Kinetics of Obtaining Microalgal Biomass and Removal of Organic Contaminants in Photobioreactors Operated with Microalgae—Study Case: Treatment of Wastewater from a Poultry Slaughterhouse
Abstract
:1. Introduction
2. Materials and Methods
2.1. Production of the Microalgae Chlorella spp. and Spirulina maxima
2.2. Propagation of Microalgal Species
2.3. Sampling and Physicochemical Characterization of Poultry Wastewater
2.4. Cultivation of Microalgae in Poultry Wastewater
2.5. Addition of Micronutrients to Microalgal Cultures in Poultry Wastewater
2.6. Mathematical Modeling of Microalgal Growth
2.7. Experimental Design
3. Results and Discussion
3.1. Wastewater Characteristics
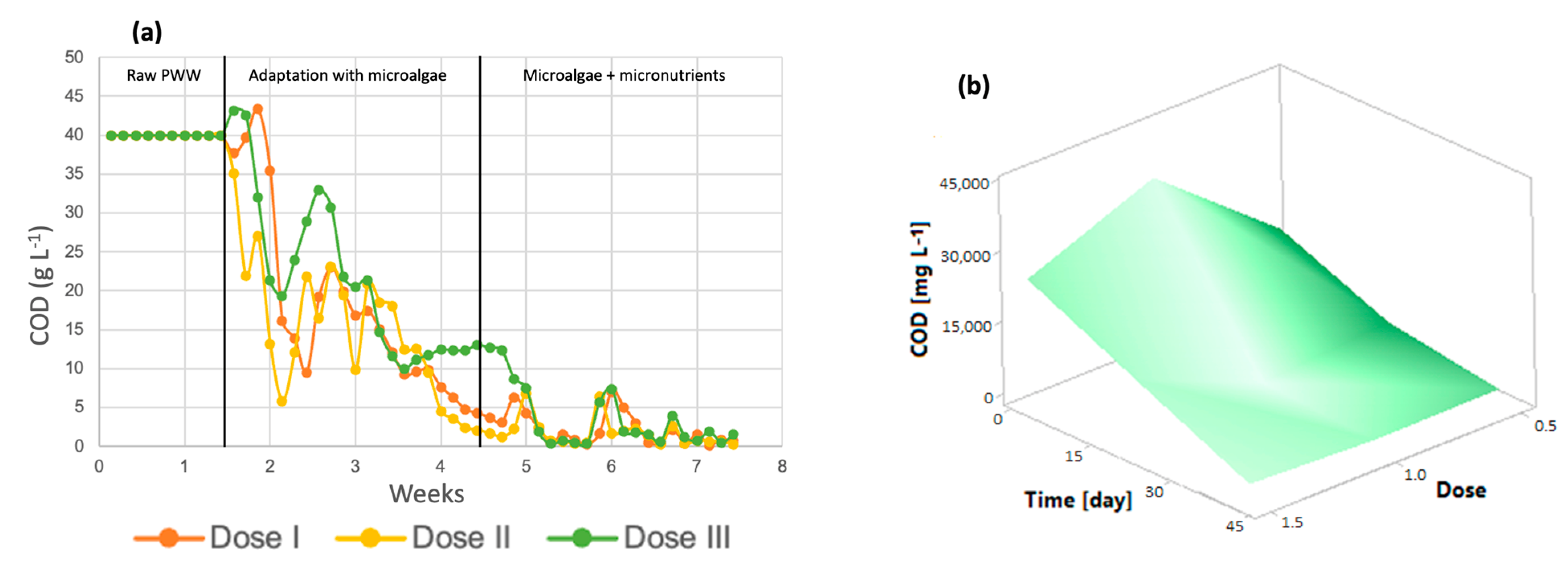
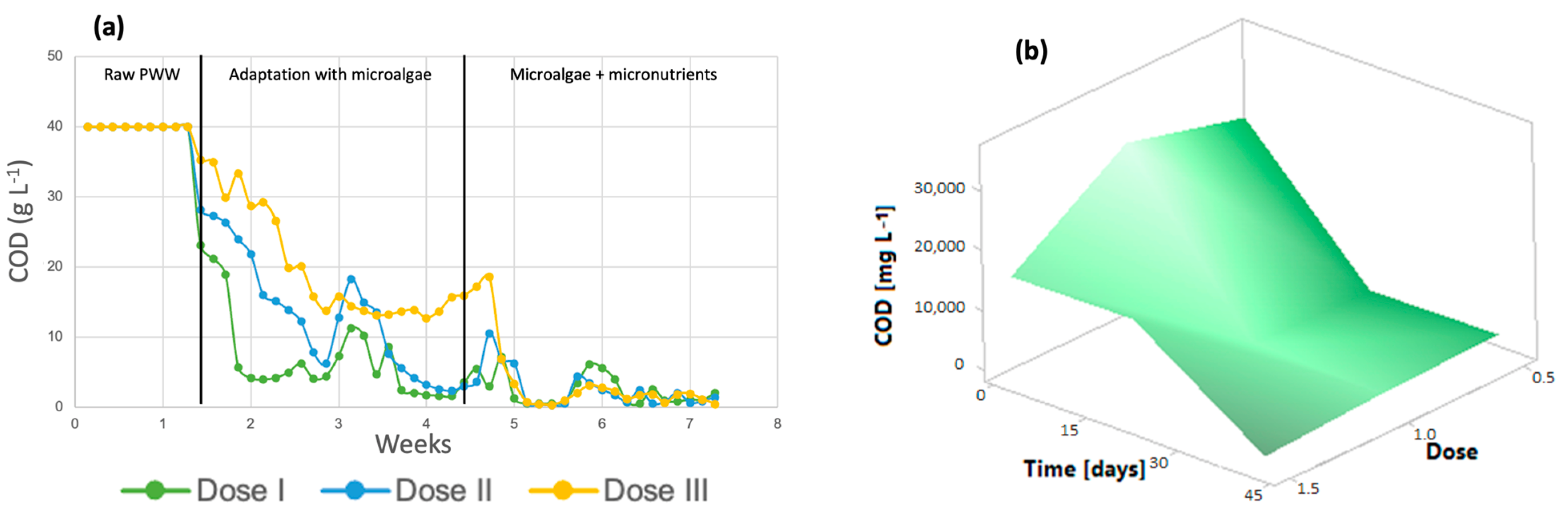
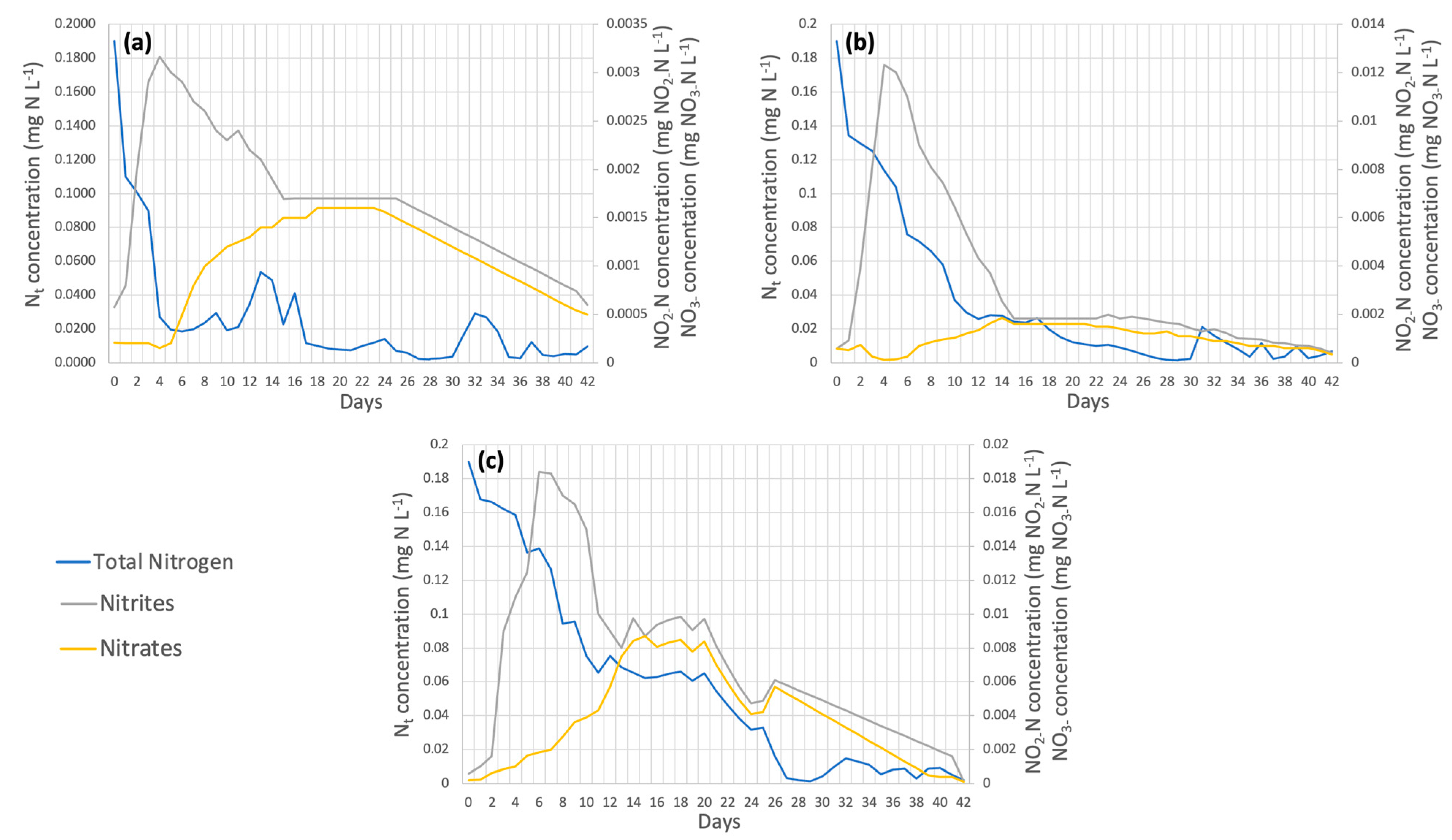
3.2. Effect of Micronutrients on the Removal of Organic Pollutants by Microalgae
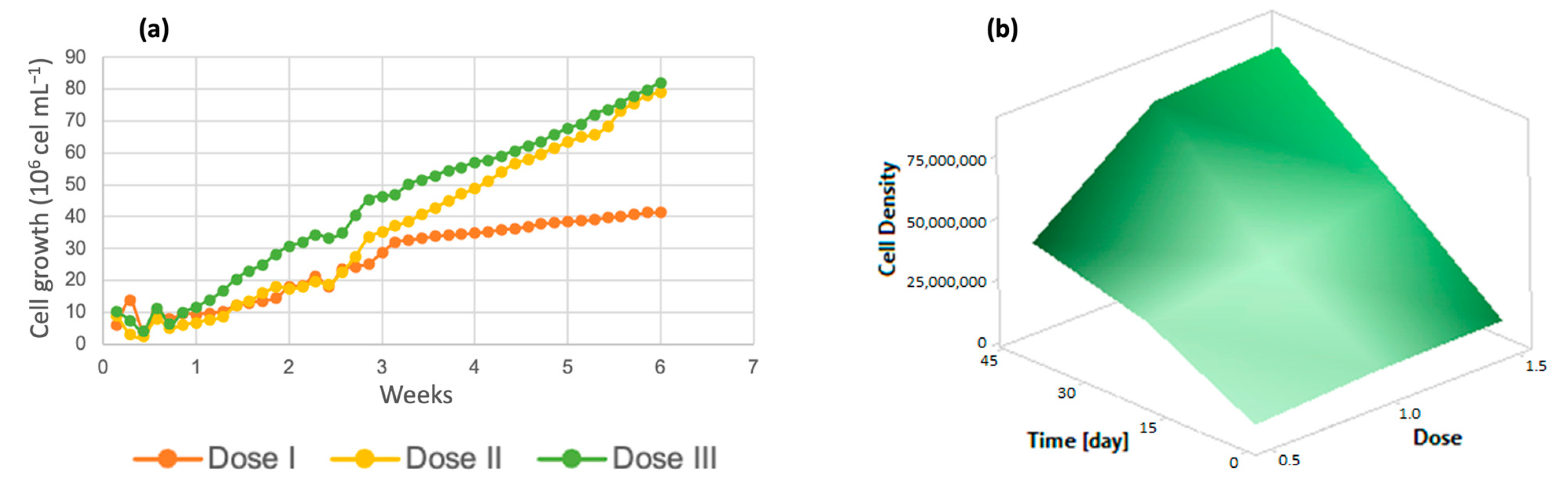
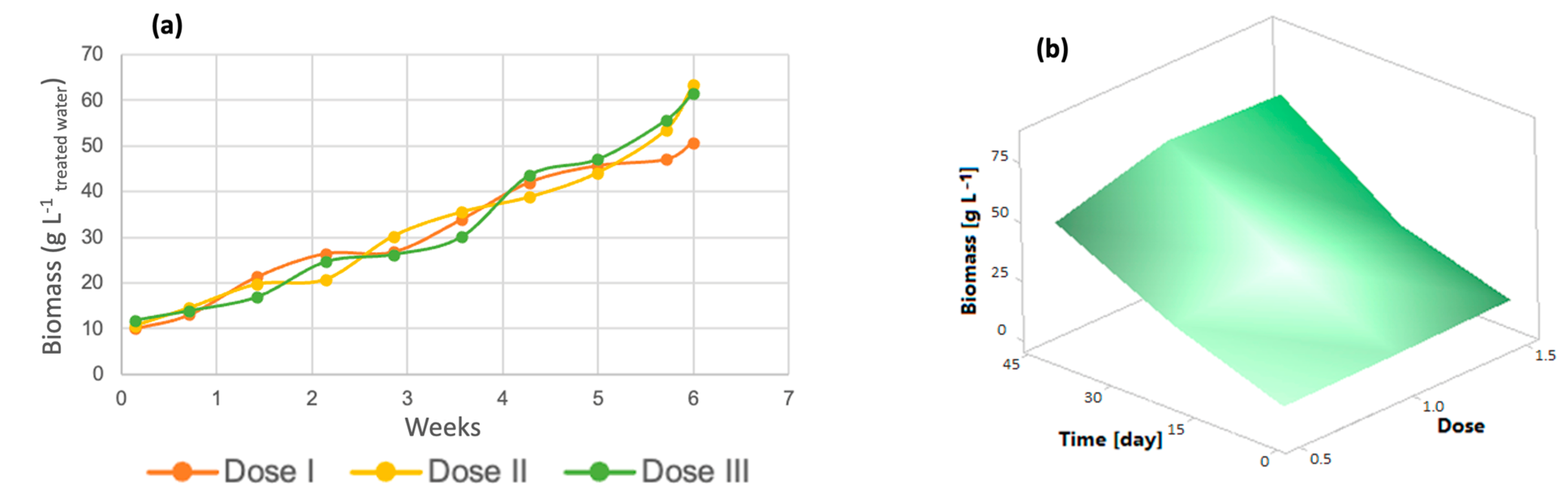
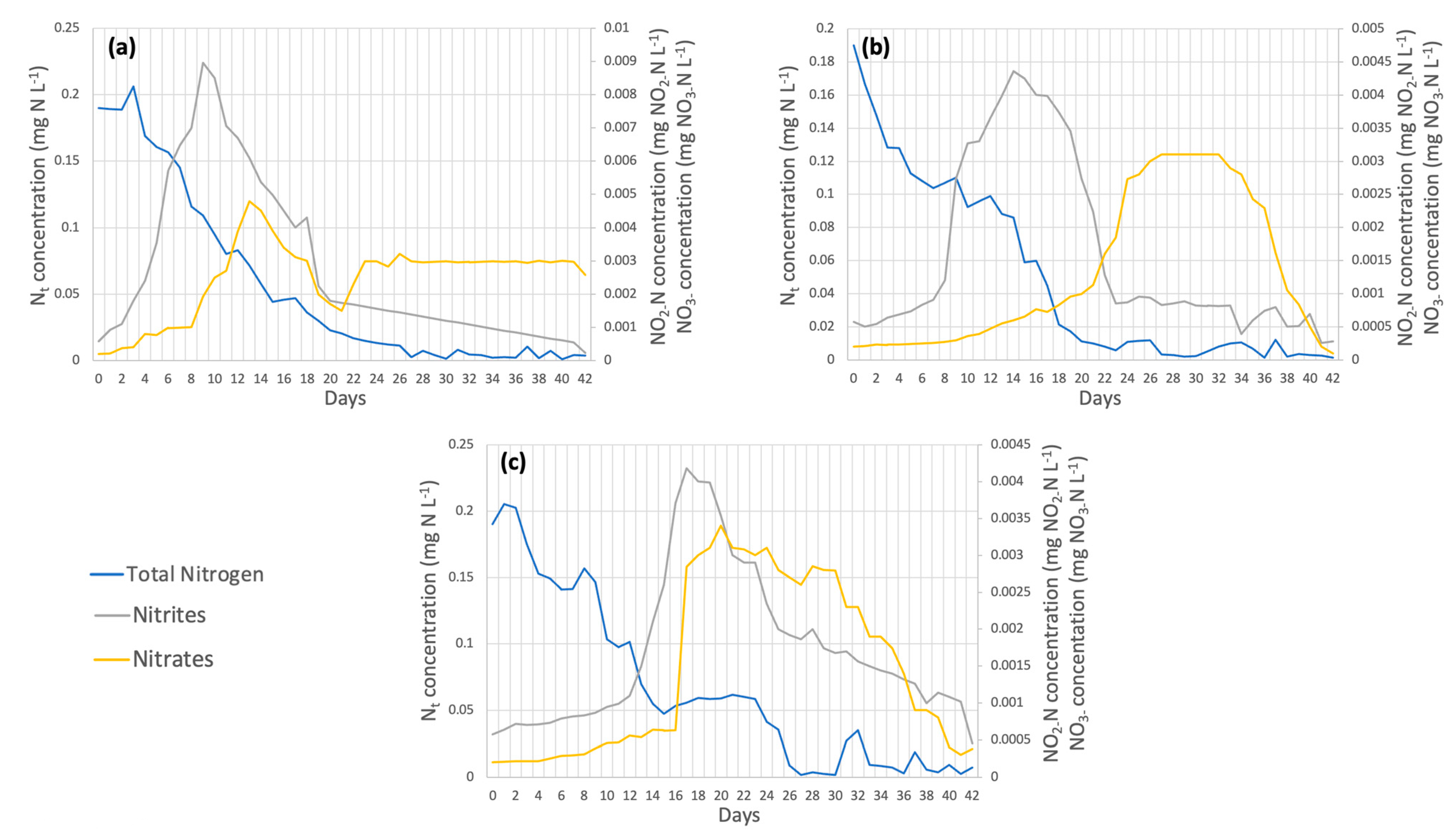
3.2.1. Chlorella spp.
3.2.2. Spirulina maxima
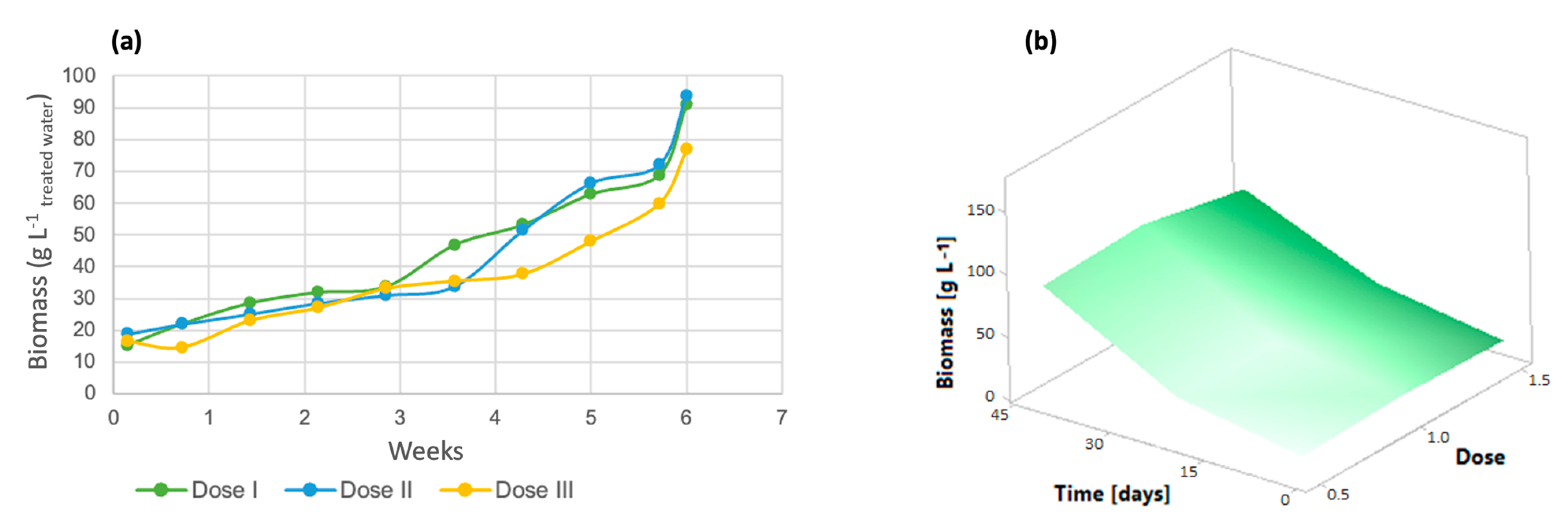
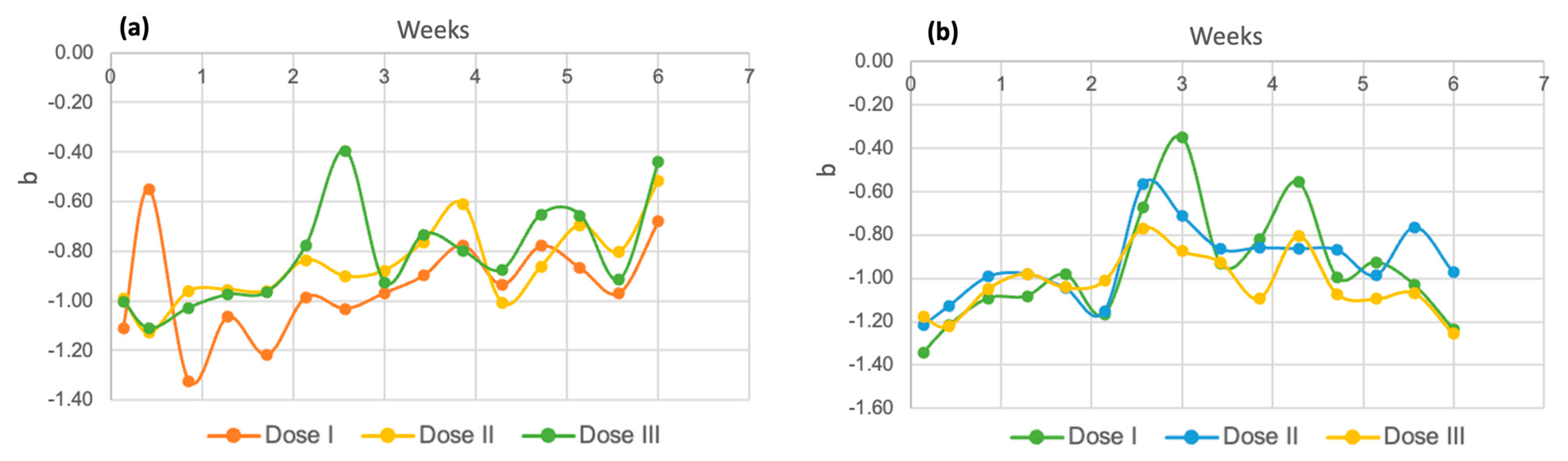
3.3. Kinetic Parameters
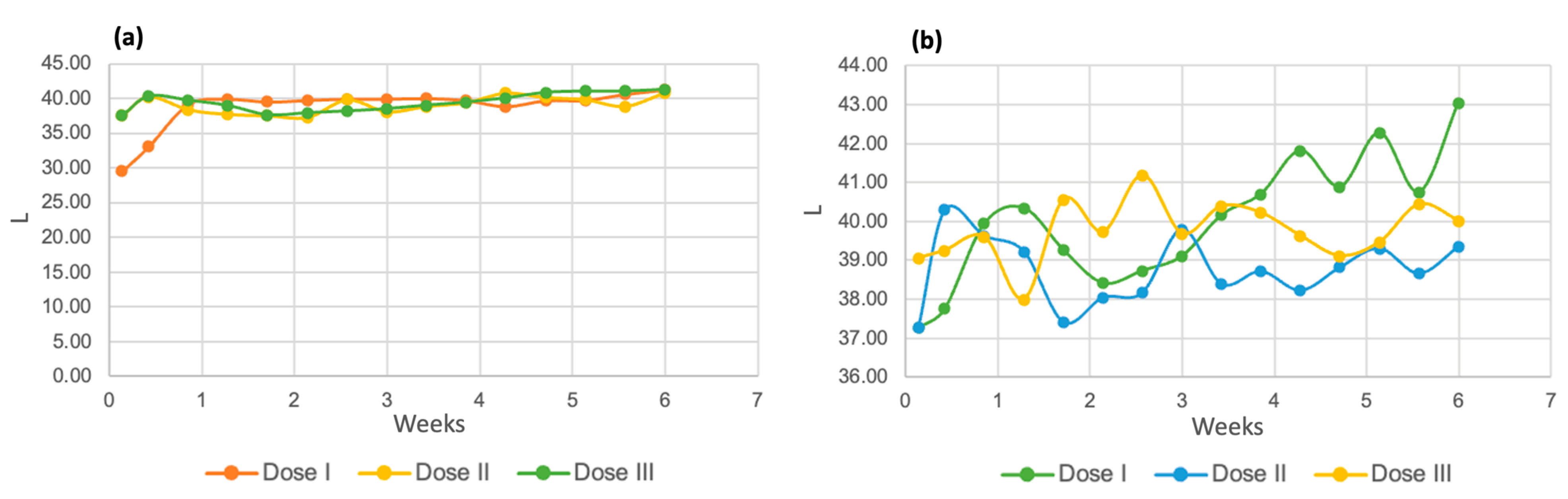
3.4. Color and Turbidity Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdul-Aziz, H.; Ahmad-Puat, N.N.; Alazaiza, M.Y.D.; Hung, Y.T. Poultry Slaughterhouse Wastewater Treatment Using Submerged Fibers in an Attached Growth Sequential Batch Reactor. Int. J. Environ. Res. Public Health 2018, 15, 1734. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Mondal, T.; Sharma, R.; Mahalakshmi, N.; Gupta, M. Poultry Waste Management. Int. Curr. Microbiol. Appl. Sci. 2018, 7, 701–712. [Google Scholar] [CrossRef]
- Yaakob, M.A.; Saphira Radin Mohamed, R.M.; Saeed Al-Gheethi, A.A.; Mohd Kassim, A.H. Characteristics of Chicken Slaughterhouse Wastewater. Chem. Eng. Trans. 2018, 63, 637–642. [Google Scholar] [CrossRef]
- Fatima, F.; Du, H.; Kommalapati, R.R. Treatment of poultry Slaughterhouse Wastewater with Membrane Technologies: A Review. Water 2021, 13, 1905. [Google Scholar] [CrossRef]
- Sabando-Fraile, C.; Corral-Bobadilla, M.; Lostado-Lorza, R.; Somovilla-Gomez, F. Multiresponse Performance Evaluation and Life Cycle Assessment for the Optimal Elimination of Pb (II) from Industrial Wastewater by Adsorption Using Vine Shoot Activated Carbon. Sustainability 2023, 15, 11007. [Google Scholar] [CrossRef]
- Baker, B.R.; Mohamed, R.; Al-Gheethi, A.; Aziz, H.A. Advanced technologies for poultry slaughterhouse wastewater treatment: A systematic review. J. Dispers. Sci. Technol. 2020, 42, 880–899. [Google Scholar] [CrossRef]
- Terán-Hilares, R.; Atoche-Garay, D.F.; Pinto-Pagaza, D.A.; Ahmed, M.A.; Colina-Andrade, G.J.; Santos, J.C. Promising physicochemical technologies for poultry slaughterhouse wastewater treatment: A critical review. J. Environ. Chem. Eng. 2021, 9, 105174. [Google Scholar] [CrossRef]
- Menaa, F.Z.; Arbib, Z.; Perales, J.A. Urban wastewater photobiotreatment with microalgae in a continuously operated photobioreactor: Growth, nutrient removal kinetics and biomass coagulation—Flocculation. Environ. Technol. 2019, 40, 342–355. [Google Scholar] [CrossRef] [PubMed]
- Geremia, E.; Rippa, M.; Catone, C.M.; Ulgiati, S. A Review about Microalgae Wastewater Treatment for Bioremediation and Biomass Production—A New Challenge for Europe. Environments 2021, 8, 136. [Google Scholar] [CrossRef]
- Hernández, E.; Lobato-Benítez, C.; Hernández, C.A. Algal enzymes, biotechnological potential uses: A review. Cymbella 2017, 3, 1–15. [Google Scholar]
- Emparan, Q.; Harun, R.; Danquah, M.K. Role of phycoremediation for nutrient removal from wastewaters: A review. Appl. Ecol. Environ. Res. 2019, 17, 889–915. [Google Scholar] [CrossRef]
- Kartik, A.; Akhil, D.; Lakshmi, D.; Gopinath, K.P.; Arun, J.; Sivaramakrishnan, R.; Pugazhendhi, A. A critical review on production of biopolymers from algae biomass and their applications. Bioresour. Technol. 2021, 329, 124868. [Google Scholar] [CrossRef] [PubMed]
- NMX-AA-008-SCFI-2016; Análisis de Aguas—Medición del pH en Aguas Naturales, Residuales y Residuales Tratadas. Método de Prueba. Diario Oficial de la Federación: Mexico City, Mexico, 2016.
- NMX-AA-007-SCFI-2013; Análisis de Agua—Medición de la Temperatura en Aguas Naturales, Residuales y Residuales Tratadas. Método de Prueba. Diario Oficial de la Federación: Mexico City, Mexico, 2013.
- NMX-AA-038-SCFI-2001; Análisis de Agua—Determinación de Turbiedad en Aguas Naturales, Residuales y Residuales Tratadas. Método de Prueba. Diario Oficial de la Federación: Mexico City, Mexico, 2001.
- NMX-AA-034-SCFI-2015; Análisis de Agua—Medición de Sólidos y Sales Disueltas en Aguas Naturales, Residuales y Residuales Tratadas. Método de Prueba. Diario Oficial de la Federación: Mexico City, Mexico, 2015.
- NMX-AA-012-SCFI-2001; Análisis de Agua—Determinación de Oxígeno Disuelto en Aguas Naturales, Residuales y Residuales Tratadas. Método de Prueba. Diario Oficial de la Federación: Mexico City, Mexico, 2001.
- NMX-AA-093-SCFI-2000; Análisis de Agua—Determinación de la Conductividad Electrolítica. Método de Prueba. Diario Oficial de la Federación: Mexico City, Mexico, 2000.
- NMX-AA-029-SCFI-2001; Diario Oficial de la Federación. Análisis de aguas—Determinación de fósforo total en aguas naturales, residuales y residuales tratadas. Método de prueba. Diario Oficial de la Federación: Mexico City, Mexico, 2001.
- NMX-AA-030-SCFI-2001; Análisis de Agua—Determinación de la Demanda Química de Oxígeno en Aguas Naturales, Residuales y Residuales Tratadas. Método de Prueba. Diario Oficial de la Federación: Mexico City, Mexico, 2001.
- NMX-AA-026-SCFI-2010; Análisis de Agua—Medición de Nitrógeno Total Kjeldahl en Aguas Naturales, Residuales y Residuales Tratadas. Método de Prueba. Diario Oficial de la Federación: Mexico City, Mexico, 2010.
- NMX-AA-113-SCFI-2012; Análisis de Agua—Medición del Número de Huevos de Helminto en Aguas Residuales y Residuales Tratadas por Observación Microscópica. Método de Prueba. Diario Oficial de la Federación: Mexico City, Mexico, 2012.
- NMX-AA-042-SCFI-2015; Análisis de Agua—Enumeración de Organismos Coliformes Totales, Organismos Coliformes Fecales (Termotolerantes) y Escherichia coli—Método del Número más Probable en Tubos Múltiples. Diario Oficial de la Federación: Mexico City, Mexico, 2015.
- Ghafari, M.; Rashidi, B.; Haznedaroglu, B.Z. Effects of macro and micronutrients on neutral lipid accumulation in oleaginous microalgae. Biofuels 2016, 9, 147–156. [Google Scholar] [CrossRef]
- Hamed, S.M.; Selim, S.; Klöck, G.; AbdElgawad, H. Sensitivity of two green microalgae to copper stress: Growth, oxidative and antioxidants analyses. Ecotoxicol. Environ. Saf. 2017, 144, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Sousa, C.A.; Sousa, H.; Vale, F.; Simões, M. Microalgae-based bioremediation of wastewaters—Influencing parameters and mathematical growth modeling. Chem. Eng. J. 2021, 425, 131412. [Google Scholar] [CrossRef]
- Basitere, M.; Njoya, M.; Rinquest, Z.; Ntwampe, S.K.O.; Sheldon, M.S. Performance evaluation and kinetic parameter analysis for static granular bed reactor (SGBR) for treating poultry slaughterhouse wastewater at mesophilic condition. Water Pract. Technol. 2019, 14, 259–268. [Google Scholar] [CrossRef]
- Ángel-Isaza, J.; Mesa-Salgado, N.; Narváez-Solarte, W. Ácidos orgánicos, una alternativa en la nutrición avícola: Una revisión. Rev. CES Med. Zootec. 2019, 14, 45–58. [Google Scholar] [CrossRef]
- NOM-001-SEMARNAT-2021; Límites Permisibles de Contaminantes en las Descargas de Aguas Residuales en Cuerpos Receptores Propiedad de la Nación. Diario Oficial de la Federación: Mexico City, Mexico, 2021.
- Meiramkulova, K.; Bazarbayeva, T.; Orynbassar, R.; Tleukulov, A.; Madina, N.; Mashan, T.; Dariya, A.; Apendina, A.; Nurmukhanbetova, N. Assesing the Influence of Electrode Polarity on the Treatment of Poultry Slaughterhouse Wastewater. Molecules 2022, 27, 1014. [Google Scholar] [CrossRef] [PubMed]
- Caldera, Y.; Sánchez, M.; Gutiérrez, E. Calidad física de aguas residuales de una industria avícola en un sistema de flotación por aire disuelto con coagulantes. Rev. Tecnocientífica 2017, 13, 57–66. [Google Scholar]
- Oyewale, A.T.; Adesakin, T.A.; Oyedeji, O.; Aduwo, A.I.; Bakare, M.K. Impact Assessment of Poultry Discharge on the Physico-Chemical and Microbiological Water Quality of Olosuru Stream in Ikire, Southwestern Nigeria. J. Water Resour. Prot. 2018, 10, 1061–1082. [Google Scholar] [CrossRef]
- Bozorg-Haddad, O.; Delpasand, M.; Loáiciga, H.A. 10—Water quality, hygiene, and health. In Economical, Political, and Social Issues in Water Resource; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Pinto, L.A.D.M.; Pinto, M.D.M.; Bovo, J.; Mateus, G.A.P.; Tavres, F.D.O.; Baptista, A.T.A.; Hirata, A.K. Aspectos ambientais do abate de aves: Uma revisão. Uningá Rev. 2015, 22, 44–50. [Google Scholar]
- Bunce, J.T.; Ndam, E.; Ofiteru, I.D.; Moore, A.; Graham, D.W. A Review of Phosphorus Removal Technologies and Their Applicability to Small-Scale Domestic Wastewater Treatment Systems. Front. Environ. Sci. 2018, 6, 8. [Google Scholar] [CrossRef]
- Sánchez, M.; Caldera, Y.; Gutiérrez, E. Eficiencia de coagulantes durante el tratamiento de aguas residuales de la industria avícola en un sistema de flotación. Impacto Cient. 2017, 12, 201–214. [Google Scholar]
- Phoon, B.L.; Ong, C.C.; Saheed, M.S.M.; Show, P.L.; Chang, J.S.; Ling, T.C.; Lam, S.S.; Juan, J.C. Conventional and emerging technologies for removal of antibiotics from wastewater. J. Hazard. Mater. 2020, 400, 122961. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Morales, J.L.; Gutiérrez-González, E.C.; Colina-Andrade, G.J. Obtención y caracterización de carbón activado obtenido de lodos de plantas de tratamiento de agua residual de una industria avícola. Ing. Investig. Tecnol. 2016, 17, 453–462. [Google Scholar] [CrossRef]
- Oliveira, A.C.; Barata, A.; Batista, A.P.; Gouveia, L. Scenedesmus obliquus in poultry wastewater bioremediation. Environ. Technol. 2019, 40, 3735–3744. [Google Scholar] [CrossRef] [PubMed]
- Ramos, R.; Pizarro, R. Crecimiento y capacidad de bioremedicación de Chlorella vulgaris (Trebouxiophycea Chlorophyta) cultivada en aguas residuales generadas en el cultivo del pez dorado Seriola lalandi (Perciformes: Carangidae). Rev. Biol. Mar. Oceanogr. 2018, 53, 75–86. [Google Scholar] [CrossRef]
- Rodriguez, I.B.; Ho, T.Y. Trace Metal Requirements and Interactions in Symbiodinium kawagutii. Front. Microbiol. 2018, 9, 142. [Google Scholar] [CrossRef]
- Malagón-Micán, M.L.; Suárez-Chaparro, M.Y. Influencia en la concentración inicial de Chlorella vulgaris y CO2 en la producción de lípidos. Rev. Lasallista Investig. 2020, 17, 56–69. [Google Scholar] [CrossRef]
- Geddes, L.; Kunihiro, K.; Turner, E. Simplified Procedures for Water Examination; AWWA: Denver, CO, USA, 2014. [Google Scholar]
- Gutiérrez-Casiano, N.; Hernández-Aguilar, E.; Alvarado-Lassman, A.; Méndez-Contreras, J.M. Removal of carbon and nitrogen in wastewater from a poultry processing plant in a photobioreactor cultivated with the microalga Chlorella vulgaris. J. Environ. Sci. Health Part A 2022, 57, 620–633. [Google Scholar] [CrossRef]
- Tan, X.B.; Zhao, X.C.; Zhang, Y.L.; Zhou, Y.Y.; Yang, L.B.; Zhang, W.W. Enhanced lipid and biomass production using alcohol wastewater as carbon resource for Chlorella pyrenoidosa cultivation in anaerobically digested starch wastewater in outdoors. Bioresour. Technol. 2018, 247, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.L.; Pires, J.C.M.; Simões, M. A review on the use of microalgal consortia for wastewater treatment. Algal Res. 2017, 24, 403–415. [Google Scholar] [CrossRef]
- Dębowski, M.; Rusanowska, P.; Zieliński, M.; Dudek, M.; Romanowska-Duda, Z. Biomass Production and Nutrient Removal by Chlorella vulgaris from Anaerobic Digestion Effluents. Energies 2018, 11, 1654. [Google Scholar] [CrossRef]
- Carrasquedo-Ferrer, S.J.; Rincón-Lizardo, N.C.; Díaz-Montiel, A.R.; Pire-Sierra, M.C. Monitoreo de la remoción biológica de nitrógeno en efluentes de tenerías usando un reactor por carga secuencial. Ing. Investig. Tecnol. 2014, 15, 287–298. [Google Scholar] [CrossRef]
- Hudson, R.; De Graaf, R.; Rodin, M.S.; Ohno, A.; Lane, N.; McGlynn, S.E.; Yamada, Y.M.A.; Nakamura, R.; Barge, L.M.; Braun, D.; et al. CO2 reduction driven by a pH gradient. Proc. Natl. Acad. Sci. USA 2020, 117, 22873–22879. [Google Scholar] [CrossRef] [PubMed]
- Al-Homaidan, A.A.; Alabdullatif, J.A.; Al-Hazzani, A.A.; Al-Ghanayem, A.A.; Alabbad, A.F. Adsorptive removal of cadmium ions by Spirulina platensis dry biomass. Saudi J. Biol. Sci. 2015, 22, 795–800. [Google Scholar] [CrossRef]
- Suastes-Rivas, J.K.; Hernández-Altamirano, R.; Mena-Cervantes, V.Y.; Chairez, I. Simultaneous Optimization of Biomass and Metabolite Production by a Microalgae-Yeast Coculture Under Inorganic Micronutrients. Bioenergy Res. 2020, 13, 974–985. [Google Scholar] [CrossRef]
- Samorì, G.; Samorì, C.; Guerrini, F.; Pistocchi, R. Growth and nitrogen removal capacity of Desmodesmus communis and of a natural microalgae consortium in a batch culture system in view of urban wastewater: Part I. Water Res. 2013, 2, 791–801. [Google Scholar] [CrossRef]
- Sarkar, S.; Sarkar, S.; Manna, M.S.; Gayen, K.; Bhowmick, T.K. Chapter 12—Extraction of carbohydrates and proteins from algal resources using supercritical and subcritical fluids for high-quality products. In Innovative and Emerging Technologies in the Biomarine Food Sector; Academic Press: Cambridge, MA, USA, 2022. [Google Scholar] [CrossRef]
- Mahlangu, D.; Mphahlele, K.; De Paola, F.; Mthombeni, N.H. Microalgae-Mediated Biosorption for Effective Heavy Metals Removal from Wastewater: A Review. Water 2024, 16, 718. [Google Scholar] [CrossRef]
- Sandgruber, F.; Gieldsdorf, A.; Baur, A.C.; Schenz, B.; Müller, S.M.; Schwerdtle, T.; Stangl, G.I.; Griehl, C.; Lorkowski, S.; Dawczynski, C. Variability in Macro- and Micronutrients of 15 Commercially Available Microalgae Powders. Mar. Drugs 2021, 19, 310. [Google Scholar] [CrossRef]
- Owens, B.F.; Matthew, D.; Diepenbrock, C.H.; Tiede, T.; Wu, D.; Mateos-Hernández, M.; Gore, M.A.; Rocheford, T. Genome-Wide Association Study and Pathway-Level Analysis of Kernel Color in Maize. G3 2019, 9, 1945–1955. [Google Scholar] [CrossRef] [PubMed]
- Ngobeni, P.V.; Gutu, L.; Basitere, M.; Harding, T.; Ikumi, D. Poultry Slaughterhouse Wastewater Treatment Using an Integrated Biological and Electrocoagulation Treatment System: Process Optimization Using Response Surface Methodology. Sustainability 2022, 14, 9561. [Google Scholar] [CrossRef]
- Gómez-Polo, C.; Martín-Casado, A.M.; Quispe, N.; Gallardo, E.R.; Montero, J. Color Changes of Acetal Resins (CAD-CAM) In Vivo. Appl. Sci. 2023, 13, 181. [Google Scholar] [CrossRef]
- Fernández-Honores, A.M.; Alvítez-Izquierdo, E.; Rodríguez-Izquierdo, E.F. Taxonomía e importancia de “Spirulina” Artrhospira jenneri (Cyanophyceae: Oscillatoriaceae). Arnaldoa 2019, 26, 1091–1104. [Google Scholar]
- Gilpavas, E.; Arbeláez-Castaño, P.E.; Medina-Arroyave, J.D.; Gómez-Atehortua, C.M. Tratamiento de aguas residuales de la industria textil mediante coagulación química acoplada a procesos Fenton intensificados con ultrasonido de baja frecuencia. Rev. Int. Contam. Ambient. 2017, 34, 157–167. [Google Scholar] [CrossRef]
- Abdelfattah, A.; Ali, S.S.; Ramadan, H.; El-Aswar, E.I.; Eltawab, R.; Ho, S.H.; Elsamahy, T.; Li, S.; El-Sheekh, M.M.; Schagerl, M.; et al. Microalgae-based wastewater treatment: Mechanisms, challenges, recent advances and future prospects. Environ. Sci. Ecotechnol. 2023, 13, 100205. [Google Scholar] [CrossRef] [PubMed]
- Nishshanka, G.K.S.H.; Premaratne, V.C.L.M.; Nimarshana, P.H.V.; Ariyadasa, T.U.; Kornaros, M. Wastewater-based microalgal biorefineries for the production of astaxanthin and coproducts: Current status, challenges and future perspectives. Bioresour. Technol. 2021, 342, 126018. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Ho, N.; Ogden, K.L.; Arnold, R.G. Cultivation of Nannochloropsis salina in municipal wastewater or digester centrate. Ecotoxicol. Environ. Saf. 2014, 103, 45–53. [Google Scholar] [CrossRef]
- Beuckles, A.; Smolders, E.; Muylaert, K. Nitrogen availability influences phosphorus removal in microalgae-based wastewater treatment. Water Res. 2015, 77, 98–106. [Google Scholar] [CrossRef]








| Parameter | Equipment | Reference |
|---|---|---|
| pH | Hanna® potentiometer, Mexico City, Mexico. | NMX-AA-008-SCFI-2016 [13] |
| Temperature | Taylor® thermometer, Mexico City, Mexico. | NMX-AA-007-SCFI-2013 [14] |
| Turbidity | Hatch® turbidimeter, Mexico City, Mexico. | NMX-AA-038-SCFI-2001 [15] |
| TS and TVS | Ecoshel® stove, Mexico City, Mexico. | NMX-AA-034-SCFI-2015 [16] |
| Dissolved oxygen and saturation | Hatch® multiparameter, Mexico City, Mexico. | NMX-AA-012-SCFI-2001 [17] |
| Electrical conductivity | Hatch® multiparameter, Mexico City, Mexico. | NMX-AA-093-SCFI-2000 [18] |
| Total phosphorus | Termoscientific® spectrophotometer, Mexico City, Mexico. | NMX-AA-029-SCFI-2001 [19] |
| COD | Termoscientific® spectrophotometer, Mexico City, Mexico. | NMX-AA-030-SCFI-2001 [20] |
| Total nitrogen | Labconco® microkjeldahl, Mexico City, Mexico. | NMX-AA-026-SCFI-2010 [21] |
| Helminth eggs | AmScope® microscope, Mexico City, Mexico. | NMX-AA-113-SCFI-2012 [22] |
| Fecal coliforms | Ecoshel® stove, Mexico City, Mexico. | NMX-AA-042-SCFI-2015 [23] |
| Dose | Micronutrients (g/L) | |||
|---|---|---|---|---|
| ZnCl2 | MnCl2 | NaMoO4 | CuSO4 | |
| 1 | 0.0025 | 0.006 | 0.006 | 0.01 |
| 2 | 0.005 | 0.012 | 0.012 | 0.02 |
| 3 | 0.0075 | 0.018 | 0.018 | 0.03 |
| Analysis | Result | Maximum Permissible Limits | Units |
|---|---|---|---|
| pH | 6.04 ± 0.02 | 6–9 | NA |
| Temperature | 25 ± 0.2 | 35 | °C |
| COD | 39.950 ± 0.1 | 150 | mg/L |
| Total solids | 0.78415 ± 0.01 | NA | % m/m |
| Total volatile solids | 77.6089 ± 0.01 | NA | % m/m |
| Turbidity | 952 ± 4 | NA | NTU |
| Dissolved O2 | 1.50 ± 0.05 | 5 | mg/L |
| O2 saturation | 19.10 ± 0.1 | 80 | % |
| Electric conductivity | 0.830 ± 0.02 | NA | mS/cm |
| Total phosphorus | 0.0004958 ± 0.00002 | 15 | mg/L |
| Total Kjeldahl nitrogen | 0.19 ± 0.01 | NA | % |
| % Proteins | 1.19 ± 0.01 | NA | % |
| Helminth eggs | Not found | 0 | |
| Total coliforms | 1.1 × 1011 ± 0.1 | NA | NMP/100 mL |
| a | b | c | μm | λ | G (days) | R2 (%) | Sum of Squares | |
|---|---|---|---|---|---|---|---|---|
| Control | 7.984 | 1.581 | 0.028 | 0.225 | 20.544 | 3.069 | 97.63 | 0.081 |
| Dose 1 | 7.726 | 1.891 | 0.062 | 0.481 | 14.290 | 1.438 | 97.56 | 0.075 |
| Dose 2 | 8.069 | 1.468 | 0.055 | 0.449 | 8.400 | 1.541 | 99.13 | 0.061 |
| Dose 3 | 7.932 | 1.686 | 0.078 | 0.620 | 8.770 | 1.117 | 99.4 | 0.027 |
| a | b | c | μm | λ | G (days) | R2 (%) | Sum of Squares | |
|---|---|---|---|---|---|---|---|---|
| Control | 8.274 | 1.952 | 0.034 | 0.281 | 27.967 | 2.461 | 96.61 | 0.077 |
| Dose 1 | 8.227 | 2.463 | 0.071 | 0.588 | 20.438 | 1.177 | 99.32 | 0.0080 |
| Dose 2 | 8.143 | 2.488 | 0.071 | 0.582 | 6.822 | 1.19 | 99.06 | 0.010 |
| Dose 3 | 8.048 | 1.938 | 0.065 | 0.525 | 14.377 | 1.32 | 99 | 0.030 |
| COD Removal (%) | N Removal (%) | Cell Density × 106 (cel/mL) | μm | λ | G (d) | |
|---|---|---|---|---|---|---|
| Chlorella spp. | ||||||
| Dose 1 | 96 | 98.08 | 41.38 | 0.481 | 14.290 | 1.438 |
| Dose 2 | 99 | 99.33 | 78.96 | 0.449 | 8.400 | 1.541 |
| Dose 3 | 98 | 96.11 | 81.96 | 0.620 | 8.770 | 1.117 |
| Spirulina maxima | ||||||
| Dose 1 | 88 | 94.84 | 159.56 | 0.588 | 20.438 | 1.177 |
| Dose 2 | 94 | 96.42 | 131.59 | 0.582 | 6.822 | 1.19 |
| Dose 3 | 98 | 98.87 | 92.18 | 0.525 | 14.377 | 1.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Guzmán, S.M.; Hernández-Aguilar, E.; Alvarado-Lassman, A.; Méndez-Contreras, J.M. Kinetics of Obtaining Microalgal Biomass and Removal of Organic Contaminants in Photobioreactors Operated with Microalgae—Study Case: Treatment of Wastewater from a Poultry Slaughterhouse. Water 2024, 16, 1558. https://doi.org/10.3390/w16111558
Pérez-Guzmán SM, Hernández-Aguilar E, Alvarado-Lassman A, Méndez-Contreras JM. Kinetics of Obtaining Microalgal Biomass and Removal of Organic Contaminants in Photobioreactors Operated with Microalgae—Study Case: Treatment of Wastewater from a Poultry Slaughterhouse. Water. 2024; 16(11):1558. https://doi.org/10.3390/w16111558
Chicago/Turabian StylePérez-Guzmán, Solmaría Mandi, Eduardo Hernández-Aguilar, Alejandro Alvarado-Lassman, and Juan Manuel Méndez-Contreras. 2024. "Kinetics of Obtaining Microalgal Biomass and Removal of Organic Contaminants in Photobioreactors Operated with Microalgae—Study Case: Treatment of Wastewater from a Poultry Slaughterhouse" Water 16, no. 11: 1558. https://doi.org/10.3390/w16111558






