Abstract
Phytoremediation is one of the effective technologies for removing pollutants from the aquatic environment. Toxic compounds such as chlorpyrifos can affect the physiological processes of aquatic plants, causing secondary oxidative stress in plant tissues. Macrophytes, like other organisms inhabiting the contaminated ecosystem, have developed a system of defense mechanisms, thanks to which plants can still exist in their natural ecosystem. Our research is a summary of the previously presented results of the effectiveness of purifying contaminated water with chlorpyrifos in the phytoremediation process and the second type of phytoremediation supported by microorganisms, which intensify the process of removing contaminants from the environment. This research concerned changes in nonenzymatic and enzymatic antioxidants in Canadian seaweed, needle spikerush and water mint caused by chlorpyrifos. The research determines changes in the total concentration of polyphenols, flavonoids and dyes (chlorophyll A, chlorophyll B, anthocyanins and carotenoids) as well as differences in the activity of guaiacol peroxidase and glutathione S-transferase. The analysis of the results showed an increase in the content of polyphenols and flavonoids. The reverse trend was observed in the case of the pigment content. The appearance of chlorpyrifos in the environment caused an increase in the activity of the examined enzymes. The process involving microorganisms that were obtained from places contaminated with pesticide proved to be more effective. This shows the cooperation of species living in an investigated ecosystem.
1. Introduction
The intensive development of civilization caused the high negative effects on the environment. The increase in awareness and the desire to improve the quality of life, which initiated the dynamic development of industry with the appearance of synthetic compounds, have a negative impact on the aquatic ecosystems. This required the development of new and effective processes based on biological, chemical and thermal methods, whose utilitarian use will allow to get safety products [1,2,3]. One of the technologies based on biological methods used in the degradation of organic compounds is a phytoremediation that uses plants for reduction, degradation, assimilation and metabolizing environmental pollution such as heavy metals, hydrocarbons or pesticides [4,5]. Some water plants that appear naturally in the aquatic ecosystems in Poland are able to participate in biological processes to decrease or move the pollutants amounts [6]. The native plants are able to change their physiological functions to exist in a polluted environment; this is their response to abiotic stress. This phenomenon allows the plants to participate in water treatment as a main tool for phytoremediation. This process occurs naturally and can be intensified using microbial consortia to increase the effectiveness of pollution removal [7,8,9].
Plant protection products that have been used for years have a beneficial effect on inhibiting the development of microorganisms. However, intensive use of pesticides in agriculture may pose threats to the proper functioning of aquatic and terrestrial ecosystems, both fauna and flora [10,11,12]. Improperly selected doses of chemical compounds may remain in the environment and migrate within ecosystems. The presence of plant protection products was found in all types of flowing waters—rainwater, surface and groundwater, which may increase during agrochemical treatments. Organophosphate pesticides were very popular in the world and used intensely. These include, among others, chlorpyrifos which was distributed by the Dow Chemical Company since 1965 as a foliar pesticide [13]. These compounds were used to control pests in industrial crops, in vegetable and orchards [14]. Organophosphate pesticides are esters of phosphoric acid and its derivatives. Their activities relate to the electrophilic nature of the phosphorus ester bond [15]. The presence of organophosphorus compounds in the environment may cause unfavorable changes in the physiological processes of plants such as: photosynthesis, respiration, cell division, synthesis of growth regulators, water uptake, as well as changes in the structure of cell organelles and limiting plant productivity [16]. The presence of pesticides in the environment is one of the causes of secondary oxidative stress in plant tissues, characterized by intensive production of reactive oxygen species (ROS). An undesirable effect caused by toxic pesticide particles may be a change in the proper functioning of protein, lipid or nucleic acid molecules [17].
One of the groups found in aquatic ecosystems are aquatic plants called macrophytes. The presence of toxic chemical compounds such as pesticides resulted in the activation of defense mechanisms in plants tissues [18]. The ability to quickly adapt macrophytes to new environmental conditions enabled the initiation of research using them. The phytoremediation process was used to optimize the system of macrophytes antioxidant mechanisms, consisting of nonenzymatic and enzymatic antioxidants [19]. The system of enzymes that remove free radicals include guaiacol peroxidase (GPX) and glutathione S-transferase (GST) [20,21,22]. Biochemical reactions involving enzymes, e.g., GPX, contribute to detoxification processes that remove various types of toxic compounds [23]. This enzyme participates in various physiological processes, such as auxin catabolism, wound healing and defense mechanisms against infections caused by pathogens [24]. GPX may also participate in the capture of pollutants in the root sphere and the oxidative degradation of compounds found in the plant’s environment [25]. The second enzyme that enters the system of enzymatic mechanisms is glutathione S-transferase (GST). In 1970, a reaction involving GST was first described, catalyzing the decomposition of atrazine by conjugation with glutathione tripeptide in Sorghum and corn plants [26]. The results initiated intensive research on the participation of GST in the detoxification processes of herbicides and other plant xenobiotics [27]. According to the literature, the GPX and GST activities can be induced in plants by abiotic factors including insecticides, heavy metals, infection caused by pathogens. Both the enzymes can be treated as stress markers [28].
The nonenzymatic antioxidant system of plants consists of low molecular weight compounds such as phenols, flavonoids, pigments—chlorophyll A and B, anthocyanins, carotenoids [29,30,31]. Phenolic compounds participate in reactions leading to the removal of reactive oxygen species and inhibition of the formation of free radicals [32]. Research on the properties of flavonoids has proven that they are responsible for the color of plants, the smell of flowers and the taste of fruit [33,34]. This feature qualifies them as natural repellents, and any action of toxic compounds, including pesticides, causes changes in their proper functioning [35].
In our research, we focused on the chosen macrophytes use in a pure phytoremediation process and a phytoremediation assisted by microorganisms to remove chlorpyrifos from an aquatic environment. We compared effectiveness both types of the processes. Because the enzymatic and nonenzymatic antioxidants defense system is responsible for water plants’ stress tolerance we also investigated the polyphenols, flavonoids and pigments (chlorophyll a, chlorophyll b, anthocyanins and carotenoids) contents as well as the activities changes of guaiacol peroxidase (GPX) and glutathione S-transferase (GST) caused by chlorpyrifos presence during the phytoremediation process. There are many works in the literature describing research on the biological degradation of pesticides. However, there are many questions about specific compounds such as chlorpyrifos. Our research focused on the answers to such questions regarding a single compound of chlorpyrifos as an example of an organophosphorus pesticide, and, more precisely, on the use of phytoremediation appearing in natural conditions as a part of the processes taking place to clean aquatic ecosystems. In addition, we confirmed that in the natural environment, water treatment occurs as a complex process where all organisms that live in the habitat participate in it. It is also important that the plants and microorganisms conducting the water purification process are characteristic for a given climate zone—in our case, a moderate zone. The above shows the novelty of our applied research, which brings elements of innovation to the general knowledge.
2. Materials and Methods
2.1. Materials
2.1.1. Water Plants
Three species of macrophytes were used in our studies: Canadian waterweed (Elodea canadensis Michx.), needle spikerush (Eleocharis acicularis L.) and water mint (Mentha aquatica L.), which originated from organic farming “Ogrody Wodne”, Miedzychod, Poland. The plants were chosen because of their natural location. They are characteristic for the moderate climate zone and are often found in areas of water reservoirs.
2.1.2. Microorganisms
In the process of supported phytoremediation the autochthonous microorganisms isolated from soils were used in the studies: Bacillus cereus, Bacillus licheniformis, Oerskovia paurometabola. The details concerning the species are presented in Table 2, Results.
2.1.3. Chlorpyrifos
In our research, we used chlorpyrifos, a commercial plant protection product. For the analytical part, we used the chlorpyrifos standard (Sigma-Aldrich Production GmbH, Buchs, Switzerland; CAS: 2921-88-2). Selected physicochemical properties of the chlorpyrifos are presented in Table 1.

Table 1.
Chlorpyrifos chosen properties.
2.2. Experiments
2.2.1. Isolation of Microorganisms
Soil samples were taken from agricultural areas where the following crops were supported by pesticide spraying: A—corn (central Poland), B—celery (central Poland), C—strawberries (Germany), D—apple trees (Germany). The collected soil samples were sieved through sieves with a mesh size of 2 mm. The sieved fractions were used for further studies. An enrichment procedure was used to isolate bacteria that effectively degrade insecticides. The process was carried out in a mineral medium: (NH4)2SO4 2.0 g/dm3 (Pol-Aura sp z o.o., Lodz, Poland), Na2HPO4 12H2O 1.5 g/dm3 (Pol-Aura sp z o.o., Lodz, Poland), KH2PO4 1.5 g/dm3 (Pol-Aura sp z o.o., Lodz, Poland), MgSO4 ·7H2O 0.01 g/dm3 (Pol-Aura sp z o.o., Lodz, Poland), FeSO4 ·7H2O 0.01 g/dm3 (Pol-Aura sp z o.o., Lodz, Poland), CaCl2 2H2O 0.001 g/dm3 (Pol-Aura sp z o.o., Lodz, Poland). Chlorpyrifos was added to the medium at a concentration of 50 mg/dm3.
To 90 mL of the medium prepared in this way, 10 g of soil was added and culturing was carried out at 30 °C for 72 h. After this time, the culture of microorganisms was passaged on a medium for the determination of the total number of microorganisms, using Plate Count Agar (PCA) with the following composition: casein peptone 5.0 g/dm3, yeast extract 2.5 g/dm3 (Pol-Aura sp z o.o., Lodz, Poland), glucose 1.0 g/dm3 (Pol-Aura sp z o.o., Lodz, Poland), agar 12 g/dm3 (Pol-Aura sp z o.o., Lodz, Poland). The plates were incubated in an incubator at 37 °C for 48 h.
After this time, three strains of morphologically different bacteria were isolated from each soil sample. Pure bacterial cultures were stored in cryobanks at −30 °C. The strains from the pure culture were then inoculated into 100 mL mineral medium, which contained 50 mg/ dm3 of chlorpyrifos. After 72 h of incubation at 30 °C, the concentration of chlorpyrifos was measured. The optical density of the culture (OD550) was also measured using a DEN-1B densitometer (Biosan). Three strains with the highest biodegradation activity were selected for further research.
The bacteria were identified using molecular methods based on 16S rRNA gene analysis. Genomic DNA was extracted using the Genomic Mini kit (A&A Biotechnology) according to the methodology provided by the manufacturer. The reaction mixture was prepared in a volume of 25 µL containing 12 µL of polymerase (1.5 units) REDTaq™ ReadyMix™ (Sigma), 0.2 µL of each universal primer (27F and 1492R) and 11.6 µL of water and 1 µL of DNA. The 16S rRNA gene was amplified via PCR in the MJ Mini Gradient Thermal Cycler (Bio-Rad) in a cycle consisting of initial denaturation at 94 °C for 2 min, denaturation at 94 °C for 1 min, primer annealing at 50 °C for 1 min (34 repetitions), extension 72 °C for 3 min and final extension at 72 °C for 3 min.
The PCR reaction products were analyzed with horizontal electrophoresis in 1% (w/v) agarose gel in 0.5 × TBE buffer (Sigma-Aldrich Sp z o.o., Poznan, Poland). The amplified PCR products were purified employing the Clean-Up AX kit (A&A Biotechnology) and then subjected to a sequencing reaction. The obtained nucleotide sequences were compared with the BLAST 2.10.0+ program (the Basic Local Alignment Search Tool) with the sequences available in the NCBI database (the National Center of Biotechnology Information database).
2.2.2. Phytoremediation Process
All details concerning the cultivation process were described in the previously published paper of Sobiecka et al. [36]. The pure phytoremediation process (F1) was carried out in an aquatic environment polluted by different concentrations of chlorpyrifos: 50 μg/dm3, 100 μg/dm3, 150 μg/dm3. Macrophytes were also cultivated in the medium without adding the tested pesticide, as a reference test. The second phytoremediation process was conducted with assistance of microbial consortia (F2). In this process, the plant cultivation environment was enriched with inoculum of three isolated microbial consortia. To prepare the inoculum, each strain was activated on PCA medium. After 72 h of culturing in 37 °C, suspensions of the tested strains were made in physiological saline. The density of each suspension was determined densitometrically so that the final number of cells was 1.0 × 106 cells/cm3. Then, 0.650 cm3 of each of the suspensions prepared in this way was added to 1 dm3 of the medium in which the phytoremediation process was carried out. The initial abundance of each strain in the environment was approximately 2.0 × 103 cells/cm3.
2.3. Determination of Chlorpyrifos
After the end of phytoremediation supported by microorganisms, the bacterial suspension had to be removed before performing the chromatographic analysis. For this purpose, a sample containing bacterial cells was extracted by Solid Phase Extraction (SPE). The extraction was carried out in extraction columns filled with a C-18 bed from Phenomenex on a 12-station SPE system from J.T. Baker. After conditioning the column by passing 5 mL of methylene chloride and then 10 mL of distilled water, the samples were applied to the column. A sample of the bacterial suspension was loaded onto the column under pressure 5mBar.
Methylene chloride (Pol-Aura sp z o.o., Lodz, Poland) was used to extract the chlorpyrifos from the column. Elution was carried out twice using 10 mL of reagent. Each portion of the solvent remained in contact with the stationary phase from 20 s to 1 min, because then the leaching effect is most effective.
Chlorpyrifos concentrations before and after both phytoremediation processes were determined with gas chromatography on a two-dimensional gas chromatograph coupled with a mass spectrometer, with a TOF ion time-of-flight detector (Pegasus 4D, LECO Corp., St. Joseph, MI, USA). An extremely important advantage of this electron capture detector is its high sensitivity and selectivity to impurities containing elements with high electronegativity.
After the extraction process, the obtained eluates were dosed to the chromatographic column in various temperature programs of the oven, which enabled the determination of the effect of the oven temperature on the chromatographic separation of chlorpyrifos. The retention time of the pesticide with a positive response was then recorded. The following temperature program was used: initial temperature 70 °C, then increase of 15 °C/min to 300 °C, at which chlorpyrifos in the tested samples gave a positive response with the correct peak shape.
2.4. Determination of Enzymatic Antioxidants
The enzymatic antioxidants system analytical details were described in a previous paper of Sobiecka et al. [37]. The information concerned the determination of a glutathione S-transferase (GST) and guaiacol peroxidase (GPX). The standards came from the Polish Office of Sigma-Aldrich Sp z o.o., Poznan, Poland.
2.5. Determination of Nonenzymatic Antioxidants
The nonenzymatic antioxidants system analytical details were described in a previous paper of Sobiecka et al. and concerned the determination of polyphenols, flavonoids and pigments has been repeated [36]. All the required standards were bought in the Polish Office of Sigma-Aldrich Sp z o.o., Poznan, Poland.
2.6. Statistical Analysis
The STATISTICA Version 10 (StatSoft, Cracow, Poland) was used for statistical calculations. The results present the average of three biological samples measurements. The single-factor analysis of ANOVA variance was used. The Duncan multiple-range post hoc test (p < 0.05) in order to show statistically significant differences between the tested samples was used to analysis.
3. Results
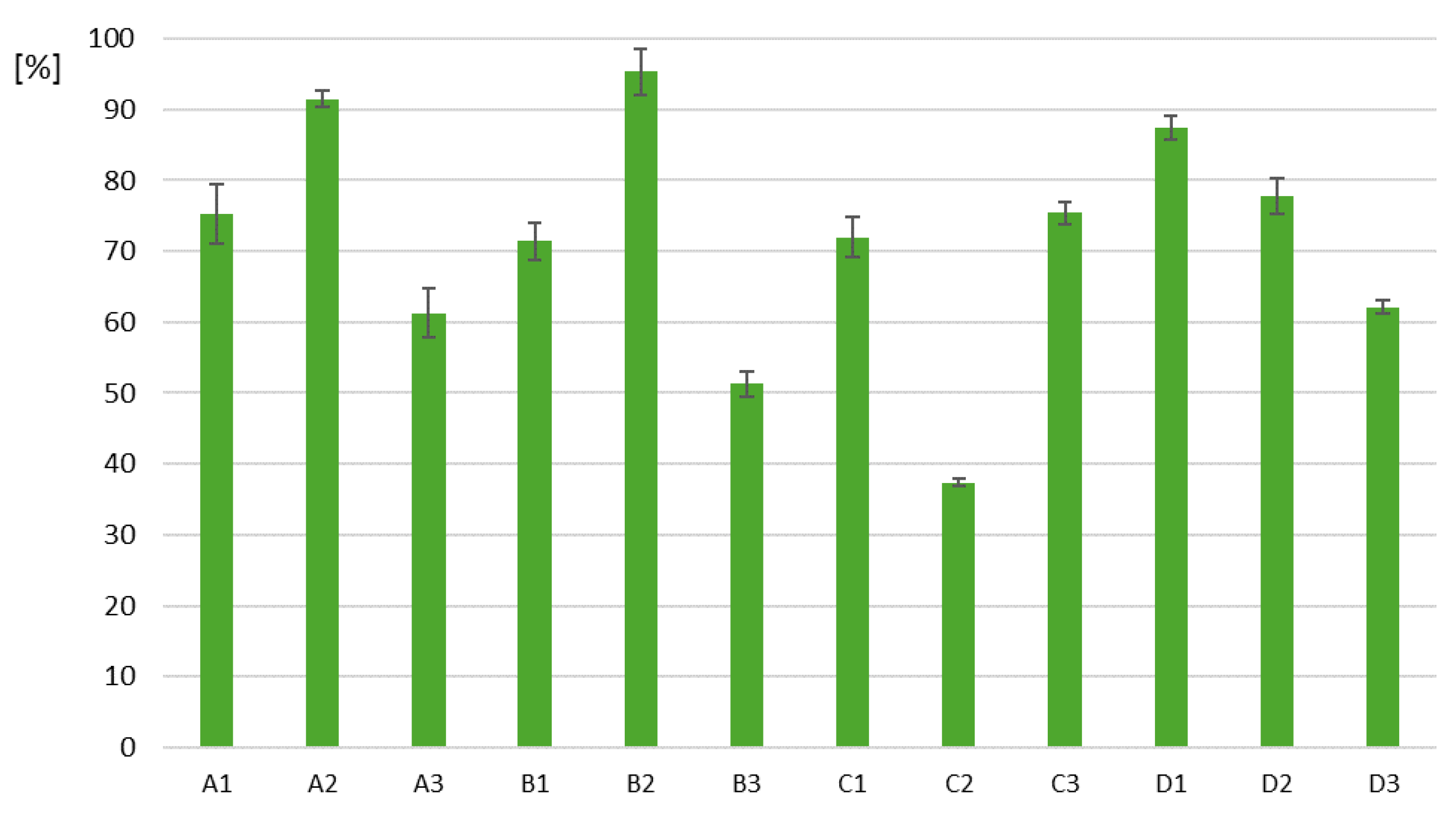
Our research on the purification of water contaminated with chlorpyrifos was preceded by the isolation of bacterial strains naturally occurring in temperate climate zones—including Poland. The bacteria were isolated from places contaminated with pesticides. Next, they were cultivated in a solution polluted by chlorpyrifos to check their biodegradation effectiveness, as seen in Figure 1. The three most effective strains were identified, and these results are summarized in Table 2.

Figure 1.
Biodegradation of chlorpyrifos by soil bacteria originating from A—a corn field, B—celery cultivation, C—a strawberry field, D—an apple orchard.

Table 2.
Summary of the most efficient strains.
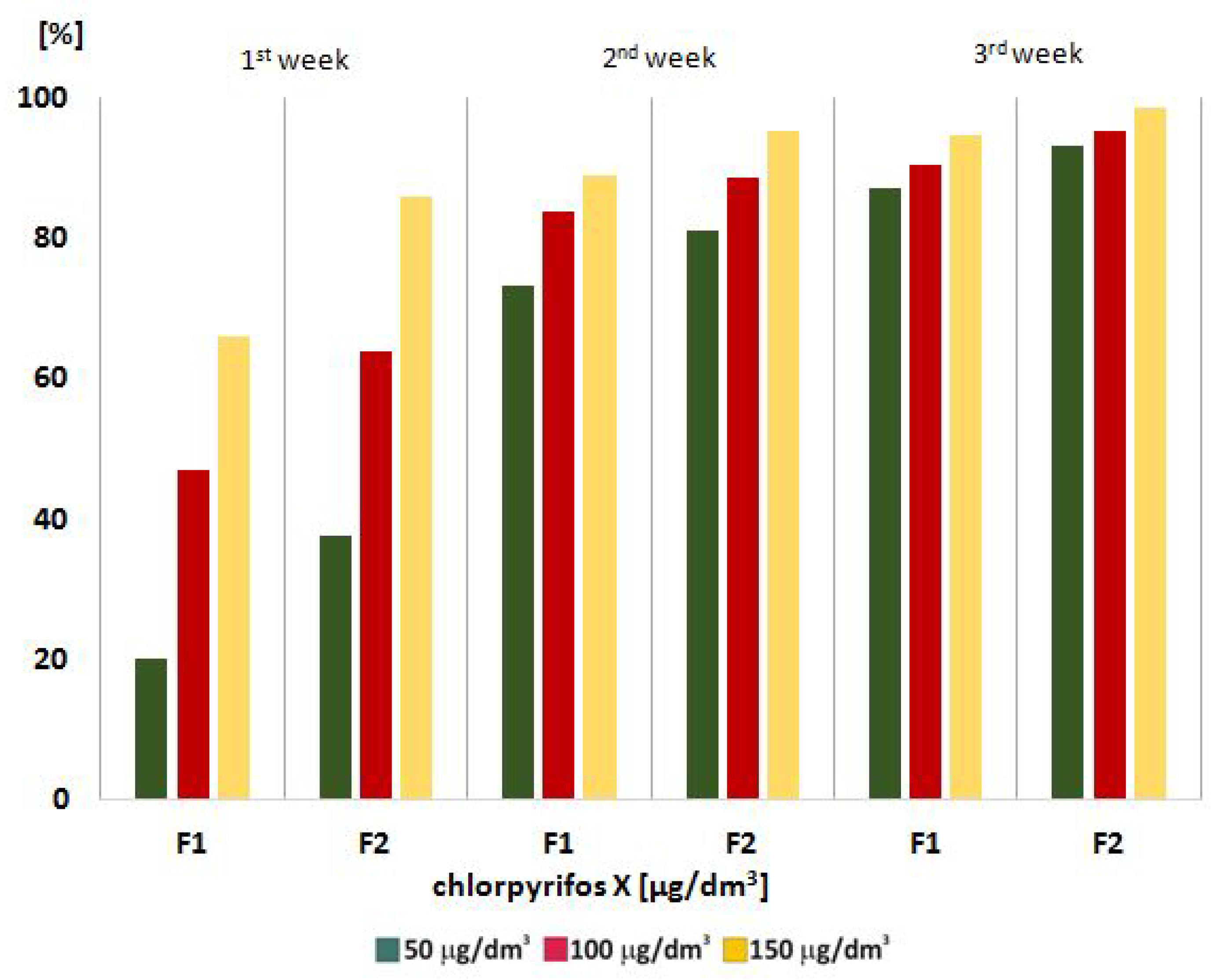
In the main part of our studies we estimated the effectiveness of phytoremediation—one of the biological technologies for pollutant removal from aquatic environment. During the phytoremediation process (F1) and phytoremediation supported by microorganisms (F2) various concentrations of chlorpyrifos: 50 μg/dm3, 100 μg/dm3 and 150 μg/dm3, in the aquatic environment were monitoring (Figure 2). The enrichment of the aquatic environment with the bacteria species (Bacillus cereus, Bacillus licheniformis, Oerskovia paurometabola) intensified the biological process, which resulted in an increase in the effectiveness of the phytoremediation process. The presence of macrophytes and microorganisms enlarged their scope to get rid of toxins from the environment. The investigated macrophytes reduced the concentration of chlorpyrifos to a large extent after the end of F1 and F2 processes according to the initial concentrations, Figure 2.

Figure 2.
Chlorpyrifos removal from an aquatic environment in F1—phytoremediation; and F2—phytoremediation supported by microorganisms.
The used of a hybrid phytoremediation method in the removal of chlorpyrifos significantly decreased the pesticide amount from the aquatic environment. After the F2 process, the pesticide concentration was from 1.55% to 14.00% of its initial values, and after the F1 process from 5.35% to 34.00%. Analyzing the results, it was found that the phytoremediation process supported by microorganisms conducted by macrophytes is more effective. The plants selected for this research, Canadian waterweed, needle spikerush and water mint, showed increased activity of an enzymatic antioxidant systems in response to abiotic stress caused by the presence of chlorpyrifos in the environment. These results confirmed that biological methods, including commonly occurring aquatic plants, can be effective in cleaning an aquatic environment contaminated with chlorpyrifos.
Water plants have developed the ability to accumulate pollutants in their tissues in order to grow in difficult environmental conditions in the presence of toxic substances. In order to protect against oxidative stress, macrophytes have developed mechanisms consisting of nonenzymatic and enzymatic antioxidants system.
For the second part of the presented results of our studies, we focused on the behavior of the chosen water plants (Canadian waterweed (A), needle spikerush (B) and water mint (C)) in the presence of three various concentrations of chlorpyrifos: 50 µg/dm3, 100 µg/dm3 and 150 µg/dm3. One of the processes conducted in the aquatic environment was enriched by an inoculum of bacterial consortia. We analyzed a few nonenzymatic and enzymatic antioxidants that can be markers of the abiotic stress caused by various concentrations of chlorpyrifos.
The contents of polyphenols and flavonoids were measured in leaves of the chosen macrophytes (Table 3). The increased concentration of chlorpyrifos in the cultures intensified the concentration of both investigated compounds in the tissues of the tested plants. The increasing amounts of polyphenols and flavonoids correlated with the increasing chlorpyrifos concentrations in the water solution. All of the macrophytes were able to grow and develop in the presence of toxic substances.

Table 3.
The content of polyphenols and flavonoids in chosen macrophytes after phytoremediation processes F1and F2.
Table 4 shows the average content of four pigments in the leaves of Canadian seaweed, needle spikerush and water mint. The presence of chlorpyrifos in the plant growth environment resulted in a reduction in the content of pigments in the tissues of the tested plants. This correlation was directly proportional and the concentration of chlorpyrifos 150 µg/dm3 significantly inhibited the pigment content in plant tissues compared to the control sample.

Table 4.
The content of nonenzymatic antioxidants in chosen macrophytes after phytoremediation processes.
Our research also analyzed changes in the activities of selected enzymatic oxidants, glutathione S-transferase (GST) and guaiacol peroxidase (GPX), in leaf and root tissues. The results are presented in Table 5. It was observed that the concentration of chlorpyrifos 150 µg/dm3 intensifies the enzyme activity the most. Activity increased more than three times compared to the initial value in all plant species. For water mint the highest increase in GST activity was observed in leaves and roots. In a case of Canadian waterweed and needle spikerush the activity intensification was observed in leaves. Generally, the similar tendency was observed in activity changes of GXP. The highest concentration of pollutant caused the most intensive activity of the enzyme. We observed more intensified activities in leaves compared roots.

Table 5.
The enzymes activities changes in chosen macrophytes after phytoremediation processes.
4. Discussion
The results presented in this paper compare the changes of chosen nonenzymatic and enzymatic antioxidants defense system for pure phytoremediation process (F1) and the phytoremediation assisted by microorganisms (F2). Changes in the activities and concentrations of compounds that are part of the plant defense system enable the removal of pollutants from the environment. Living organisms, both plants and microorganisms, are exposed to toxic compounds which induce the response to stress. In the presence of chlorpyrifos, the plants activated the cell organelles that induce biochemical processes of transcriptional up-regulation of phenylpropanoid pathway [38]. The activation of biosynthetic enzymes and up-regulation of key genes of phenylpropanoid branch allowed to stimulate a phenolic biosynthesis [39,40].
The presence of chlorpyrifos resulted in a decrease in the content of chloroplast pigments in the studied macrophytes, caused by oxidative stress. The toxic compound caused an increased content of free radicals. The direct effect of these reactions was the inhibition of the activity of enzymes responsible for the process of chlorophyll synthesis [41]. Similar results were obtained by the Shixiang and Yadav groups [42,43]. Scientists have also observed that chlorophyll concentrations decrease as the concentration of pesticides in the environment increases. Higher concentrations of pollutants adversely affect the physiology of macrophytes.
Abiotic stress caused by the presence of pesticides in the environment also affects the enzymatic defense system of plants. Our studies have proven that the activities of both enzymes: glutathione S-transferase (GST) and guaiacol peroxidase (GPX) in plant tissues increase with increasing concentration of chlorpyrifos. Both enzymes are involved in the biochemical reactions of plants responsible for the detoxification process of xenobiotics such as pesticides. The plant defends itself against accumulating pollutants in its tissues [44]. Our study results regarding changes in GST activity were similar to those obtained in Tlidjen’s study [45]. In the case of the activity of guaiacol peroxidase (GPX), which is responsible for the proper course of physiological processes in plant tissues, a correlation with the growth of chlorpyrifos in the environment was observed. The increased activity of GPX was also observed in research of Sharma group [46] and Bertrand, in which different concentrations of chlorpyrifos were tested in culture in small pondweed (Potamogeton pusillus) tissue [14]. The obtained results of the increase in activity confirm that guaiacol peroxidase is one of the enzymes responsible for the plant response to abiotic stress caused by chlorpyrifos. The analysis of the results also confirms that both enzymes—GPX and GST—are involved in the process of removing organic peroxides and hydrogen peroxide, which are products of aerobic metabolism [47,48]. These enzymes significantly reduce the oxidative stress effects on plant tissues.
The proper management of waste disposal is one of the elements of the economy that requires not only technological, but also economic and social activities, taking into account the needs and interests of man together with balancing adverse impact on the natural environment.
During the research, the hypothesis that the concentration of abiotic factors directly affects the activity of enzymes was also confirmed. The highest concentration of chlorpyrifos stimulated enzyme activity most intensely.
The investigated nonenzymatic antioxidative stress system compounds: polyphenols, flavonoids and pigments (chlorophyll a, chlorophyll b, anthocyanins and carotenoids) effected in amount changes which was correlated to the chlorpyrifos concentration. The aquatic environment polluted by the highest chlorpyrifos concentration (150 μg/dm3) caused an increase in polyphenols and flavonoids in plants tissues while the content of pigments diminished.
The ability of the chosen macrophytes to adapt in the presence of toxic chlorpyrifos molecules allowed for the testing of a remediation process to clean a polluted ecosystem [49,50]. For the study we used three species of plants naturally occurring in the aquatic environment of our temperate climate in Poland.
5. Conclusions
To summarize our research, we confirm the proper choice of macrophytes used in our studies: Canadian waterweed, needle spikerush and water mint. The chosen water plants provided an efficient process of pollutant removal. The phytoremediation process was intensified in the presence of the following microbial consortia: Bacillus cereus, Bacillus licheniformis, Oerskovia paurometabola, selected from the polluted sites. The investigated method was a useful technology for cleansing the aquatic environment of chlorpyrifos, resulting in chlorpyrifos removals of 86% to 98% after the F2 process, compared to its initial values, and 66% to 94% after the F1 process.
Author Contributions
Conceptualization, E.S. and M.M.; methodology, E.S., M.M. and A.N.; validation, M.M. and T.P.O.; formal analysis, M.M. and A.N.; investigation, M.M. and A.N.; writing—original draft preparation, E.S. and T.P.O.; writing—review and editing, E.S., T.P.O. and A.N.; visualization, T.P.O.; supervision, E.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rani, K.; Dhania, G. Bioremediation and biodegradation of pesticide from contaminated soil and water—A noval approach. Int. J. Curr. Microbiol. App. Sci. 2014, 3, 23–33. [Google Scholar]
- Meng, X.; Guo, Y.; Wang, Y.; Fan, S.; Wang, K.; Han, W. A systematic review of photolysis and hydrolysis degradation modes, degradation mechanisms, and identification methods of pesticides. J. Chem. 2022, 2022, 9552466. [Google Scholar] [CrossRef]
- Sobiecka, E.; Kołaciński, Z.; Rincón, J.M.; Szymański, Ł.; Olejnik, T.P. Coloured sintered glass-ceramics from hospital incineration fly ash. Mat. Lett. 2019, 252, 34–37. [Google Scholar] [CrossRef]
- Susarla, S.; Medina, V.F.; McCutcheon, S.C. Phytoremediation: An ecological solution to organic chemical contamination. Ecol. Eng. 2002, 18, 647–658. [Google Scholar] [CrossRef]
- Seridou, P.; Fyntrilakis, K.; Syranidou, E.; Kalogerakis, N. Hydroponic phytoremediation of antimony by Tamarix smyrnensis and Nerium oleander. J. Chem. Technol. Biotechnol. 2023, 98, 2214–2223. [Google Scholar] [CrossRef]
- Futughe, A.E.; Purchase, D.; Jones, H. Phytoremediation using Native Plants. In Phytoremediation: In-Situ Applications, 1st ed.; Shmaefsky, B., Ed.; Concepts and Strategies in Plant Sciences; Springer Nature: Cham, Switzerland, 2020; pp. 285–327. [Google Scholar]
- Shilev, S.; Babrikova, I.; Babrikov, T. Consortium of plant growth-promoting bacteria improves spinach (Spinacea oleracea L.) growth under heavy metal stress conditions. J. Chem. Technol. Biotechnol. 2020, 95, 932–939. [Google Scholar] [CrossRef]
- Arora, N.K.; Fatima, T.; Mishra, J.; Mishra, I.; Verma, S.; Verma, R.; Verma, M.; Bhattacharya, A.; Verma, P.; Mishra, P. Ha-lo-tolerant plant growth promoting rhizobacteria for improving productivity and remediation of saline soils. J. Adv. Res. 2020, 26, 69–82. [Google Scholar] [CrossRef]
- Tchuisseu Tchakounté, G.V.; Berger, B.; Patz, S.; Becker, M.; Turecková, V.; Novák, O.; Tarkowská, D.; Fankem, H.; Silke, R. The response of maize to inoculation with Arthrobacter sp. and Bacillus sp. in phosphorus-deficient, salinity-affected soil. Microorganisms 2020, 8, 1005. [Google Scholar] [CrossRef] [PubMed]
- Tudi, M.; Daniel Ruan, H.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture development, pesticide application and its impact on the environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef] [PubMed]
- De Souza, R.M.; Seibert, D.; Quesada, H.B.; de Jesus Bassetti, F.; Fagundes-Klen, M.R.; Bergamasco, R. Occurrence, impacts and general aspects of pesticides in surface water: A review. Process Saf. Environ. Prot. 2020, 135, 22–37. [Google Scholar] [CrossRef]
- Rumschlag, S.L.; Mahon, M.B.; Hoverman, J.T.; Raffel, T.R.; Carrick, H.J.; Hudson, P.J.; Rohr, J.R. Consistent effects of pesticides on community structure and ecosystem function in freshwater systems. Nat. Commun. 2020, 11, 6333. [Google Scholar] [CrossRef] [PubMed]
- Espinoza-Navarro, O.; Ponce-LaRosa, C.; Bustos-Obregón, E. Organophosphorous Pesticides: Their Effects on Biosentinel Species and Humans. Control and Application in Chile. Int. J. Morphol. 2017, 35, 1069–1074. [Google Scholar] [CrossRef][Green Version]
- Bertrand, L.; Marinob, D.J.; Monferránc, M.V.; Améa, M.V. Can a low concentration of an organophosphate insecticide cause negative effects on an aquatic macrophyte? Exposure of Potamogeton pusillus at environmentally relevant chlorpyrifos concentrations. Environ. Exp. Bot. 2017, 198, 139–147. [Google Scholar] [CrossRef]
- Pehkonen, S.O.; Zhang, Q. The degradation of organophosphorus pesticides in natural waters: A critical review. Crit. Rev. Environ. Sci. Technol. 2002, 32, 17–72. [Google Scholar] [CrossRef]
- Zhang, H.; Yuan, X.; Xiong, T.; Wang, H.; Jiang, L. Bioremediation of co-contaminated soil with heavy metals and pesticides: Influence factors, mechanisms and evaluation methods. Chem. Eng. J. 2020, 398, 125657. [Google Scholar] [CrossRef]
- Elerŝek, T.; Filipič, M. Chapter 12: Organophosphorus Pesticides—Mechanism of Their Toxicity. In The Impacts of Pesticide Exposure; Stoytcheva, M., Ed.; IntechOpen Limited: London, UK, 2011; pp. 243–260. [Google Scholar]
- Singh, A.; Kumar, A.; Yadav, S.; Singh, I.K. Reactive oxygen species-mediated signaling during abiotic stress. Plant Gene 2019, 18, 100173. [Google Scholar] [CrossRef]
- Anee, T.I.; Nahar, K.; Rahman, A.; Mahmud, J.A.; Bhuiyan, T.F.; Alam, M.U.; Fujita, M.; Hasanuzzaman, M. Oxidative damage and antioxidant defense in Sesamum indicum after different waterlogging durations. Plants 2019, 8, 196. [Google Scholar] [CrossRef]
- Nianiou-Obeidat, I.; Madesis, P.; Kissoudis, C.; Voulgari, G.; Chronopoulou, E.; Tsaftaris, A.; Labrou, N.E. Plant glutathione transferase-mediated stress tolerance: Functions and biotechnological applications. Plant Cell Rep. 2017, 36, 791–805. [Google Scholar] [CrossRef] [PubMed]
- Lotfi, N.; Vahdati, K.; Hassani, D.; Kholdebarin, B.; Amiri, R. Peroxidase, guaiacol peroxidase and ascorbate peroxidase activity accumulation in leaves and roots of walnut trees in response to drought stress. Acta Hortic. 2010, 861, 309–316. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Kawano, T. Roles of the reactive oxygen species-generating peroxidase reactions in plant defense and growth induction. Plant Cell Rep. 2003, 21, 829–837. [Google Scholar] [CrossRef]
- Bela, K.; Horváth, E.; Gallé, Á.; Szabados, L.; Tari, I.; Csiszár, J. Plant glutathione peroxidases: Emerging role of the antioxidant enzymes in plant development and stress responses. J. Plant Physiol. 2015, 176, 192–201. [Google Scholar] [CrossRef]
- Hemeda, H.M.; Klein, B.P. Effects of naturally occurring antioxidants on peroxidase activity of vegetable extracts. J. Food Sci. 1990, 55, 184–185. [Google Scholar] [CrossRef]
- Varga, B.; Janda, T.; László, E.; Veisz, O. Influence of abiotic stresses on the antioxidant enzyme activity of cereals. Acta Physiol. Plant. 2012, 34, 849–858. [Google Scholar] [CrossRef]
- Edwards, R.; Dixon, D.D. Metabolism of Natural and Xenobiotics Substrates by the Plant Glutathione S-Transferase Superfamily. In Ecological Studies, Molecular Ecotoxicology of Plants; Sandermann, H., Ed.; Springer-Verlag: Berlin/Heidelberg, Germany, 2004; Volume 170, pp. 17–50. [Google Scholar]
- Hasanuzzaman, M.; Nahar, K.; Anee, T.I.; Fujita, M. Glutathione in plants: Biosynthesis and physiological role in environmental stress tolerance. Physiol. Mol. Biol. Plants 2017, 23, 249–268. [Google Scholar] [CrossRef]
- Uarrota, V.G.; Moresco, R.; Schmidt, E.C.; Bouzon, Z.L.; da Costa Nunes, E.; de Oliveira Neubert, E.; Peruch, L.A.M.; Rocha, M.; Maraschin, M. The role of ascorbate peroxidase, guaiacol peroxidase, and polysaccharides in cassava (Manihot esculenta Crantz) roots under postharvest physiological deterioration. Food Chem. 2016, 197, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.R. Toxic Effect of Chlorpyrifos and Dimethoate on Protein and Chlorophyll-a Content of Spirulina platensis. Int. J. Eng. Sci. Adv. Res. 2015, 1, 24–26. [Google Scholar]
- Dvořáková Březinová, T.; Vymazal, J. Phenolic compounds in wetland macrophytes. Sci. Agric. Bohem. 2018, 49, 1–8. [Google Scholar]
- Ghasemzadeh, A.; Ghasemzadeh, N. Flavonoids and phenolic acids: Role and biological activity in plants and humans. J. Med. Plants Res. 2011, 5, 6697–6703. [Google Scholar]
- Belščak-Cvitanović, A.; Durgo, K.; Huđek, A.; Bačun-Družina, V.; Komes, D. Overview of Polyphenols and Their Properties. In Polyphenols: Properties, Recovery, and Applications; Woodhead Publishing: New York, NY, USA, 2018; pp. 3–44. [Google Scholar]
- Solovchenko, A.; Yahia, E.M.; Chen, C. Pigments. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Yahia, E.M., Ed.; Woodhead Publishing (Elsevier): Duxford, UK, 2019; pp. 225–252. [Google Scholar]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant flavonoids: Chemical characteristics and biological activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef] [PubMed]
- Sobiecka, E.; Mroczkowska, M.; Olejnik, T.P. The Influence of Chlorpyrifos on the Nonenzymatic Antioxidants Content in Macrophytes Leaves. Antioxidants 2022, 11, 684. [Google Scholar] [CrossRef] [PubMed]
- Sobiecka, E.; Mroczkowska, M.; Olejnik, T.P. The Enzymatic Antioxidants Activities Changes in Water Plants Tissues Exposed to Chlorpyrifos Stress. Antioxidants 2022, 11, 2104. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef]
- Sharma, A.; Thakur, S.; Kumar, V.; Kanwar, M.K.; Kesavan, A.K.; Thukral, A.K.; Bhardwaj, R.; Alam, P.; Ahmad, P. Presowing Seed Treatment with 24-Epibrassinolide Ameliorates Pesticide Stress in Brassica juncea L. through the Modulation of Stress Markers. Front. Plant Sci. 2016, 7, 1569. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Yuan, H.; Kanwar, M.K.; Bhardwaj, R.; Thukral, A.K.; Zheng, B. Jasmonic Acid Seed Treatment Stimulates Insecticide Detoxification in Brassica juncea L. Front. Plant Sci. 2018, 9, 1609. [Google Scholar] [CrossRef] [PubMed]
- Shixiang, G.A.O.; Huiyun, P.; Xiaolu, L.I.; Xiaohua, X.U. Phytotoxicity of four herbicides on Ceratophyllum demersum, Vallisneria natans and Elodea nuttallii. J. Environ. Sci. 2009, 21, 307–312. [Google Scholar]
- Komives, T.; Gullner, G. Phase I Xenobiotic Metabolic Systems in Plants. Z. Nat. 2005, 60, 179–185. [Google Scholar]
- Tlidjen, S.; Meksem Amara, L.; Bouchlaghem, S.; Sbartai, H.; Djebar, M.R. Oxidative stress in Elodea canadensis and Lemna minor exposed to Calliofop 36EC. Glob. J. Biodivers. Sci. Manag. 2012, 2, 29–37. [Google Scholar]
- Vighi, I.; Benitez, L.; Amaral, M.; Moraes, G.; Auler, P.; Rodrigues, G.; Deuner, S.; Maia, L.; Braga, E. Functional characterization of the antioxidant enzymes in rice plants exposed to salinity stress. Biol. Plant. 2017, 61, 540–550. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, L.A.; Corpas, F.J.; Sandalio, L.M.; Palma, J.M.; Gomez, M.; Barroso, J.B. Reactive oxygen species, antioxidant systems and nitric oxide in peroxisomes. J. Exp. Bot. 2002, 372, 1255–1272. [Google Scholar] [CrossRef]
- Lv, T.; Carvalho, P.N.; Zhang, L.; Zhang, Y.; Button, M.; Arias, C.A.; Weber, K.P.; Brix, H. Functionality of microbial communities in constructed wetlands used for pesticide remediation: Influence of system design and sampling strategy. Water Res. 2017, 110, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Shukla, A.K.; Srivastva, N.; Upadhyay, S.N.; Dubey, S.K. Utilization of microbial community potential for removal of chlorpyrifos: A review. Crit. Rev. Biotechnol. 2016, 36, 727–742. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).