The Use of Aquatic Macrophytes as a Nature-Based Solution to Prevent Ciprofloxacin Deleterious Effects on Microalgae
Abstract
1. Introduction
2. Materials and Methods
2.1. Cultures
2.2. Bioassays
2.2.1. Phytoremediation Using Aquatic Macrophytes
2.2.2. Toxicological Evaluations in Microalgae
2.3. Data Analysis
3. Results
3.1. Phytoremediation Capacity of Aquatic Macrophytes
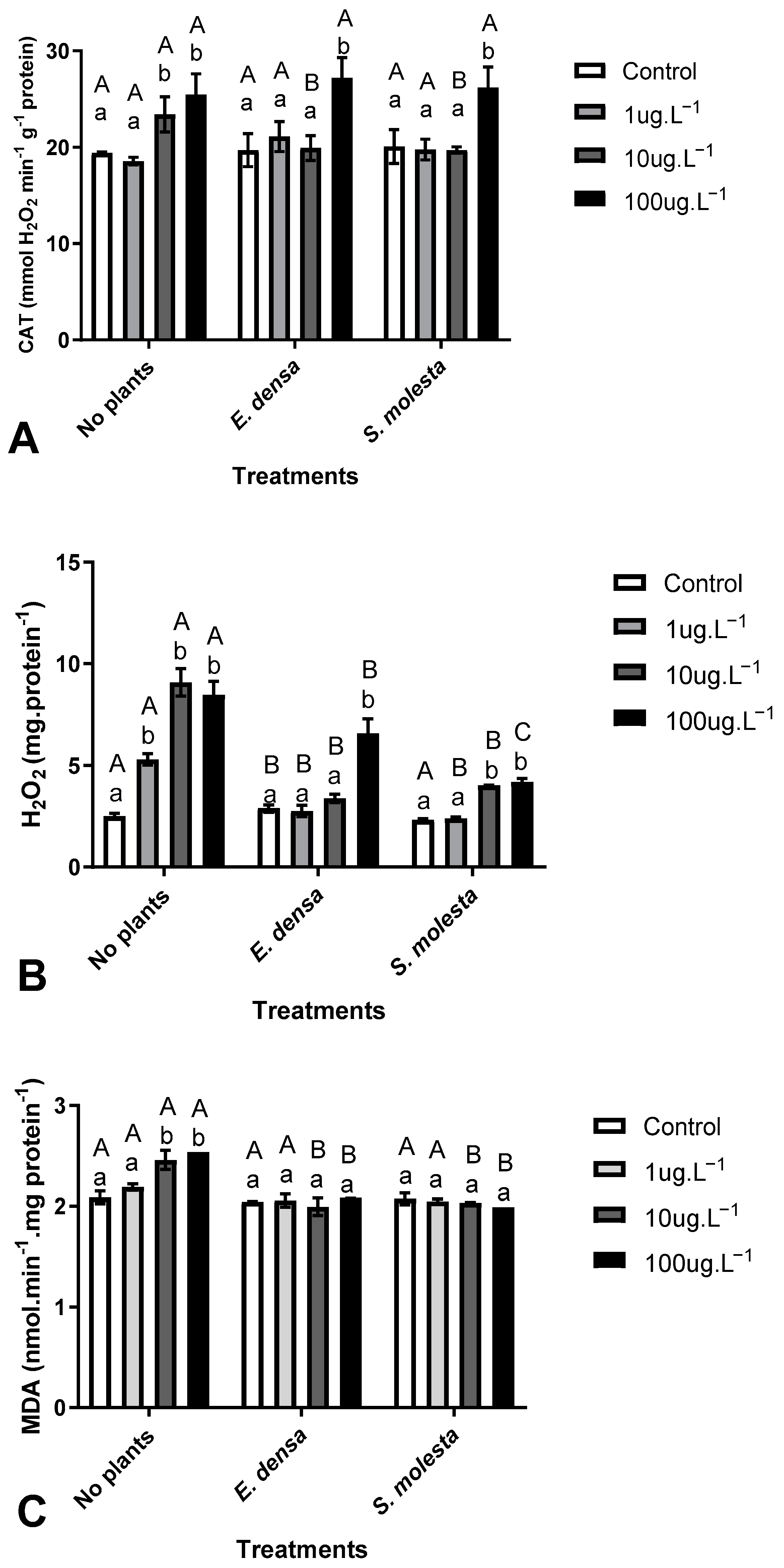
3.2. Toxicological Effects of Cipro on D. subcapitatus
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gomes, M.P.; Brito, J.C.M.; Vieira, F.; Kitamura, R.; Juneau, P. Emerging Contaminants in Streams of Doce River Watershed, Minas Gerais, Brazil. Front. Environ. Sci. 2022, 9, 801599. [Google Scholar] [CrossRef]
- Kelly, K.R.; Brooks, B.W. Global Aquatic Hazard Assessment of Ciprofloxacin: Exceedances of Antibiotic Resistance Development and Ecotoxicological Thresholds. Prog. Mol. Biol. Transl. Sci. 2018, 159, 59–77. [Google Scholar] [PubMed]
- Sarafraz, M.; Ali, S.; Sadani, M.; Heidarinejad, Z.; Bay, A.; Fakhri, Y.; Mousavi Khaneghah, A. A Global Systematic, Review-Meta Analysis and Ecological Risk Assessment of Ciprofloxacin in River Water. Int. J. Environ. Anal. Chem. 2020, 102, 5106–5121. [Google Scholar] [CrossRef]
- Wang, J.; Chu, L.; Wojnárovits, L.; Takács, E. Occurrence and Fate of Antibiotics, Antibiotic Resistant Genes (ARGs) and Antibiotic Resistant Bacteria (ARB) in Municipal Wastewater Treatment Plant: An Overview. Sci. Total Environ. 2020, 744, 140997. [Google Scholar] [CrossRef]
- Mathur, P.; Sanyal, D.; Callahan, D.L.; Conlan, X.A.; Pfeffer, F.M. Treatment Technologies to Mitigate the Harmful Effects of Recalcitrant Fluoroquinolone Antibiotics on the Environment and Human Health. Environ. Pollut. 2021, 291, 118233. [Google Scholar] [CrossRef] [PubMed]
- Pazda, M.; Kumirska, J.; Stepnowski, P.; Mulkiewicz, E. Antibiotic Resistance Genes Identified in Wastewater Treatment Plant Systems—A Review. Sci. Total Environ. 2019, 697, 134023. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, E.; Cummins, E. Antibiotic Resistance in Surface Water Ecosystems: Presence in the Aquatic Environment, Prevention Strategies, and Risk Assessment. Hum. Ecol. Risk Assess. Int. J. 2017, 23, 299–322. [Google Scholar] [CrossRef]
- Dalhoff, A. Global Fluoroquinolone Resistance Epidemiology and Implictions for Clinical Use. Interdiscip. Perspect. Infect. Dis. 2012, 2012, 1–37. [Google Scholar] [CrossRef]
- Elizalde-Velázquez, G.A.; Rosas-Ramírez, J.R.; Raldua, D.; García-Medina, S.; Orozco-Hernández, J.M.; Rosales- Pérez, K.; Islas-Flores, H.; Galar-Martínez, M.; Guzmán-García, X.; Gómez-Oliván, L.M. Low Concentrations of Ciprofloxacin Alone and in Combination with Paracetamol Induce Oxidative Stress, Upregulation of Apoptotic-Related Genes, Histological Alterations in the Liver, and Genotoxicity in Danio Rerio. Chemosphere 2022, 294, 133667. [Google Scholar] [CrossRef]
- Kitamura, R.S.A.; Vicentini, M.; Bitencourt, V.; Vicari, T.; Motta, W.; Brito, J.C.M.; Cestari, M.M.; Prodocimo, M.M.; de Assis, H.C.S.; Gomes, M.P. Salvinia Molesta Phytoremediation Capacity as a Nature-Based Solution to Prevent Harmful Effects and Accumulation of Ciprofloxacin in Neotropical Catfish. Environ. Sci. Pollut. Res. 2023, 30, 41848–41863. [Google Scholar] [CrossRef]
- Peltzer, P.M.; Lajmanovich, R.C.; Attademo, A.M.; Junges, C.M.; Teglia, C.M.; Martinuzzi, C.; Curi, L.; Culzoni, M.J.; Goicoechea, H.C. Ecotoxicity of Veterinary Enrofloxacin and Ciprofloxacin Antibiotics on Anuran Amphibian Larvae. Environ. Toxicol. Pharmacol. 2017, 51, 114–123. [Google Scholar] [CrossRef]
- Kitamura, R.S.A.; Brito, J.C.M.; Silva de Assis, H.C.; Gomes, M.P. Physiological Responses and Phytoremediation Capacity of Floating and Submerged Aquatic Macrophytes Exposed to Ciprofloxacin. Environ. Sci. Pollut. Res. 2023, 30, 622–639. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, R.S.A.; Vicentini, M.; Perussolo, M.C.; Lirola, J.R.; Cirilo dos Santos, C.F.; Moreira Brito, J.C.; Cestari, M.M.; Prodocimo, M.M.; Gomes, M.P.; Silva de Assis, H.C. Sublethal Biochemical, Histopathological and Genotoxicological Effects of Short-Term Exposure to Ciprofloxacin in Catfish Rhamdia Quelen. Environ. Pollut. 2022, 300, 118935. [Google Scholar] [CrossRef]
- Marques, R.Z.; Wistuba, N.; Brito, J.C.M.; Bernardoni, V.; Rocha, D.C.; Gomes, M.P. Crop Irrigation (Soybean, Bean, and Corn) with Enrofloxacin-Contaminated Water Leads to Yield Reductions and Antibiotic Accumulation. Ecotoxicol. Environ. Saf. 2021, 216, 112193. [Google Scholar] [CrossRef]
- Adikwu, E.; Brambaifa, N. Ciprofloxacin Cardiotoxicity and Hepatotoxicity in Humans and Animals. Pharmacol. Pharm. 2012, 3, 207–213. [Google Scholar] [CrossRef]
- Rosas-Ramírez, J.R.; Orozco-Hernández, J.M.; Elizalde-Velázquez, G.A.; Raldúa, D.; Islas-Flores, H.; Gómez-Oliván, L.M. Teratogenic Effects Induced by Paracetamol, Ciprofloxacin, and Their Mixture on Danio Rerio Embryos: Oxidative Stress Implications. Sci. Total Environ. 2022, 806, 150541. [Google Scholar] [CrossRef]
- Yang, C.; Song, G.; Lim, W. A Review of the Toxicity in Fish Exposed to Antibiotics. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 237, 108840. [Google Scholar] [CrossRef]
- Ramesh, M.; Sujitha, M.; Anila, P.A.; Ren, Z.; Poopal, R.K. Responses of Cirrhinus Mrigala to Second-generation Fluoroquinolone (Ciprofloxacin) Toxicity: Assessment of Antioxidants, Tissue Morphology, and Inorganic Ions. Environ. Toxicol. 2021, 36, 887–902. [Google Scholar] [CrossRef]
- Diniz, V.; Rath, G.; Rath, S.; Rodrigues-Silva, C.; Guimarães, J.R.; Cunha, D.G.F. Long-Term Ecotoxicological Effects of Ciprofloxacin in Combination with Caffeine on the Microalga Raphidocelis Subcapitata. Toxicol. Rep. 2021, 8, 429–435. [Google Scholar] [CrossRef]
- Gomes, M.P.; Gonçalves, C.A.; de Brito, J.C.M.; Souza, A.M.; da Silva Cruz, F.V.; Bicalho, E.M.; Figueredo, C.C.; Garcia, Q.S. Ciprofloxacin Induces Oxidative Stress in Duckweed (Lemna Minor L.): Implications for Energy Metabolism and Antibiotic-Uptake Ability. J. Hazard. Mater. 2017, 328, 140–149. [Google Scholar] [CrossRef]
- Gomes, M.P.; Kitamura, R.S.A.; Marques, R.Z.; Barbato, M.L.; Zámocký, M. The Role of H2O2-Scavenging Enzymes (Ascorbate Peroxidase and Catalase) in the Tolerance of Lemna Minor to Antibiotics: Implications for Phytoremediation. Antioxidants 2022, 11, 151. [Google Scholar] [CrossRef]
- Nunes, B.; Veiga, V.; Frankenbach, S.; Serôdio, J.; Pinto, G. Evaluation of Physiological Changes Induced by the Fluoroquinolone Antibiotic Ciprofloxacin in the Freshwater Macrophyte Species Lemna Minor and Lemna Gibba. Environ. Toxicol. Pharmacol. 2019, 72, 103242. [Google Scholar] [CrossRef]
- Gomes, M.P.; de Brito, J.C.M.; Bicalho, E.M.; Silva, J.G.; de Fátima Gomides, M.; Garcia, Q.S.; Figueredo, C.C. Ciprofloxacin vs. Temperature: Antibiotic Toxicity in the Free-Floating Liverwort Ricciocarpus Natans from a Climate Change Perspective. Chemosphere 2018, 202, 410–419. [Google Scholar] [CrossRef]
- Sodhi, K.K.; Singh, D.K. Insight into the Fluoroquinolone Resistance, Sources, Ecotoxicity, and Degradation with Special Emphasis on Ciprofloxacin. J. Water Process Eng. 2021, 43, 102218. [Google Scholar] [CrossRef]
- Furley, T.H.; Brodeur, J.; Silva de Assis, H.C.; Carriquiriborde, P.; Chagas, K.R.; Corrales, J.; Denadai, M.; Fuchs, J.; Mascarenhas, R.; Miglioranza, K.S.B.; et al. Toward Sustainable Environmental Quality: Identifying Priority Research Questions for Latin America. Integr. Environ. Assess. Manag. 2018, 14, 344–357. [Google Scholar] [CrossRef]
- Liu, S.; Dong, G.; Zhao, H.; Chen, M.; Quan, W.; Qu, B. Occurrence and Risk Assessment of Fluoroquinolones and Tetracyclines in Cultured Fish from a Coastal Region of Northern China. Environ. Sci. Pollut. Res. 2018, 25, 8035–8043. [Google Scholar] [CrossRef]
- McCorquodale-Bauer, K.; Grosshans, R.; Zvomuya, F.; Cicek, N. Critical Review of Phytoremediation for the Removal of Antibiotics and Antibiotic Resistance Genes in Wastewater. Sci. Total Environ. 2023, 870, 161876. [Google Scholar] [CrossRef]
- Miksch, K.; Cema, G.; Corvini, P.F.X.; Felis, E.; Sochacki, A.; Surmacz-Górska, J.; Wiszniowski, J.; Zabczynski, S. R&D Priorities in the Field of Sustainable Remediation and Purification of Agro-Industrial and Municipal Wastewater. N. Biotechnol. 2015, 32, 128–132. [Google Scholar] [CrossRef]
- Song, Y.; Kirkwood, N.; Maksimović, Č.; Zhen, X.; O’Connor, D.; Jin, Y.; Hou, D. Nature Based Solutions for Contaminated Land Remediation and Brownfield Redevelopment in Cities: A Review. Sci. Total Environ. 2019, 663, 568–579. [Google Scholar] [CrossRef]
- Wei, Z.; Van Le, Q.; Peng, W.; Yang, Y.; Yang, H.; Gu, H.; Lam, S.S.; Sonne, C. A Review on Phytoremediation of Contaminants in Air, Water and Soil. J. Hazard. Mater. 2021, 403, 123658. [Google Scholar] [CrossRef]
- Fletcher, J.; Willby, N.; Oliver, D.M.; Quilliam, R.S. Phytoremediation Using Aquatic Plants. In Phytoremediation. Concepts and Strategies in Plant Sciences; Springer: Berlin/Heidelberg, Germany, 2020; pp. 205–260. [Google Scholar]
- Ansari, A.A.; Naeem, M.; Gill, S.S.; AlZuaibr, F.M. Phytoremediation of Contaminated Waters: An Eco-Friendly Technology Based on Aquatic Macrophytes Application. Egypt J. Aquat. Res. 2020, 46, 371–376. [Google Scholar] [CrossRef]
- Gebeyehu, A.; Shebeshe, N.; Kloos, H.; Belay, S. Suitability of Nutrients Removal from Brewery Wastewater Using a Hydroponic Technology with Typha Latifolia. BMC Biotechnol. 2018, 18, 74. [Google Scholar] [CrossRef]
- Gomes, M.P.; Tavares, D.S.; Richardi, V.S.; Marques, R.Z.; Wistuba, N.; Moreira de Brito, J.C.; Soffiatti, P.; Sant’Anna-Santos, B.F.; Navarro da Silva, M.A.; Juneau, P. Enrofloxacin and Roundup® Interactive Effects on the Aquatic Macrophyte Elodea Canadensis Physiology. Environ. Pollut. 2019, 249, 453–462. [Google Scholar] [CrossRef]
- Yan, Y.; Deng, Y.; Li, W.; Du, W.; Gu, Y.; Li, J.; Xu, X. Phytoremediation of Antibiotic-Contaminated Wastewater: Insight into the Comparison of Ciprofloxacin Absorption, Migration, and Transformation Process at Different Growth Stages of E. Crassipes. Chemosphere 2021, 283, 131192. [Google Scholar] [CrossRef]
- Yan, Y.; Pengmao, Y.; Xu, X.; Zhang, L.; Wang, G.; Jin, Q.; Chen, L. Migration of Antibiotic Ciprofloxacin during Phytoremediation of Contaminated Water and Identification of Transformation Products. Aquat. Toxicol. 2020, 219, 105374. [Google Scholar] [CrossRef]
- Deng, Y.; Qian, X.; Wu, Y.; Ma, T.; Xu, X.; Li, J.; Wang, G.; Yan, Y. Effects of Ciprofloxacin on Eichhornia Crassipes Phytoremediation Performance and Physiology under Hydroponic Conditions. Environ. Sci. Pollut. Res. 2022, 29, 47363–47372. [Google Scholar] [CrossRef]
- Gomes, M.P.; Brito, J.C.M.; Rocha, D.C.; Navarro-Silva, M.M.A.M.; Juneau, P.; Moreira Brito, J.C.; Cristina Rocha, D.; Navarro-Silva, M.M.A.M.; Juneau, P.; Brito, J.C.M.; et al. Individual and Combined Effects of Amoxicillin, Enrofloxacin, and Oxytetracycline on Lemna Minor Physiology. Ecotoxicol. Environ. Saf. 2020, 203, 111025. [Google Scholar] [CrossRef]
- Gostyńska, J.; Pankiewicz, R.; Romanowska-Duda, Z.; Messyasz, B. Overview of Allelopathic Potential of Lemna Minor L. Obtained from a Shallow Eutrophic Lake. Molecules 2022, 27, 3428. [Google Scholar] [CrossRef]
- Bich, T.T.N.; Kato-Noguchi, H. Allelopathic Potential of Two Aquatic Plants, Duckweed (Lemna minor L.) and Water Lettuce (Pistia stratiotes L.), on Terrestrial Plant Species. Aquat. Bot. 2012, 103, 30–36. [Google Scholar] [CrossRef]
- Rocha, C.S.; Kochi, L.Y.; Ribeiro, G.B.; Rocha, D.C.; Carneiro, D.N.M.; Gomes, M.P. Evaluating Aquatic Macrophytes for Removing Erythromycin from Contaminated Water: Floating or Submerged? Int. J. Phytoremediation 2021, 24, 995–1003. [Google Scholar] [CrossRef]
- Vilvert, E.; Contardo-Jara, V.; Esterhuizen-Londt, M.; Pflugmacher, S. The Effect of Oxytetracycline on Physiological and Enzymatic Defense Responses in Aquatic Plant Species Egeria Densa, Azolla Caroliniana, and Taxiphyllum Barbieri. Toxicol. Environ. Chem. 2017, 99, 104–116. [Google Scholar] [CrossRef]
- Hussain, N.; Abbasi, T.; Abbasi, S.A. Vermiremediation of an Invasive and Pernicious Weed Salvinia (Salvinia molesta). Ecol. Eng. 2016, 91, 432–440. [Google Scholar] [CrossRef]
- Senavirathna, M.D.H.J.; Muhetaer, G.; Atapaththu, K.S.S.; Fujino, T. Egeria Densa Allelopathy on Microcystis Aeruginosa Under Different Light Intensities and Preliminary Insight into Inter-Parameter Relationships. Water Air Soil Pollut. 2021, 232, 135. [Google Scholar] [CrossRef]
- Li, B.; Yin, Y.; Kang, L.; Feng, L.; Liu, Y.; Du, Z.; Tian, Y.; Zhang, L. A Review: Application of Allelochemicals in Water Ecological Restoration—Algal Inhibition. Chemosphere 2021, 267, 128869. [Google Scholar] [CrossRef] [PubMed]
- Ceschin, S.; Bellini, A.; Scalici, M. Aquatic Plants and Ecotoxicological Assessment in Freshwater Ecosystems: A Review. Environ. Sci. Pollut. Res. 2021, 28, 4975–4988. [Google Scholar] [CrossRef]
- Albert, C.; Brillinger, M.; Guerrero, P.; Gottwald, S.; Henze, J.; Schmidt, S.; Ott, E.; Schröter, B. Planning Nature-Based Solutions: Principles, Steps, and Insights. Ambio 2021, 50, 1446–1461. [Google Scholar] [CrossRef]
- Cohen-Shacham, E.; Walters, G.; Janzen, C.; Maginnis, S. (Eds.) Nature-Based Solutions to Address Global Societal Challenges; IUCN International Union for Conservation of Nature: Gland, Switzerland, 2016; ISBN 9782831718125. [Google Scholar]
- Prigioniero, A.; Zuzolo, D.; Niinemets, Ü.; Guarino, C. Nature-Based Solutions as Tools for Air Phytoremediation: A Review of the Current Knowledge and Gaps. Environ. Pollut. 2021, 277, 116817. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, P.N.; Finger, D.C.; Masi, F.; Cipolletta, G.; Oral, H.V.; Tóth, A.; Regelsberger, M.; Exposito, A. Nature-Based Solutions Addressing the Water-Energy-Food Nexus: Review of Theoretical Concepts and Urban Case Studies. J. Clean. Prod. 2022, 338, 130652. [Google Scholar] [CrossRef]
- Oral, H.V.; Carvalho, P.; Gajewska, M.; Ursino, N.; Masi, F.; van Hullebusch, E.D.; Kazak, J.K.; Exposito, A.; Cipolletta, G.; Andersen, T.R.; et al. A Review of Nature-Based Solutions for Urban Water Management in European Circular Cities: A Critical Assessment Based on Case Studies and Literature. Blue-Green Syst. 2020, 2, 112–136. [Google Scholar] [CrossRef]
- OECD (Organization for Economic Co-operation and Development). Test No. 221: Lemna sp. Growth Inhibition Test; OECD Guidelines for the Testing of Chemicals, Section 2: Effects on Biotic Systems; OECD: Paris, France, 2006; ISBN 9789264016194. [Google Scholar]
- Shi, L.; Zhou, X.F.; Zhang, Y.L.; Gu, G.W. Simultaneous Determination of 8 Fluoroquinolone Antibiotics in Sewage Treatment Plants by Solid-Phase Extraction and Liquid Chromatography with Fluorescence Detection. Water Sci. Technol. 2009, 59, 805–813. [Google Scholar] [CrossRef]
- Migliore, L.; Cozzolino, S.; Fiori, M. Phytotoxicity to and Uptake of Enrofloxacin in Crop Plants. Chemosphere 2003, 52, 1233–1244. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative Stress and Some Antioxidant Systems in Acid Rain-Treated Bean Plants. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the Thiobarbituric Acid-Reactive-Substances Assay for Estimating Lipid Peroxidation in Plant Tissues Containing Anthocyanin and Other Interfering Compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Aebi, H. [13] Catalase in Vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- OECD (Organization for Economic Co-operation and Development). Test No. 201: Freshwater Alga and Cyanobacteria, Growth Inhibition Test; OECD Guidelines for the Testing of Chemicals, Section 2: Effects on Biotic Systems; OECD Publishing: Paris, France, 2011; ISBN 9789264069923. [Google Scholar]
- Adedara, I.A.; Godswill, U.-A.S.; Mike, M.A.; Afolabi, B.A.; Amorha, C.C.; Sule, J.; Rocha, J.B.T.; Farombi, E.O. Chronic Ciprofloxacin and Atrazine Co-Exposure Aggravates Locomotor and Exploratory Deficits in Non-Target Detritivore Speckled Cockroach (Nauphoeta cinerea). Environ. Sci. Pollut. Res. 2021, 28, 25680–25691. [Google Scholar] [CrossRef] [PubMed]
- Yisa, A.G.; Chia, M.A.; Sha’aba, R.I.; Gauji, B.; Gadzama, I.M.K.; Oniye, S.J. The Antibiotic Ciprofloxacin Alters the Growth, Biochemical Composition, and Antioxidant Response of Toxin-Producing and Non-Toxin-Producing Strains of Microcystis. J. Appl. Phycol. 2021, 33, 2145–2155. [Google Scholar] [CrossRef]
- Evans-Roberts, K.M.; Mitchenall, L.A.; Wall, M.K.; Leroux, J.; Mylne, J.S.; Maxwell, A. DNA Gyrase Is the Target for the Quinolone Drug Ciprofloxacin in Arabidopsis Thaliana. J. Biol. Chem. 2016, 291, 3136–3144. [Google Scholar] [CrossRef]
- Lu, T.; Zhu, Y.; Ke, M.; Peijnenburg, W.J.G.M.; Zhang, M.; Wang, T.; Chen, J.; Qian, H. Evaluation of the Taxonomic and Functional Variation of Freshwater Plankton Communities Induced by Trace Amounts of the Antibiotic Ciprofloxacin. Environ. Int. 2019, 126, 268–278. [Google Scholar] [CrossRef]
- Gomes, M.P.; Le Manac’h, S.G.; Hénault-Ethier, L.; Labrecque, M.; Lucotte, M.; Juneau, P. Glyphosate-Dependent Inhibition of Photosynthesis in Willow. Front. Plant. Sci. 2017, 8, 207. [Google Scholar] [CrossRef]
- Panja, S.; Sarkar, D.; Datta, R. Removal of Tetracycline and Ciprofloxacin from Wastewater by Vetiver Grass (Chrysopogon zizanioides (L.) Roberty) as a Function of Nutrient Concentrations. Environ. Sci. Pollut. Res. 2020, 27, 34951–34965. [Google Scholar] [CrossRef] [PubMed]
- Panja, S.; Sarkar, D.; Li, K.; Datta, R. Uptake and Transformation of Ciprofloxacin by Vetiver Grass (Chrysopogon zizanioides). Int. Biodeterior. Biodegrad. 2019, 142, 200–210. [Google Scholar] [CrossRef]
- Adesanya, T.; Zvomuya, F.; Farenhorst, A. Phytoextraction of Ciprofloxacin and Sulfamethoxaxole by Cattail and Switchgrass. Chemosphere 2021, 279, 130534. [Google Scholar] [CrossRef]
- Kochi, L.Y.; Freitas, P.L.; Maranho, L.T.; Juneau, P.; Gomes, M.P. Aquatic Macrophytes in Constructed Wetlands: A Fight against Water Pollution. Sustainability 2020, 12, 9202. [Google Scholar] [CrossRef]
- Kitamura, R.S.A.; Marques, R.Z.; Kubis, G.C.; Kochi, L.Y.; Barbato, M.L.; Maranho, L.T.; Juneau, P.; Gomes, M.P. The Phytoremediation Capacity of Lemna minor Prevents Deleterious Effects of Anti-HIV Drugs to Nontarget Organisms. Environ. Pollut. 2023, 329, 121672. [Google Scholar] [CrossRef] [PubMed]

| System | Treatments (µg L−1) | Cipro Concentration in Water (µg L−1) | Degradation (%) | Phytoremediation Efficiency (%) | |
|---|---|---|---|---|---|
| Initial (T0) | 96 h | 96 h | 96 h | ||
| Natural degradation (No plants) | 0 | n.d | n.d | n.d | - |
| 1 | 1.03 ± 0.05 Aa | 0.98 ± 0.04 Aa | 2.40 ± 1.29 a | - | |
| 10 | 10.43 ± 0.28 Ab | 8.80 ± 0.13 Ab* | 15.44 ± 1.07 b | - | |
| 100 | 100.63 ± 9.58 Ac | 74.01 ± 5.81 Ac* | 21.70 ± 5.48 a | ||
| Water treatment (Salvinia molesta) | 0 | n.d | n.d | - | n.d |
| 1 | 1.01 ± 0.04 Aa | n.d Ba* | - | 97.60 ± 1.29 Aa | |
| 10 | 11.76 ± 0.66 Ab | 0.56 ± 0.03 Bb* | - | 79.67 ± 0.71 Ab | |
| 100 | 100.63 ± 9.58 Ac | 2.47 ± 0.17 Bb* | 76.61 ± 3.21 Ab | ||
| Water treatment (Egeria densa) | 0 | n.d | n.d | - | n.d |
| 1 | 1.01 ± 0.51 Aa | 0.17 ± 0.03 C* | - | 81.40 ± 2.71 Ba | |
| 10 | 10.81 ± 0.05 Ab | 2.44 ± 0.26 Bb* | - | 57.96 ± 2.75 Bb | |
| 100 | 106.00 ± 3.91 Ac | 12.08 ± 1.33 Cb* | 66.60 ± 3.01 Bb | ||
| F Values | D.F | Pn | Respiration | DGR | IR | CAT | H2O2 | MDA |
|---|---|---|---|---|---|---|---|---|
| Cipro concentration | 6 | 83.12 *** | 47.00 *** | 433.15 *** | 1638.0 *** | 12.31 *** | 66.49 *** | 4.43 * |
| Systems | 2 | 146.30 *** | 66.15 *** | 812.70 *** | 4828.0 *** | 0.12 | 75.46 *** | 40.27 *** |
| Cipro × Systems | 3 | 14.26 *** | 7.03 ** | 96.69 *** | 562.9 *** | 0.92 | 14.78 *** | 7.27 *** |
| Comparison of means | ||||||||
| Systems | ||||||||
| Tukey Test, p < 0.05 | ||||||||
| No plants | 3.01 ± 0.93 a | 2.16 ± 0.51 a | −34,750 ± 23,100 a | 53.56 ± 18.39 a | 21.73 ± 3.28 a | 6.34 ± 1.52 a | 2.32 ± 0.23 a | |
| E. densa | 4.94 ± 0.38 b | 2.91 ± 0.21 b | 16,118 ± 11,095 b | 12.78 ± 8.90 b | 21.98 ± 3.54 a | 3.90 ± 0.90 b | 2.04 ± 0.04 b | |
| S. molesta | 5.30 ± 0.39 c | 3.36 ± 0.11 c | 24,048 ± 11,333 c | 1.34 ± 0.62 c | 21.43 ± 3.17 a | 3.23 ± 0.51 c | 2.03 ± 0.03 b | |
| Cipro (µg L−1) | ||||||||
| Tukey Test, p < 0.05 | ||||||||
| Control | 5.62 ± 0.07 a | 3.55 ± 0.07 a | 32,435 ± 1925 a | 0 ± 0 a | 19.73 ± 0.19 a | 2.57 ± 0.16 a | 2.07 ± 0.01 a | |
| 1 | 4.91 ± 0.80 b | 2.90 ± 0.34 b | 5769 ± 2609 b | 21.08 ± 20.88 b | 19.82 ± 0.73 ab | 3.48 ± 0.91 b | 2.10 ± 0.04 ab | |
| 10 | 3.99 ± 1.01 c | 2.66 ± 0.49 c | 2769 ± 2111 c | 24.21 ± 22.06 c | 21.02 ± 1.20 ab | 5.49 ± 1.80 c | 2.16 ± 0.14 b | |
| 100 | 3.15 ± 0.97 d | 2.12 ± 0.52 d | −33,093 ± 19,752 d | 45.04 ± 20.78 d | 26.30 ± 0.50 b | 6.41 ± 1.23 d | 2.20 ± 0.16 b |
| Plants | Phytoremediation Efficiency | Treatment Time | Ecotoxicity Evaluation | References |
|---|---|---|---|---|
| S. molesta | 76 to 97% | 96 h | Prevents deleterious effects on microalgae Desmodesmus subspicatus | Present work |
| E. densa | 57 to 81% | |||
| S. molesta | 79 to 97% | 96 h | Prevents deleterious effects to catfish Rhamdia quelen and human health | [10] |
| S. molesta | 63 to 76% | 96 h | No | [12] |
| S. molesta | 69 to 93% | 7 days | No | [12] |
| E. densa | 58 to 75% | 96 h | No | [12] |
| E. densa | 68 to 90% | 7 days | No | [12] |
| Eichhornia crassipes | Up to 84% | 7 Days | No | [37] |
| Chrysopogon zizanioides | 94% | 60 days | No | [65] |
| Chrysopogon zizanioides | 97% | 30 days | No | [66] |
| Typha latifolia | 34% | 7 days | No | [67] |
| Panicum virgatum | 10% | 7 days | No | [67] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kitamura, R.S.A.; Fusaro, T.; Marques, R.Z.; Brito, J.C.M.; Juneau, P.; Gomes, M.P. The Use of Aquatic Macrophytes as a Nature-Based Solution to Prevent Ciprofloxacin Deleterious Effects on Microalgae. Water 2023, 15, 2143. https://doi.org/10.3390/w15122143
Kitamura RSA, Fusaro T, Marques RZ, Brito JCM, Juneau P, Gomes MP. The Use of Aquatic Macrophytes as a Nature-Based Solution to Prevent Ciprofloxacin Deleterious Effects on Microalgae. Water. 2023; 15(12):2143. https://doi.org/10.3390/w15122143
Chicago/Turabian StyleKitamura, Rafael Shinji Akiyama, Tayna Fusaro, Raizza Zorman Marques, Julio Cesar Moreira Brito, Philippe Juneau, and Marcelo Pedrosa Gomes. 2023. "The Use of Aquatic Macrophytes as a Nature-Based Solution to Prevent Ciprofloxacin Deleterious Effects on Microalgae" Water 15, no. 12: 2143. https://doi.org/10.3390/w15122143
APA StyleKitamura, R. S. A., Fusaro, T., Marques, R. Z., Brito, J. C. M., Juneau, P., & Gomes, M. P. (2023). The Use of Aquatic Macrophytes as a Nature-Based Solution to Prevent Ciprofloxacin Deleterious Effects on Microalgae. Water, 15(12), 2143. https://doi.org/10.3390/w15122143








