistance in China: 2018 report. Chin. J. Infect. Chemother. 2020, 20, 1–10. [Google Scholar]
- Alm, E.W.; Zimbler, D.; Callahan, E.; Plomaritis, E. Patterns and persistence of antibiotic resistance in faecal indicator bacteria from freshwater recreational beaches. J. Appl. Microbiol. 2014, 117, 273–285. [Google Scholar] [CrossRef]
- Alves, M.; Pereira, A.; Araújo, S.M.; Castro, B.; Correia, A.; Henriques, I. Seawater is a reservoir of multi-resistant Escherichia coli, including strains hosting plasmid-mediated quinolones resistance and extended-spectrum beta-lactamases genes. Front. Microbiol. 2014, 5, 426. [Google Scholar] [CrossRef]
- Pan, M.; Chu, L. Occurrence of antibiotics and antibiotic resistance genes in soils from wastewater irrigation areas in the Pearl River Delta region, southern China. Sci. Total Environ. 2018, 624, 145–152. [Google Scholar] [CrossRef]
- Kaushik, M.; Kumar, S.; Kapoor, R.K.; Gulati, P. Integrons and antibiotic resistance genes in water-borne pathogens: Threat detection and risk assessment. J. Med. Microbiol. 2019, 68, 679–692. [Google Scholar] [CrossRef]
- Leonard, A.F.; Zhang, L.; Balfour, A.J.; Garside, R.; Hawkey, P.M.; Murray, A.K.; Ukoumunne, O.C.; Gaze, W.H. Exposure to and colonisation by antibiotic-resistant E. coli in UK coastal water users: Environmental surveillance, exposure assessment, and epidemiological study (Beach Bum Survey). Environ. Int. 2018, 114, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Turgeon, P.; Michel, P.; Levallois, P.; Chevalier, P.; Daignault, D.; Crago, B.; Irwin, R.; McEwen, S.A.; Neumann, N.F.; Louie, M. Antimicrobial-resistant Escherichia coli in public beach waters in Quebec. Infect. Dis. Med. Microbiol. 2012, 23, e20–e25. [Google Scholar] [CrossRef]
- Kotlarska, E.; Łuczkiewicz, A.; Pisowacka, M.; Burzyński, A. Antibiotic resistance and prevalence of class 1 and 2 integrons in Escherichia coli isolated from two wastewater treatment plants, and their receiving waters (Gulf of Gdansk, Baltic Sea, Poland). Environ. Sci. Pollut. Res. 2014, 22, 2018–2030. [Google Scholar] [CrossRef] [PubMed]
- EPA-821-R-14-010; Method 1603: Escherichia coli (E. coli) in Water by Membrane Filtration Using Modified Membrane-Thermotolerant Escherichia coli Agar (Modified mTEC). U.S. Environmental Protection Agency: Washington, DC, USA, 2014.
- Merchant, I.A.; Packer, R.A. Veterinary bacteriology and virology. Q. Rev. Biol. 1950, 4, 211–305. [Google Scholar]
- Barrow, G.I.; Feltham, R.K. Cowan and Steel’s Manual for the Identification of Medical Bacteria, 3rd ed.; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar]
- CLSI Document M100-S25; Performance Standards for Antimicrobial Susceptibility Testing. Twenty-Fifth Informational Supplement; Clinical and Laboratory Standard Institute: Malvern, PA, USA, 2015.
- Heinrich; Manjusha, S.; Sarita, G.B.; Elyas, K.K.; Chandrasekaran, M. Multiple Antibiotic Resistances of Vibrio Isolates from Coastal and Brackish Water Areas. Am. J. Biochem. Biotechnol. 2005, 1, 193–198. [Google Scholar] [CrossRef][Green Version]
- Mohanta, T.; Goel, S. Prevalence of antibiotic-resistant bacteria in three different aquatic environments over three seasons. Environ. Monit. Assess. 2014, 186, 5089–5100. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Eils, R.; Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 2016, 32, 2847–2849. [Google Scholar] [CrossRef]
- Ekundayo, T.C.; Okoh, A.I. Antimicrobial resistance in freshwater Plesiomonas shigelloides isolates: Implications for environmental pollution and risk assessment. Environ. Pollut. 2020, 257, 493. [Google Scholar] [CrossRef] [PubMed]
- Andrade, V.D.C.; Zampieri, B.D.B.; Ballesteros, E.R.; Pinto, A.B.; De Oliveira, A.J.F.C. Densities and antimicrobial resistance of Escherichia coli isolated from marine waters and beach sands. Environ. Monit. Assess. 2015, 187, 342. [Google Scholar] [CrossRef]
- McCusker, M.P.; Ferreira, D.A.; Cooney, D.; Alves, B.M.; Fanning, S.; Pagès, J.-M.; Martins, M.; Davin-Regli, A. Modulation of antimicrobial resistance in clinical isolates of Enterobacter aerogenes: A strategy combining antibiotics and chemosensitisers. J. Glob. Antimicrob. Resist. 2018, 16, 187–198. [Google Scholar] [CrossRef]
- Na, G.; Fang, X.; Cai, Y.; Ge, L.; Zong, H.; Yuan, X.; Yao, Z.; Zhang, Z. Occurrence, distribution, and bioaccumulation of antibiotics in coastal environment of Dalian, China. Mar. Pollut. Bull. 2013, 69, 233–237. [Google Scholar] [CrossRef]
- Zhang, Q.; Ying, G.; Pan, C.; Liu, Y.; Zhao, J. Comprehensive evaluation of antibiotics emission and fate in the river basins of China: Source analysis, multimedia modeling, and linkage to bacterial resistance. Environ. Sci. Technol. 2015, 49, 6772–6782. [Google Scholar] [CrossRef]
- Hu, J.; Shi, J.; Chang, H.; LI, D.; Yang, M.; Kamagata, Y. Phenotyping and genotyping of antibiotic-resistant Escherichia coli isolated from a natural river rasin. Environ. Sci. Technol. 2008, 42, 3415–3420. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, G.; Wang, C.; Lu, N.; Yuan, X.; Zhu, X. Pollution characteristics of antibiotics and antibiotic resistance of coliform bacteria in the Yitong River, China. Environ. Monit. Assess. 2019, 191, 516. [Google Scholar] [CrossRef]
- Rizzo, L.; Fiorentino, A.; Anselmo, A. Effect of solar radiation on multidrug resistant E. coli strains and antibiotic mixture photodegradation in wastewater polluted stream. Sci. Total Environ. 2012, 427, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Américo-Pinheiro, J.H.P.; Bellatto, L.C.; Mansano, C.F.M.; Vilar, D.D.S.; Ferreira, L.F.R.; Torres, N.H.; Bilal, M.; Iqbal, H.M.N. Monitoring microbial contamination of antibiotic resistant Escherichia coli isolated from the surface water of urban park in southeastern Brazil. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100438. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, D.; He, S.; Ye, H.; Zhang, L.; Wenhui, Z.; Zhang, W.; Shuchang, C.; Chen, S. Prevalence of Antibiotic-Resistant Escherichia coli in Drinking Water Sources in Hangzhou City. Front. Microbiol. 2017, 8, 1133. [Google Scholar] [CrossRef]
- Dang, H.; Song, L.; Chen, M.; Chang, Y. Concurrence of cat and tet Genes in Multiple Antibiotic-Resistant Bacteria Isolated from a Sea Cucumber and Sea Urchin Mariculture Farm in China. Microb. Ecol. 2006, 52, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.R.; Na, G.S.; Lu, Z.H.; Gao, H.; Li, R.J.; Wu, X.; Zu, G.R.; Yao, Z. Distribution of sulfonamides and sulfonamide-resistant Escherichia coli in the coastal marine environment of northern yellow sea, China. Chin. J. Appl. Environ. Biol. 2014, 20, 401–406. [Google Scholar]
- Wu, N.; Qiao, M.; Zhang, B.; Cheng, W.-D.; Zhu, Y.-G. Abundance and Diversity of Tetracycline Resistance Genes in Soils Adjacent to Representative Swine Feedlots in China. Environ. Sci. Technol. 2010, 44, 6933–6939. [Google Scholar] [CrossRef]
- Wang, Y. Study on Drug-Resistance and Resistance Gene of Integron Mediated in Escherichia coli from Seawater. Master’s Thesis, Dalian Ocean University, Dalian, China, 2015. [Google Scholar]
- Wang, C.; Gu, X.; Zhang, S.; Wang, P.; Guo, C.; Gu, J.; Hou, J. Characterization of Antibiotic-Resistance Genes in Antibiotic Resistance Escherichia coli Isolates from a Lake. Arch. Environ. Contam. Toxicol. 2013, 65, 635–641. [Google Scholar] [CrossRef]
- Zhang, S.H.; Lv, X.; Han, B.; Gu, X.C.; Wang, P.F.; Wang, C.; He, Z.L. Prevalence of antibiotic resistance genes in antibiotic-resistant Escherichia coli isolates in surface water of Taihu lake basin, China. Environ. Sci. Pollut. 2015, 22, 11412–11421. [Google Scholar] [CrossRef]
- Standley, L.J.; Rudel, R.A.; Swartz, C.H.; Attfield, K.R.; Christian, J.; Erickson, M.; Brody, J.G. Wastewater-contaminated groundwater as a source of endogenous hormones and pharmaceuticals to surface water ecosystems. Environ. Toxicol. Chem. 2008, 27, 2457–2468. [Google Scholar] [CrossRef]
- Yang, Y.-Y.; Toor, G.S.; Williams, C.F. Pharmaceuticals and organochlorine pesticides in sediments of an urban river in Florida, USA. J. Soils Sediments 2015, 15, 993–1004. [Google Scholar] [CrossRef]
- Kumar, A.; Xagoraraki, I. Pharmaceuticals, personal care products and endocrine-disrupting chemicals in U.S. surface and finished drinking waters: A proposed ranking system. Sci. Total Environ. 2010, 408, 5972–5989. [Google Scholar] [CrossRef]
- Kolpin, D.W.; Furlong, E.; Meyer, M.; Thurman, E.M.; Zaugg, S.D.; Barber, L.B.; Buxton, H.T. Pharmaceuticals, Hormones, and Other Organic Wastewater Contaminants in U.S. Streams, 1999−2000: A National Reconnaissance. Environ. Sci. Technol. 2002, 36, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Emilie, L.; Barbara, P.; Thierry, B.; David, S.; Fabienne, P. Occurrence of antibiotic resistance and class 1, 2 and 3 integrons in Escherichia coli isolated from a densely populated estuary (seine, france). FEMS Microbiol. Ecol. 2009, 1, 118–130. [Google Scholar]
- Rosser, S.J.; Hilary-Kay, Y. Identification and characterization of class 1 integrons in bacteria from an aquatic, environment. Antimicrob. chemother. 1999, 44, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Tennstedt, T.; Szczepanowski, R.; Braun, S.; Pühler, A.; Schlüter, A. Occurrence of integron-associated resistance gene cassettes located on antibiotic resistance plasmids isolated from a wastewater treatment plant. FEMS Microbiol. Ecol. 2003, 45, 239–252. [Google Scholar] [CrossRef]
- Skurnik, D.; Le Menac’h, A.; Zurakowski, D.; Mazel, D.; Courvalin, P.; Denamur, E.; Andremont, A.; Ruimy, R. Integron-associated antibiotic resistance and phylogenetic grouping of Escherichia coli isolates from healthy subjects free of recent antibiotic exposure. Antimicrob. Agents Chemother. 2005, 49, 3062–3065. [Google Scholar] [CrossRef]
- Silva, M.; Vaz-Moreira, I.; Gonzalez-Pajuelo, M.; Nunes, O.C.; Manaia, C.M. Antimicrobial resistance patterns in Enterobacteriaceae isolated from an urban waste water treatment plant. FEMS Microbiol. Ecol. 2007, 60, 166–176. [Google Scholar] [CrossRef]


| Antibiotics | % of Resistance (No. of Isolates) | ||||
|---|---|---|---|---|---|
| S1 (n = 46) | S2 (n = 18) | S3 (n = 5) | S4 (n = 8) | S5 (n = 5) | |
| Tetracycline (TCY) | 26.1 (12) | 27.8 (5) | 0 | 0 | 0 |
| Sulfamethoxazole-Trimethoprim (SXT) | 23.9 (11) | 22.2 (4) | 20 (1) | 0 | 0 |
| Trimethoprim (TMP) | 26.1 (12) | 22.2 (4) | 20 (1) | 0 | 40 (2) |
| Gentamicin (GEN) | 8.7 (4) | 11.1 (2) | 20 (1) | 0 | 0 |
| Streptomycin (STR) | 6.5 (3) | 5.6 (1) | 20 (1) | 0 | 0 |
| Ciprofloxacin (CIP) | 8.7 (4) | 11.1 (2) | 0 | 37.5 (3) | 0 |
| Levofloxacin (LVX) | 2.2 (1) | 5.6 (1) | 0 | 0 | 0 |
| Cephalothin (CEP) | 4.3 (2) | 16.7 (3) | 0 | 0 | 0 |
| Aztreonam (ATM) | 0 | 0 | 0 | 0 | 0 |
| Chloramphenicol (CHL) | 4.3 (2) | 5.6 (1) | 0 | 0 | 0 |
| Gene | Tetracyclines | Sulfonamides | Aminoglycosides | β-Lactams | Quinolones | Phenicols | |||
|---|---|---|---|---|---|---|---|---|---|
| TCY | TMP | SXT | STR | GEN | CEP | CIP | LVX | CHL | |
| Class 1 integrase | 0.921 * | 1.000 ** | 1.000 ** | 0.974 ** | 0.860 | 0.040 | 0.585 | 0.967 ** | 0.262 |
| E. coli Strain | Pattern | Size of Amplicon(bp) | Gene Cassette Array |
|---|---|---|---|
| A16 | TCY-TMP-SXT-GEN-STR-CIP-CHL-LVX | 1800 | dfrA12-orfF-aadA2 |
| B14 | TCY-TMP-SXT | 1500 | dhfr12-orfF-aadA2 |
| B27 | TCY-TMP-SXT-GEN-STR-CIP-CHL-LVX | 1900 | aadA2-linF |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, J.; Fan, J.; Ming, H.; Guo, G.; Fu, Y.; Zhao, X.; Zhao, S.; Chen, Q.; Guan, D.; Jin, Y.; et al. The Municipal Sewage Discharge May Impact the Dissemination of Antibiotic-Resistant Escherichia coli in an Urban Coastal Beach. Water 2022, 14, 1639. https://doi.org/10.3390/w14101639
Su J, Fan J, Ming H, Guo G, Fu Y, Zhao X, Zhao S, Chen Q, Guan D, Jin Y, et al. The Municipal Sewage Discharge May Impact the Dissemination of Antibiotic-Resistant Escherichia coli in an Urban Coastal Beach. Water. 2022; 14(10):1639. https://doi.org/10.3390/w14101639
Chicago/Turabian StyleSu, Jie, Jingfeng Fan, Hongxia Ming, Ge Guo, Yunhan Fu, Xiaohui Zhao, Sha Zhao, Quanrui Chen, Daoming Guan, Yuan Jin, and et al. 2022. "The Municipal Sewage Discharge May Impact the Dissemination of Antibiotic-Resistant Escherichia coli in an Urban Coastal Beach" Water 14, no. 10: 1639. https://doi.org/10.3390/w14101639
APA StyleSu, J., Fan, J., Ming, H., Guo, G., Fu, Y., Zhao, X., Zhao, S., Chen, Q., Guan, D., Jin, Y., & Shi, T. (2022). The Municipal Sewage Discharge May Impact the Dissemination of Antibiotic-Resistant Escherichia coli in an Urban Coastal Beach. Water, 14(10), 1639. https://doi.org/10.3390/w14101639






