Abstract
Knowledge of the historical and present dynamics of populations of migratory fish can promote our understanding of factors affecting their recruitment and abundance. Taxonomic identification of 23,802 bone remains and 13,539 scales of fish from 30 archaeological sites along Volga River revealed that they belonged to 41 different fish species. These data allow for retrospective comparisons and highlight the potential of archaeozoology in conservation biology. Sturgeons and salmonids are vulnerable to the impacts of fishery and climatic change. The sharp decline in the numbers of Starry sturgeon (Acipenser stellatus), Caspian trout (Salmo caspius), and Caspian Inconnu (Stenodus leucichthys) from the Volga in the 17th and 18th centuries was likely related to a cooling period (“Little Ice Age”). At present, the population numbers of all anadromous sturgeons and salmonids of the Volga River are critically low. In the Volga basin over the past two millennia, the sterlet (Acipenser ruthenus) has had a very large population number, high genetic (haplogroups and haplotypes) diversity, and large body sizes. Genetic analysis (aDNA and eDNA) have great potential to expand the knowledge of fish populations along large rivers and to improve long-term biomonitoring. Therefore, analyses of historical data, conventional surveys, as well as the inclusion of genetic approaches complement each other in the development of effective conservation strategies.
1. Introduction
The Volga is the longest river in Europe and the 16th longest in the world [1]. Currently, about 80 fish and lamprey species are recorded in the Volga River drainage basin [2].
The Volga River basin covers an area of 1,360,000 km². This is the territory where, over the past 2000 years, the formation and interaction of three ethnic groups of the European part of Russia have taken place: Slavic, Turkic, and Finno-Ugric. In some periods, there was also a significant influence of the Mongolian, Baltic, and Scandinavian ethnic groups. All these ethnic groups of people were engaged in fishing in one form or another. Fishing developed intensively, including the development of trade and economic relations within this territory. Starting between the 10th and 13th centuries AD, and in particular as a result of an increase in demand for fish and products from fish processing in the territory of the Volga basin, the role of collective fishing and the use of net fishing gear significantly increased [3,4]. There is a specialization of fishers in catching certain fish, primarily sturgeon and salmonid species. Catching is carried out all year round, it covers almost the entire species composition of commercial fish that inhabit the Volga and Kama rivers, both resident and anadromous [4,5].
Scientific information about the historical species distribution, as well as the biological characteristics of a particular species, cover periods no earlier than the 18th century. However, even this information is sometimes contradictory and incomplete. In the last decade, there has been significant progress in the integration of archaeozoological data into the field of conservation biology research [6,7,8,9]. It became clear that information about a particular species can be significantly supplemented and refined with the help of archaeozoological material. Archaeological material reflects information related to the strategy of exploitation of various biological resources [10,11], including fish [12,13,14,15], to a greater extent. It should be noted that the interpretation of the results obtained on the basis of archaeozoological materials is a very difficult task, since all the aggregate material in archaeological complexes has been accumulated over many years, and sometimes decades. During this time, various changes have taken place in ecosystems, which can only be revealed by conducting comprehensive and detailed studies of the natural conditions of the past (both for aquatic and for terrestrial ecosystems) for each specific region and time period. Often, this is the only source of information about the biological and ecological characteristics of various species in the past, and this knowledge contributes to the elaboration of sustainable conservation strategies. The method of comparing samples of archaeoichthyological collections made it possible to reliably reconstruct long-term changes in the ichthyofauna of the former USSR from the Paleolithic to the Modern Age [16,17]; in the Polish Lowlands from the Mesolithic to the Modern Age [18]; and along the Austrian and Hungarian parts of the river Danube with records from the prehistoric, Roman, medieval, and late/post-medieval periods [19]. At the same time, changes in the ichthyofauna can also be largely determined by local and regional events [4,19,20,21].
This article presents new results of the study of fish in the Volga basin in a historical time frame. In addition, the data obtained through the study of subfossil fish remains are directly related to conservation biology, as they contain information about the state of fish species in the past.
2. Materials and Methods
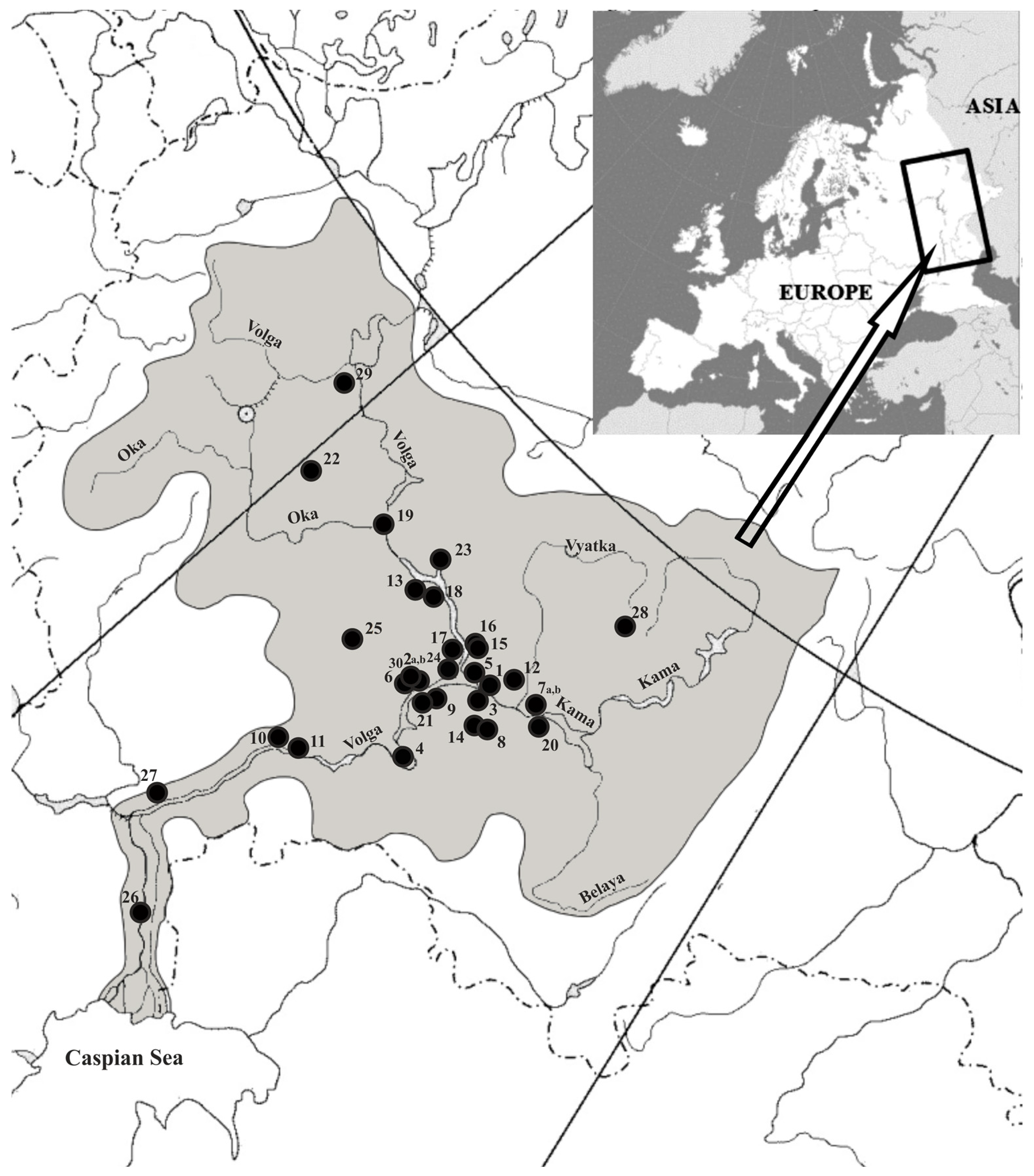
Our research is based on the subfossil fish remains from 30 archaeological sites (32 collections) between the 3rd and early 19th centuries AD located in the Volga region (Figure 1 and list of archaeological sites (Supplementary S1)). The greater part of the material was obtained in the course of excavations between 2000 and 2022. Fish bone collections from archaeological sites excavated in the 1950s and 1980s were also studied. The material was recovered mostly from household pits; from remains of residential and commercial buildings; and, to a lesser extent, from cultural layers of archaeological sites. Most fish bone remains from archaeological sites were hand-collected. Additionally, in combination with hand collection, the sieving method and the flotation method were used at a number of archaeological sites. The dating of the cultural layers of archaeological sites from which bone remains were recovered was made on the basis of typologies of ceramics, coins, and material cultural typologies; radiocarbon dating (AMS) was used for a number of archaeological sites. The material used in this article was partially published earlier [4,5,14,22].
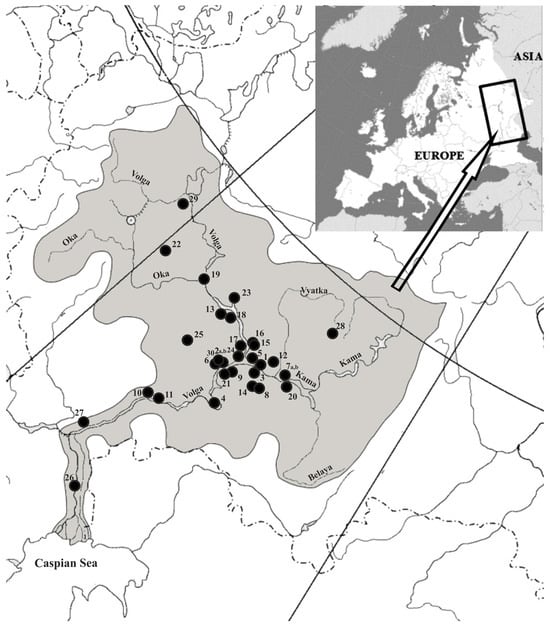
Figure 1.
The locations of archaeological sites from the layers of which the remains of fish were studied (list of archaeological sites in Supplementary S1).
The identification of species as well as the anatomical, morphological, and age diagnostics of the osteological material were performed with the aid of the reference skeletal collection of modern fish species (60 species) from the Volga River basin of the Laboratory of Biomonitoring, The Institute of Problems in Ecology and Mineral Wealth, Tatarstan Academy of Sciences. All material was processed and studied by the authors. Quantitative analysis was performed using a counting unit: the number of identified specimens of bone remains or scales—NISP (number of identified specimens) and determined the minimum number of individuals (MNI) for each fish species (Table 1). Fish remains from archaeological sites provide substantial information on the biology and ecology of fish from past historical epochs, including the estimated length, age, and period of death (catch) of subfossil fish individuals [4,23,24,25,26,27]. The estimation of fish body length for the species of the families Acipenseridae, Siluridae, and Lotidae included the total length (TL) reconstruction; for Esocidae, Cyprinidae, and Percidae, the standard length (SL); and for Salmonidae, Coregonidae, and Clupeidae, the fork length (FL).

Table 1.
Representation of the number of archaeozoological collections in the Volga region where fish species were identified: NISP (number of identified specimens) and MNI (minimum number of individuals).
The size reconstructions were based on regression equations of the forms
where XL is the measurement of the bone size (mm).
SL(TL, FL) = aXL + b
or
or
SL(TL, FL) = aXLb,
Bones of the fish skeletons were measured according to published guidelines [4,27,28,29,30]. Calculations were made in MSExcel and PAST based on a reference database of measurements of bone/scale sizes and lengths of modern fish species. The regression equations were obtained from the database of an osteological collection of bones and scales from recent (20th–21st century) specimens from the Volga River basin [4,14,27,30]. The type of regression model and the assessment of its accuracy were determined by the coefficient of determination, R2 [31]. All regressions were highly significant (p < 0.001). The regression model selection used the Akaike Information Criterion (AIC), where lower values for AIC imply the best choice of the model [32]. The ability of the obtained regression equations to reconstruct the fish length was assessed using ANOVA and ANCOVA. The determination of the ages of the fish was carried out according to standard methods [16,33,34,35]. The archaeozoological material was divided into four periods characterizing the historical stages of the development of society on the territory of the Volga region: First, the period of the early Middle Ages (3rd–8th century AD). This was the period of “Great Migration”, when significant movements of various ethnic groups took place throughout the Volga basin. Second, the period of the developed Middle Ages (the end of the 10th to the first half of the 13th century AD). This period was characterized by the formation of medieval Slavic principalities on the upper part of the Volga basin. In the Middle Volga region, the states of the Volga Bulgaria had close contact and interconnection with the local Finno-Ugric peoples, whereas in the Lower Volga region, there were numerous Turkic-speaking nomads. Third, the period of the Late Middle Ages (second half of the 13th to the 15th century AD). During this period, the Grand Duchy of Moscow was formed in the upper part of the Volga region and the Ulus Jochi (Golden Horde), and individual khanates (Kazan, Astrakhan) were established in the Middle and Lower Volga regions. Finally, fourth was the period from the post-Middle Ages to the Modern Age (16th to early 19th century AD). By this period, almost the entire Volga River basin territorially belonged to the Russian centralized state (the Tsardom of Russia).
Statistical processing of quantitative and qualitative data, as well as ecological interpretation of archaeoichthyological collections was carried out using approaches and methods developed by taking into account the characteristics of this material. To assess the degree of change in species composition over time, we used the Whitteker measure [36]:
where S is the total number of species identified in archaeological sites for a certain time interval; α is the average number of species identified in 1 location (archaeological site) and the Menhínik species richness [37]
where S = the total number of individuals (MNI) in the archaeological sites for a certain time interval, and N = number of identified fish species in the collections from the archaeological sites for a certain time interval as a measure of the fish population exploitation rate (field load) to assess the degree of change in species composition over time. To examine changes in the rank of fish used specifically, we calculated the fish index, which is the ratio of large fish to large fish and small fish species; the larger the ratio, the greater the contribution of large fish [38].
bw = (S/α) − 1,
DMn = S/√ N,
Fish index =
ΣMNI large fish >50 cm/ΣMNI large fish >50 cm + ΣMNI small fish < 50 cm
ΣMNI large fish >50 cm/ΣMNI large fish >50 cm + ΣMNI small fish < 50 cm
In order to estimate the dynamics of numbers from the standpoint of the Allee effect in the medieval and post-medieval periods, we used data from three anadromous fish species: starry sturgeon (Acipenser stellatus), Caspian trout (Salmo caspius) and Caspian Inconnu (Stenodus leucichthys). These species of fish are now completely extinct throughout the upper, middle, and northern parts of the lower reaches of the Volga River. In addition, the dynamics of their numbers for 200 years in these areas have shown a catastrophic decline. To compare with the indicators of these species, we used data on the most numerous and main commercial fish species—the sterlet (Acipenser ruthenus). The abundance of this species during the late Holocene was very high in the Volga basin [14,39]. Therefore, we put forward the assumption that such population dynamics have roots in earlier periods and are associated both with the intensity of fishing and with climate changes that took place in the Middle Ages. To obtain a reliable picture of the dynamics of the abundance of these species and the factors influencing its parameters, we used the following data:
- To estimate the value of each of these species in ancient catches according to historical periods, we introduced a species-index (“index Salmo caspius”, “index Acipenser stellatus”, “index Stenodus leucichthys”, “index Acipenser ruthenus”) expressing the ratio of the total number of individuals (MNI) of the special species to the total number of individuals (MNI) of other fish species. Such indices are successfully used in the analysis of archaeozoological materials, in particular, fish remains [14,20].
- To assess trends in fish size over time, the LSI, “Logarithmic size Index”, used this index, which is often used in archaeozoology, and was assessed in comparison with the sizes of fish from modern populations: LSI = log (Mx/Ms) = log (Mx) − log (Ms) (Mx: average restored size (length) of fish (TL—sturgeons and Lsm—salmonids) in samples for each historical period (Ms: average size of fish (length) in samples for the first half of the 20th century from the Volga River as a standard sample). The average sizes of fish (length) (TL—Acipenser stellatus, Acipenser ruthenus, and Lsm—salmonids) in samples from the first half of the 20th century from the Volga River are: starry sturgeon—133.3 cm [40,41]; Acipenser ruthenus—42.9 cm [42]; Salmo caspius—88.4 cm [43]; and Stenodus leucichthys—89.0 cm [41].
- One of the key environmental factors affecting fish populations is climate change. The main climate component is temperature. In the past two decades, much data have been obtained on temperature changes over the last two millennia in the Northern Hemisphere, including the Russian Plain within the Volga basin [44]. Based on these data, we reconstructed the indicators of average annual air temperature for each of the four time periods within the entire Volga basin (Table 1). For this purpose, the perennial fields of average annual air temperature (average data for 1951–1980) of meteorological stations in 22 nodal geographical squares of 250 km × 250 km on the territory of the Volga basin were analyzed (data obtained from the database of the All-Russian Research Institute of Hydrometeorology and Information Center http://meteo.ru/, accessed on 2 February 2024). For the values of the deviation of the average annual temperature from the average data of 1951 to 1980 for each century, data from the work of V. Klimenko and O. Solomina [44] were used.
- Another environmental factor related to climate change and significantly affecting the number of anadromous fish is the hydrological regime. Over the past two thousand years, the level regime of the Caspian Sea has changed significantly. The water balance of the Caspian Sea is determined mainly by river runoff and precipitation (input part) and evaporation (expenditure part). In the input part, the river Volga plays a decisive role, the share of which is approximately 80% of the total water input into the sea. It is believed that the fluctuation in the sea level is determined by climate fluctuations in the entire vast Caspian basin. The average change in the level of the Caspian Sea for each of the four time periods (Table 1) is calculated according to the data from the monograph “The Caspian Sea: Extreme Hydrological Events” [45]. Over the past 2000 years, the range of changes in the level of the Caspian Sea (by decade) was 11.2 m: from −34.5 to −23.3 m. The minimum levels over the centuries in the last 2000 years were during the Derbent regression in the 6th century AD (on average for the century—32.7 m) and in the 12th century during the period of the “Medieval Temperature Maximum” (on average for the century—30.7 m). The greatest levels occurred in the 17th and 18th centuries, during the “Little Ice Age” (on average for each century:24.8 m and 24.5 m, respectively).
Based on the data obtained on the biological parameters of the three species of anadromous fish and on the environmental factors indirectly or directly influencing the dynamics of the abundance of four species over the four historical periods of the Late Holocene, a principal components analysis (PCA) was conducted. Computer-based statistical analyses were performed using the program complex PAST, version 4.14 [32].
Ancient DNA sequencing data can be used to reconstruct genetic variation among populations. To study the genetic structure, we chose the most numerous species whose bone remains dominated in the layers of archaeological sites—the sterlet (Acipenser ruthenus). To clarify the genetic structure and continuity of sterlet populations in the Volga basin over time, as well as to determine whether they were isolated from populations from other river basins in the past, we studied the ancient DNA (490 bp fragment of mitochondrial D-loop) of 18 sterlet samples from archaeological sites in the Volga basin (Figure 1 and Supplementary S2) and compared these fragments to contemporary haplotypes from European and Asian rivers. The methodology for sterlet DNA research is given in Supplementary S2 [46,47,48,49,50,51,52,53,54,55,56]. The obtained sequences were deposited in the GenBank database under accession numbers: OM927985-OM928002 and OM928003-OM928007.
3. Results
Taxonomic identification of 23,802 bone remains and 13,539 scales of fish from 30 (32 collections) archaeological sites of the Volga River region revealed that they belonged to 41 species of fish and 3 interspecific hybrids from 10 families (Table 1). According to the number of individual (MNI) representation at archaeological sites, ten species of fish prevailed: sterlet (Acipenser ruthenus), common bream (Abramis brama), Zander (Sander lucioperca), Russian sturgeon (Acipenser gueldenstaedtii), starry Sturgeon (Acipenser stellatus), Northern Pike (Esox lucius), Beluga (Huso huso), European perch (Perca fluviatilis), wels catfish (Silurus glanis), and common roach (Rutilus rutilus) (Table 1). Of interest is the identification of bone remains of such species as Aral barbel (Luciobarbus brachycephalus) and Bulatmai barbel (Luciobarbus capito), and the bone findings of the Kutum (Rutilus kutum) at 10 sites prove that the ranges of these species in the past were more extensive in the Volga basin than previously thought. The revealed composition of the ichthyofauna indicated their existence between the 3rd and early 19th centuries AD in the Volga River, a typical river ecosystem characteristic of large rivers with a high number of anadromous rheophilic–eurytopic fish (14 species) and eurytopic freshwater fish (13 species) in combination with the presence of rheophilic (10) and limnophilic (4) species.
Biodiversity Measures from Archaeoichthyological Collections
- Calculations of the Whittaker measure showed that the highest values were observed in the period of the 10th century to the first half of the 13th century, and the lowest in the second half of the 13th–15th centuries as well as in the 16th and early 19th centuries. This confirms that, where there are fewer common species in archaeoichthyological collections, their β-diversity is greater, and that there were quite large differences in the species composition of fish at archaeological sites within a certain period. This may indicate the fishing preferences in relation to species or groups of fish over a given period of time or a certain region.
- Menhínik species richness index values showed that, in the first three historical periods, commercial exploitation of fish stocks was approximately the same, but in the period of the 16th–early 19th century, it increased significantly due to an increase in the number of species and a change in the ratio of species in catches, as well as, in connection with this, an increase in the commercial exploitation of all species of fish.
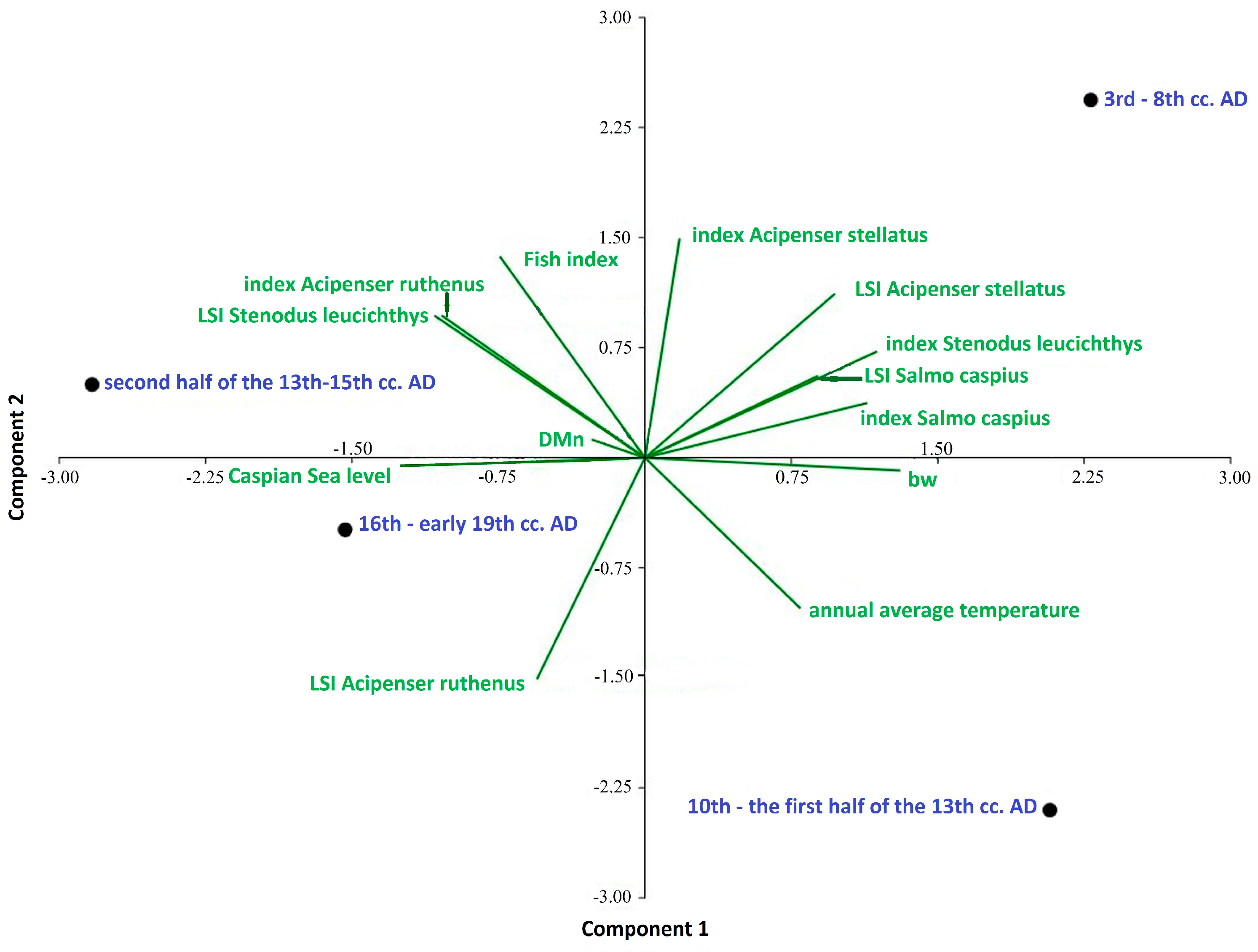
The PCA based on the biological parameters of four fish species, as well as the biodiversity and environmental factors of four historical periods, showed that the first two components of the analysis explained 82.03% of the total period’s parameter variance (Figure 2). All periods were significantly and positively correlated with the primary axis, which accounted for 50.92% of the variation. The sequence along the first axis showed that the common factors influencing the parameters were changes in the level of the Caspian Sea and the average annual temperature (Table 2). It can be seen that the high level of the Caspian Sea was negatively associated with the “index Salmo caspius”, “index Stenodus leucichthys”, the sizes of Caspian trout and starry Sturgeon (see LSI Salmo caspius and Acipenser stellatus), and the Whittaker measure (Bw). At the same time, the high level of the Caspian Sea was positively associated with size of Caspian Inconnu (LSI Stenodus leucichthys), the number of sterlet (“index Acipenser ruthenus”), and the fish index. The average annual temperature, opposite to the level of the Caspian Sea, was positively associated with an increase in indicators such as the numbers of Caspian trout (“index Salmo caspius”) and Caspian Inconnu (“index Stenodus leucichthys”), the size of starry Sturgeon (LSI Acipenser stellatus), and the level of biodiversity (Whittaker measure (Bw)). An increase in temperature was negatively associated with the size of the Caspian Inconnu (LSI Stenodus leucichthys) and the number of sterlet (“index Acipenser ruthenus”). Separate positions on this axis were determined for the periods of the 10th–13th century AD and the 16th–early 19th century AD). The second axis, apparently, reflects to a greater extent differences in trends in the number of starry sturgeon (“index Acipenser stellatus “), the size of sterlet (LSI Acipenser ruthenus), the values of the fish index, and the Menhínik species richness index (DMn). Separate positions on this axis are allocated for the periods of the 13th–15th century AD and the 3rd–8th century AD. Thus, the hypothesis regarding the important influence of environmental factors such as the level of the Caspian Sea and the temperature on the relative abundance and size of the four studied species and on the ichthyofauna as a whole is confirmed. The analysis shows that the intensity of commercial exploitation, regarding individual species and the ichthyofauna as a whole, increased significantly from the early Middle Ages to the post-Middle Ages and until modern times along the river gradient of the Volga River.
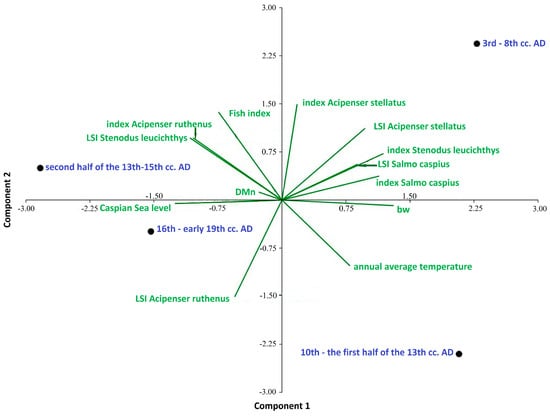
Figure 2.
Principal component analysis (PCA) of biological parameters of four fish species; commercial characteristics of fish fauna, biodiversity, and environmental factors.

Table 2.
Data of biological parameters of four fish species. Commercial characteristics of fish fauna, biodiversity, and environmental factors in four periods (n = number of reconstructed sizes of subfossil fish).
- Historical stages in the development of society in the Volga region: 3rd–8th cc. AD, 10th–the first half of the 13th cc. AD, second half of the 13th–15th cc. AD, 16th–early 19th cc. AD. bw, Whittaker measure; DMn, index of species richness of Menhinik.
- Fish index, the ratio ΣMNI large fish >50 cm to ΣMNI large fish >50 cm + ΣMNI small fish < 50 cm.
- Index Acipenser stellatus, index Salmo caspius, index Stenodus leucichthys, index Acipenser ruthenus: species indices expressing the ratio of the number of remains of a species to the number of bone remains of other fish species.
- LSI Acipenser stellatus, LSI Salmo caspius, LSI Stenodus leucichthys, LSI Acipenser ruthenus: comparative fish size indices; annual average temperature and average annual temperature indicators; Caspian Sea level and changes in the level of the Caspian Sea.
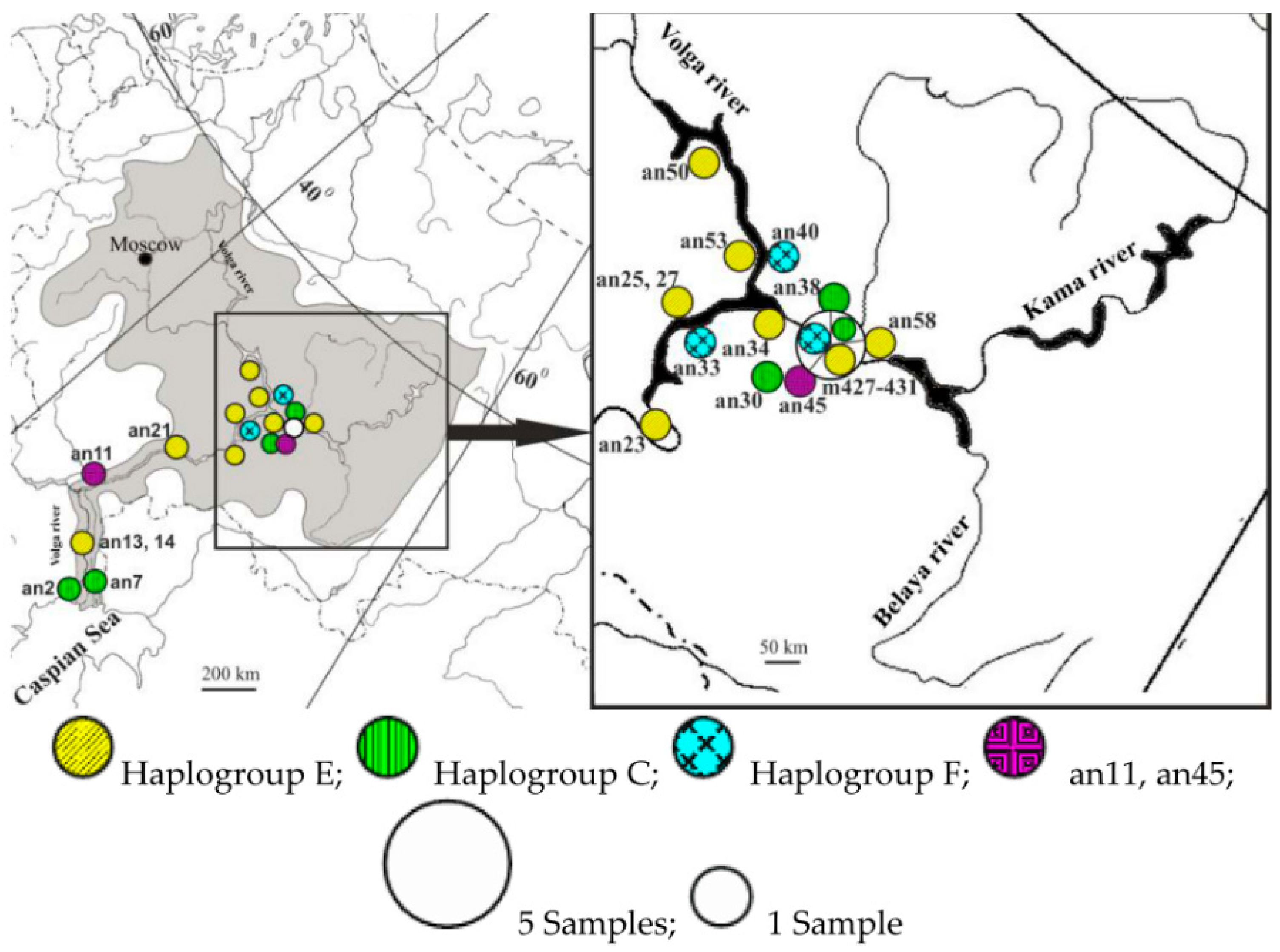
Phylogeography of ancient and contemporary sterlet in the Volga basin revealed that ancient sterlet samples with haplotypes from haplogroups C, E, and F were evenly distributed across all examined localities (Figure 3).
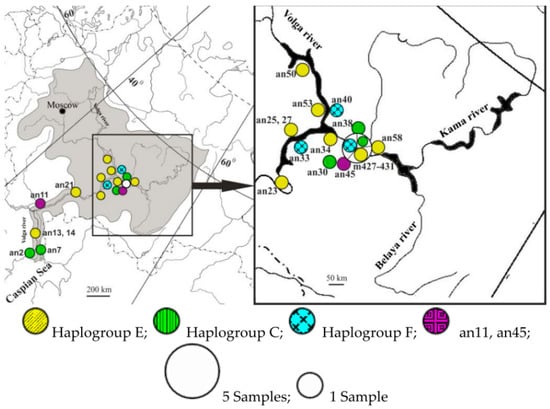
Figure 3.
Distribution of ancient (an) and contemporary (m) haplotypes closely related to major sterlet (Acipenser ruthenus) haplogroups in Volga region.
Most samples belonged to haplogroup E, which seems to be dominant in the Volga basin. Haplotypes C and F seemed to be evenly distributed across different basins, but at low frequencies. Although the contemporary samples were collected from only one locality, they demonstrated high haplotype diversity. When comparing subfossil and contemporary sterlet samples in the Volga basin, high haplotype diversity was observed among both ancient and modern samples. Analysis of the mtDNA control region sequences of the subfossil samples showed that their haplotypes were similar to the contemporary haplogroups of the Volga basin. The sampling size in this study is not sufficient to make any statistically significant conclusions regarding the genetic diversity of the contemporary population, but we can roughly estimate that the haplotype diversity of sterlet in the Volga is comparable to that of the 4th–18th century AD populations. Although the time frame of the ancient sample from the Volga River basin is quite wide, we did not find differentiation or clustering of haplotypes according to the ages of the samples. Five contemporary samples from the Kama river basin all had different, previously unpublished haplotypes—E3A, E3A1, E3G, C1B1, F2, and F3—and belonged to three haplogroups: C, E, and F. These haplogroups also occur in the Siberian rivers [46]. One of the contemporary samples from the Kama had a unique haplotype, C1B1. Previously, we described haplotypes from the haplogroup C in the Ob-Irtysh river basin only. Ancient samples an2, an30, an38 also belonged to this haplogroup. A single ancient sample, an7, was very close to haplogroup B and is widely distributed in Siberian Rivers, especially in Yenisei, the eastern part of the species range. The sample An11 was close to the central node and, thus, may represent an ancient and currently extinct lineage. The sample an45 seemed to be basal to a clade comprising haplogroups A, G, and H. Previously, we assumed that representatives of haplogroups G and H retained many ancestral positions [46].
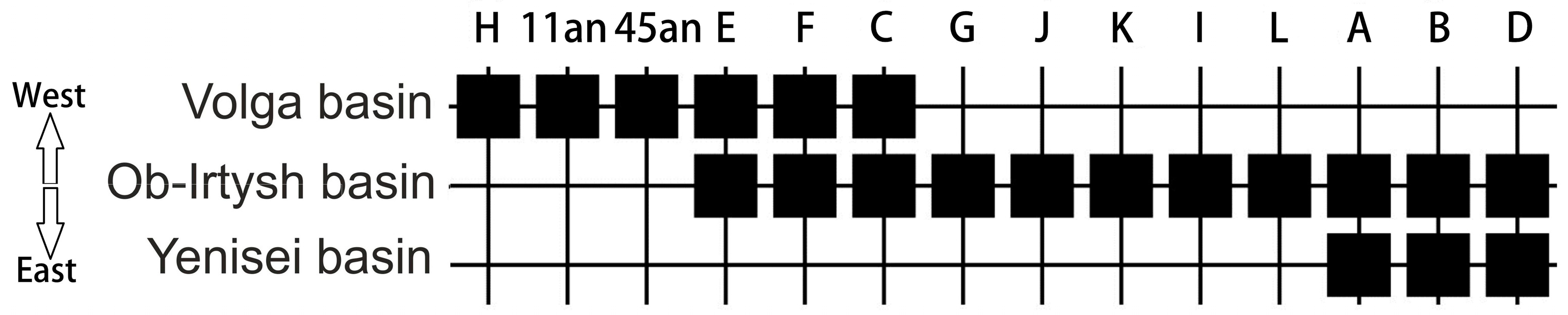
In general, our data confirm the presence of some genetic isolation in Volga River sterlet populations. Thus, in the sterlet population system, there are haplotypes (C1B1, E3A1, E3G, F2, and F3) characteristic only of the Volga basin. Also, in the Volga basin, there are so-called “common” haplogroups (C, E, F) represented in the Ob and Yenissei river systems (see [46]). This trend can be visualized by constructing a sequence matrix of the distribution of sterlet haplogroups according to the gradient of the location of the river basin from West to East, from the Volga River basin to the Yenisei River basin, using an algorithm based on the presence and absence of haplogroups along the geographic gradient (Figure 4).
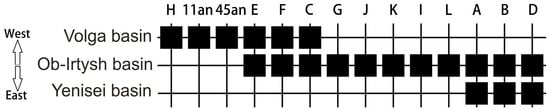
Figure 4.
Matrix of sterlet haplogroup distribution based on the presence and absence of contemporary and ancient haplogroups along the geographic gradient (West–East). The matrix was built using PAST software v. 4.12 [32].
4. Discussion
Using the example of three fish species (Acipenser stellatus, Salmo caspius, and Stenodus leucichthys), we were able to consider the issues of the historical dynamics of the population size. The fate of these species in the past is a vivid example of the extinction of species from the ichthyofauna of the region under the influence of climatic and anthropogenic factors. Sturgeons and salmonids were among the most important species of the ancient and historical Volga River fishery. These species are highly vulnerable to the impacts of fishery and climate change due to their specific behavior and life history. The spawning migration of these species can last more than a year. This has allowed some spawning groups to perform in the most remote upper reaches of the Volga River basin, for example, the upper reaches of the Kama River. Intense overfishing of populations and climate change resulted in a significant decline in catches in the upper and middle zones of the Volga River basin in the medieval and post-medieval times. The sharp decline in numbers and the disappearance of anadromous starry sturgeon (Acipenser stellatus), Caspian trout (Salmo caspius), and Caspian Inconnu (Stenodus leucichthys) from the Upper and Middle Volga seems to have occurred in the 17th and 18th centuries. We associate this with climate change in this period in the direction of sharp cooling. In this period, there was a maximum of the so-called “Little Ice Age”. Apparently, as a result of this process, there were significant difficulties in the survival of these species in the winter period. This affected both the success of extensive migration and spawning and development of juveniles afterward. In addition, overly active fishing of these species overlapped with climatic changes. As a consequence, destabilization of the populations of these species occurred, which led to the loss of extensive spawning migrations to the Upper and Middle Volga. At the same time, the numbers and sizes of non-migratory sterlet (Acipenser ruthenus) were high in all historical periods, which allowed the people of ancient settlements on the Volga River to intensively exploit the stocks of this species. Regular fishing for anadromous sturgeon and salmonids gradually faded in the middle and upper reaches of the Volga during the 19th century [57], but even until the first half of the 20th century, in the northern segment of the Lower Volga and in the lower reaches of the Kama, regular fishing was utilized for certain anadromous species such as Beluga (Huso huso), Russian sturgeon (Acipenser gueldenstaedtii), and Caspian Inconnu (Stenodus leucichthys) (see [41]).
From the beginning of the second half of the 20th century, almost the entire habitat of both the Middle and Lower Volga, especially spawning grounds, wintering pits, and migration routes, was changed as a result of extensive river engineering and giant hydraulic construction [58,59]. The catch numbers of anadromous sturgeons and salmonids continued to decline [2,58]. Indeed, the entire catch of anadromous sturgeons and salmonids in the second half of the 20th century in the northern part of the Lower Volga (from Samara to the confluence of the Kama River) was less than a one-day capture of these species on one of the fishing sites, for example, in the 15th or 16th century AD [3,4,5]. At the moment, the numbers of all anadromous sturgeon and salmonid species of the Volga River are close to extinction. And it is not known for how long these species can exist on the verge of extinction, because when the population reaches the minimum number of individuals, it degrades—this is one of the components of the Allee effect.
Recently, an opinion has emerged that the archaeological remains of fish create a somewhat distorted picture of fish assemblages of the past; fish remains from archaeological sites provide reliable comparisons only for large fish species, which are the mainstay of manual sampling from archaeological strata given that this limited material may reflect regional differences [19,20]. This opinion cannot be unambiguous, so we compare our results with the results of an assessment of long-term changes in the ichthyofauna of the second largest river system in Europe, the Danube River basin [19]. We can see that the two main river systems of Europe are characterized by fairly large differences in the species composition of fish at archaeological sites within a certain historical period, which may indicate the fishing preferences of the peoples in relation to species or groups of fish for a given period of time or region. In both river systems, a geographic (regional) gradient is clearly visible in the distribution of fish species. Data from the Volga and Danube showed that there was a trend indicating an increase in the number of species of fish caught in the fishery from the early Middle Ages to the Post-Middle Ages and the modern period. In both river systems, the existence of a typical river ecosystem in the Middle Ages and early modern period, characteristic of a large river with a high numerical content of rheophilic and anadromous fish species in the ichthyofauna, has been confirmed. The differences in the fishing ichthyofaunas of the Volga and Danube are revealed quite clearly:
- The Volga river system was characterized by four species of sturgeon, wels catfish, zander, and common bream, with the inclusion of a large number of species of cyprinids, Northern pike, European perch, and Caspian inconnu. The Danube was characterized by common carp, northern pike, cyprinids, wels catfish, and sturgeons.
- A significant change in fishing ichthyofaunas in the Volga River system occurred in the Danube during the 19th century, at the end of the Middle Ages to the beginning of the modern period.
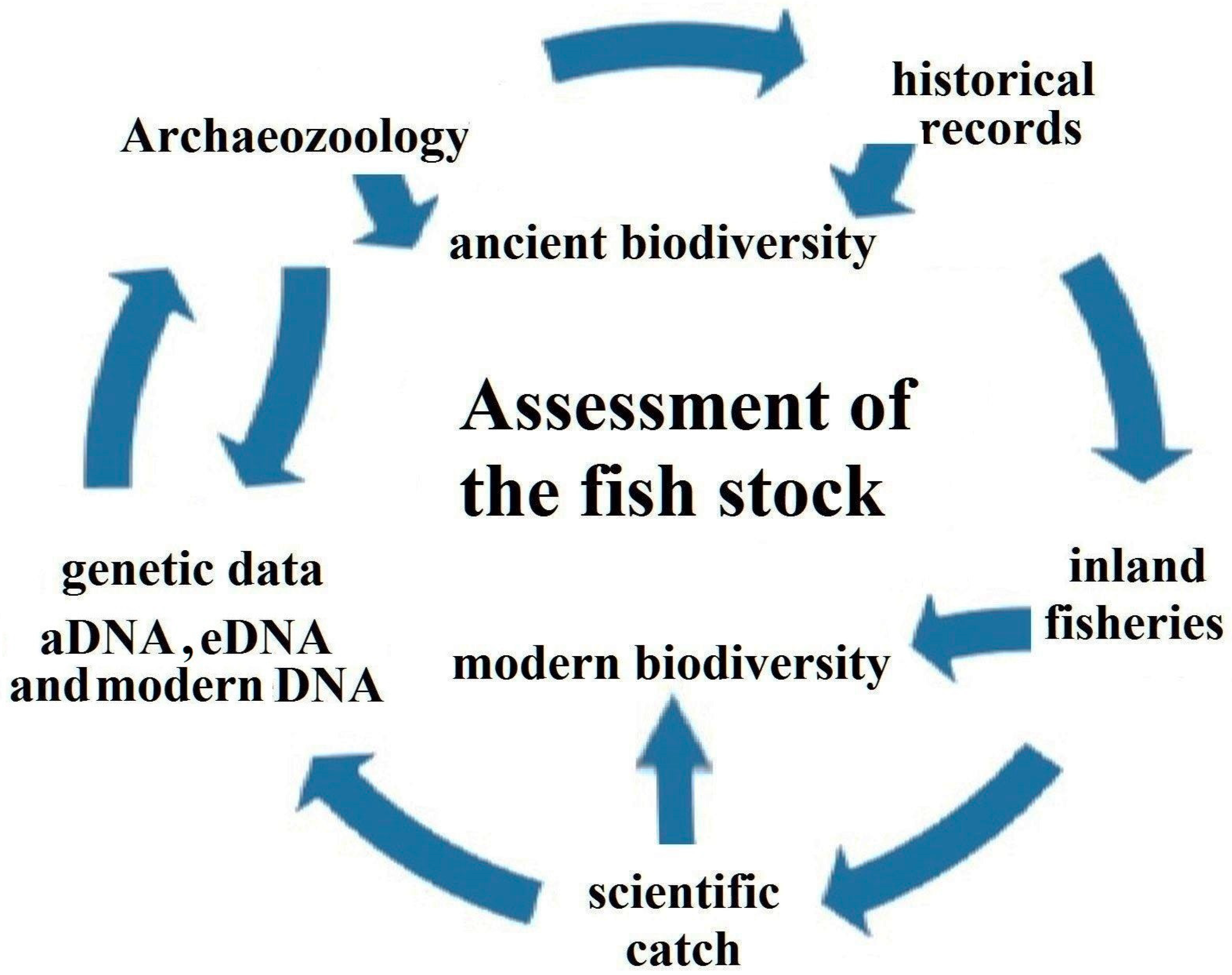
With our contribution, we want to highlight a framework for a holistic assessment of the fish stock in a large river (Figure 5), which consists of (i) archaeozoology, (ii) historical records, (iii) data from inland fisheries, (iv) scientific catch, (v) genetic data, and (vi) biodiversity assessment. In the Volga basin, all aspects were analyzed, and we highlight the need to bring this information together. Archaeozoological surveys were carried out in the Volga basin, as summarized herein (see also [4,5]). Regarding historical records, studies of the fish communities in the Volga basin date back to the Great Academic Expeditions in 1768 [60,61].
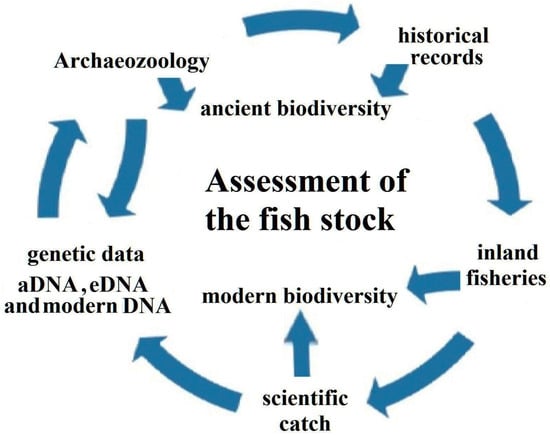
Figure 5.
Framework for a holistic assessment of the fish stock in a large river system.
The Volga River is important for inland fisheries; thus, the commercial fish catch is well documented [2,62,63,64,65]. The annual catch is about 40,000 t [57]. In the 17th century, sturgeon catches of 50,000 tons per year were reported, which decreased to 13,500 t around 1930 and 4900 t in 1946; in the 1970s and 1980s, the sturgeon catch again reached 20,000–25,000 t, with a sharp decrease to 400 t in 2004 [66]. From 1952 to 1990, the catch was never below 6000 t, and usually ranged between 7160 and 16,770 t (in 1977, it even reached 27,200 t in the entire Caspian basin). This later dropped dramatically, i.e., in 1992, the catches of Russian sturgeon, starry sturgeon, and beluga sturgeon were 3790, 2440, and 300 t, respectively [67]. Scientific catch is often carried out with gill-nets, seines, and dragnets (e.g., [68,69]). Long-term datasets are very valuable for our understanding of the dynamics of fish stock [70].
Currently, the main watercourses of the Volga River and its main tributaries are almost completely regulated by hydroelectric dams, and the floodplains are flooded with water from reservoirs. As such, there is an urgent need to study changes and long-term assessment of the biodiversity, biology, and ecology of fish populations in small- and medium-sized rivers and lakes of the Volga River basin. The results of research over the past two decades have shown that small- and medium-sized watercourses are refugia for the conservation and maintenance of rheophilic species with high biodiversity, and small lakes for typical native limnophilic fish species [71,72,73,74].
Population genetic analysis and fish ecology research have shown that, in a number of small- and medium-sized tributaries of the Middle Volga and Kama, populations of brook trout have been preserved, retaining genetic diversity of the Volga population group of Caspian trout (Salmo caspius), which has practically disappeared from the wild [75]. Furthermore, genetic diversity and ecological patterns of European populations of Siberian taimen (Hucho taimen) have been preserved in tributaries of the upper Kama and tributaries of the upper part of the river Belaya (the largest tributary of the Kama River) [74,76]. Recently, environmental DNA metabarcoding has been used to assess the fish fauna of the Volga River headwaters [77,78]. This noninvasive approach enables the determination of the number of species in an aquatic ecosystem, as well as their identity and distribution.
By examining modern and ancient DNA, a pattern of genetic divergence among Eurasian freshwater fish species can be observed. For example, for the Siberian taimen (Hucho taimen Pallas, 1773), there are only minor differences in populations between the Volga river basin and Western Siberian rivers [76], while the European grayling (Thymallus thymallus Linnaeus, 1758) demonstrates significant differences even within the Eastern European river basins [79]. Compared to other valuable commercial fish species, such as the zander (Sander lucioperca), Volga pikeperch (Sander volgensis) [80], and wels catfish (Silurus glanis) [81], that inhabit the Volga basin, in this study, the number of sterlet haplogroups and haplotype diversity was found to be very high. Our data on sterlet suggests a possible exchange of ichthyofauna between Siberia and European river basins during the second half of the Pleistocene through an extensive system of dammed lakes to the north and east of the Russian Plain and Western Siberia. This was proposed for other freshwater species as well [76,79,82,83,84,85,86]. Genetic analyses, such as ancient DNA and environmental DNA studies, have great potential to increase the knowledge of historical fish populations in large rivers and long-term changes in ichthyofauna. Sturgeon samples from archaeological excavation in the Volga region seem to have sufficient preservation for ancient DNA studies. Our study revealed the genetic continuity of sterlet from the 3rd century AD until today. This should be considered when developing programs for the restoration of this species.
5. Conclusions
At present, the population numbers of all sturgeons and salmonids of the Volga River are critically low. Therefore, the proposed framework, i.e., analyses of historical data, conventional surveys, and the inclusion of genetic approaches, is essential for the development of appropriate conservation strategies.
Reconstructions of biological parameters and data on the population characteristics of four anadromous fish species based on materials from archaeological sites can be used for operational forecasts and the development of simulation dynamics of the modern populations of these species. As such, it is necessary to rethink the meaning of subfossil animal remains, their importance, and their practical components in the conservation of the biodiversity of rare and endangered species. Their modern and ancient gene pools should also be considered, and these studies should be included in modern programs on conservation biology implemented in Russia, including those focusing on sturgeon and salmonid species. The approaches and methods used in our work for the analysis of subfossil fish remains from archaeological sites make it possible to expand the range of methods applicable to archaeoichthyological material.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w16081109/s1, Supplementary S1: List of archaeological sites; Supplementary S2: Methodology for sterlet DNA research and NCBI accession numbers; Figure S1: Map of ancient (white) and contemporary (black) sterlet sampling collection sites; Table S1. Ancient and contemporary sterlet (A. ruthenus) samples; Table S2. The list of primers used for sterlet mtDNA sequencing; Table S3. Accession numbers in GenBank and length of gap for ancient samples; Table S4. Contemporary haplotypes included in this study; Table S5. Coordinates of deamination sites in ancient samples.
Author Contributions
Conceptualization, I.V.A., V.A.T. and M.S.; methodology, I.V.A., O.V.A., A.O.A., D.N.S., S.P.M., M.A.P. and V.A.T.; formal analysis, I.V.A., O.V.A., A.O.A., D.N.S., S.P.M., M.A.P. and V.A.T.; investigation, I.V.A., O.V.A., A.O.A., D.N.S., S.P.M., M.A.P., V.A.T., K.G. and M.S.; writing—original draft preparation, I.V.A., O.V.A., A.O.A., D.N.S., S.P.M., M.A.P., V.A.T., K.G. and M.S.; writing—review and editing, I.V.A. and M.S.; visualization, I.V.A., O.V.A., A.O.A., D.N.S., S.P.M., M.A.P. and V.A.T.; funding acquisition, V.A.T. All authors have read and agreed to the published version of the manuscript.
Funding
Sterlet DNA analysis was supported by the Russian Scientific Fond, grant № 18-44-04007.
Institutional Review Board Statement
All experiments (DNA analysis and work with animal samples) were approved by the Committee on the Ethics of Animal Experiments of the Institute of Molecular and Cellular Biology (IMCB), SB RAS, Russia.
Data Availability Statement
Data will be made available upon reasonable request.
Acknowledgments
The authors gratefully acknowledge the resources provided by the “Molecular and Cellular Biology” core facility of the IMCB SB RAS. We thank Sergey Kliver for help with data analysis of DNA and Alexey Makunin for carefully reading part of the manuscript and making valuable comments on the analysis of sterlet DNA data.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mineeva, N.; Lazareva, V.; Litvinov, A.; Stepanova, I.; Chuiko, G.; Papchenkov, V.; Korneva, L.; Shcherbina, G.; Pryanichnikova, E.; Perova, S.; et al. Chapter 2—The Volga River. In Rivers of Europe, 2nd ed.; Tockner, K., Zarfl, C., Robinson, C.T., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 27–79, 922. [Google Scholar]
- Schletterer, M.; Kuzovlev, V.V.; Zhenikov, Y.N.; Tuhtan, J.A.; Haidvogl, G.; Friedrich, T.; Górski, K.; Füreder, L. Fish fauna and fisheries of large European rivers: Examples from the Volga and the Danube. Hydrobiologia 2018, 814, 45–60. [Google Scholar] [CrossRef]
- Askeyev, I.V.; Askeyev, O.V.; Galimova, D.N. Natural environment and man in the Volga-Kama and Cis-Urals (Late Paleolithic—Middle Ages). In The Middle Volga and Southern Urals: Man and Nature in Antiquity. Collection of Scientific Articles Dedicated to the 75th Anniversary of Doctor of Historical Sciences Evgeny Petrovich Kazakov; Institute of History of the Academy of Sciences of the Republic of Tatarstan: Kazan, Russia, 2009; pp. 32–112. [Google Scholar]
- Askeyev, I.V.; Galimova, D.N.; Askeyev, O.V. Ichthyofauna of the middle Volga River basin in the Late Holocene (based on archaeological excavations). Zool. Zhurnal 2013, 92, 1014–1030. [Google Scholar] [CrossRef]
- Askeyev, I.V.; Askeyev, O.V.; Galimova, D.N. Archeo-ichthyological research on the territory of the Volga-Kama region. In Archeology and Natural Sciences of Tatarstan. Book 4; Institute of History of the Academy of Sciences of the Republic of Tatarstan: Kazan, Russia, 2011; pp. 44–156. [Google Scholar]
- Butler, V.L.; Delacorte, M.G. Doing Zooarchaeology as if it mattered: Use of faunal data to address current issues in fish conservation biology in Owens Valley, California. In Zooarchaeology and Conservation Biology; Lyman, R.L., Cannon, K.P., Eds.; University of Utah Press: Salt Lake City, UT, USA, 2004; pp. 25–44. [Google Scholar]
- Braje, T.J.; Rick, T.C.; Erlandson, J.M. Rockfish in the long view: Applied zooarchaeology and conservation of Pacific red snapper (genus Sebastes) in southern California. In Conservation Biology and Applied Zooarchaeology; Wolverton, S., Lyman, R.L., Eds.; University of Arizona Press: Tucson, AZ, USA, 2012; pp. 157–178. [Google Scholar]
- Speller, C.F.; Hauser, L.; Lepofsky, D.; Moore, J.; Rodrigues, A.T.; Moss, M.L.; McKechnie, I.; Yang, D.Y. High potential for using DNA from ancient herring bones to inform modern fisheries management and conservation. PLoS ONE 2012, 7, e51122. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.H. An environmental (pre)history of European fishing: Past and future archaeological contributions to sustainable fisheries. J. Fish Biol. 2019, 94, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Grant, J.; Gorin, S.; Fleming, N. The Archaeology Coursebook—An Introduction to Study Skills, Topics and Methods; Taylor & Francis Group: London, UK; New York, NY, USA, 2002; 323p. [Google Scholar]
- Piezonka, H.; Kostyleva, E.; Zhilin, M.G.; Dobrovolskaya, M.; Terberger, T. Flesh or fish? First results of archaeometric research of prehistoric burialsfrom Sakhtysh IIa, Upper Volga region, Russia. Doc. Praehist. 2013, 40, 57–73. [Google Scholar] [CrossRef]
- Jones, T.L.; Gobalet, K.W.; Codding, B.F. The archaeology of fish and fishing on the central coast of California: The case for an under-exploited resource. J. Anthr. Archaeol. 2016, 41, 88–108. [Google Scholar] [CrossRef]
- McKechnie, I.; Moss, M.L. Meta-analysis in zooarchaeology expands perspectives on Indigenous fisheries of the Northwest Coast of North America. J. Archaeol. Sci. Rep. 2016, 8, 470–485. [Google Scholar] [CrossRef]
- Shaymuratova, D.N.; Askeyev, I.V.; Askeyev, O.V.; Monachov, S.P.; Askeyev, A.O.; Smirnov, A.A. Sterlet Acipenser ruthenus (Acipenseriformes, Acipenseridae) of Middle Volga and Lower Kama in IV–XVIII centuries AD: Size and age composition, growth and value in the ancient fishing. Vopr. Rybolov. 2017, 18, 401–421. [Google Scholar]
- Guiry, E.J.; Kennedy, J.R.; O’connell, M.T.; Gray, D.R.; Grant, C.; Szpak, P. Early evidence for historical overfishing in the Gulf of Mexico. Sci. Adv. 2021, 7, eabh2525. [Google Scholar] [CrossRef]
- Lebedev, V.D. Freshwater Quaternary Ichthyofauna of the European Part of the USSR; Moscow State University Publishing: Moscow, Russia, 1960; 402p. [Google Scholar]
- Tsepkin, E.A. Changes in the exploited fish fauna of inland water-bodies of Eastern Europe and Northern Asia in the Quaternary period. Vopr. Ikhtiologii 1995, 35, 3–17. [Google Scholar]
- Makowiecki, D. History of Fishes and Fishing in Holocene on Polish Lowland in the Light of Archaeoichthyological Studies; Institute of Archaeology and Ethnology Polish Academy of Science: Poznan, Poland, 2003; 198p. [Google Scholar]
- Galik, A.; Haidvogl, G.; Bartosiewicz, L.; Guti, G.; Jungwirth, M. Fish remains as a source to reconstruct long-term changes of fish communities in the Austrian and Hungarian Danube. Aquat. Sci. 2015, 77, 337–354. [Google Scholar] [CrossRef] [PubMed]
- Broughton, J.M.; Martin, E.P.; McEneaney, B.; Wake, T.; Simons, D.D. Late Holocene anthropogenic depression of sturgeon in San Francisco Bay, California. J. Calif. Great Basin Anthropol. 2015, 35, 3–27. [Google Scholar]
- De Cupere, B.; Van Neer, W. Faunal Remains from Archaeological Sites Document Human Impact on the Terrestrial and Aquatic Environment: Examples from the last thousand years in Belgium. Internet Archaeol. 2023, 62. [Google Scholar] [CrossRef]
- Shaymuratova, D.N.; Askeyev, I.V.; Nedashkovsky, L.F. Archaeoichthyological research of settlements of the Golden Horde period of the Saratov Volga. Volga River Reg. Archaeol. 2021, 4, 191–204. [Google Scholar] [CrossRef]
- Casteel, R.W. Some archaeological uses of fish remains. Am. Antiq. 1972, 37, 404–419. [Google Scholar] [CrossRef]
- Morey, D.F. Archaeological assessment of seasonality from freshwater fish remains: Aquantitative procedure. J. Ethnobiol. 1983, 3, 75. [Google Scholar]
- Wheeler, A.; Jones, A.K.G. Fishes; Cambridge Manuals in Archaeology; Cambridge University Press: Cambridge, UK, 1989; 210p. [Google Scholar]
- Guillaud, E.; Elleboode, R.; Mahe, K.; Bearez, P. Estimation of age, growth and fishing season of a Paleolithic population of grayling (Thymallus thymallus) using scale analysis. Int. J. Osteoarchaeol. 2017, 27, 683–692. [Google Scholar] [CrossRef]
- Askeyev, I.V.; Tarasov, A.Y.; Askeyev, A.O.; Askeyev, O.V.; Shaymuratova, D.N.; Monakhov, S.P. Highly productive fishing in Lake Onega? New data on the subsistence basis of the Late Stone age populations in Russian Karelia. J. Archaeol. Sci. Rep. 2023, 47, 103771. [Google Scholar]
- Morales Muñiz, A.; Rosenlund, K. Fish Bone Measurements: An Attempt to Standardize the Measurement of Fish Bones from Archaeological Sites; Steenstrupia: Copenhagen, Denamrk, 1979; 48p. [Google Scholar]
- Radu, V. Exploitation des Ressources Aquatiques dans les Cultures Néolithiques et Calcolithiques de la Roumanie Méridionale. Ph.D. Thesis, l’Université Aix-Marseille I, Préhistoire, Archéologie, Histoire et Civilisation de l’Antiquité et du Moyen-Age, Aix-en-Provence, France, 2003; 432p. [Google Scholar]
- Živaljević, I.; Askeyev, I.V.; Shaymuratova (Galimova), D.N.; Askeyev, O.V.; Monakhov, S.P.; Borić, D.; Stefanović, S. Size estimations of sturgeons (Acipenseridae) from the Mesolithic-Neolithic Danube Gorges. In Foraging Assemblages, Volume 2; Borić, D., Antonović, D., Mihailović, B., Eds.; Serbian Archaeological Society: Belgrade, Serbia; The Italian Academy for Advanced Studies in America, Columbia University: New York, NY, USA, 2021; pp. 422–427. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis, 4th ed.; Prentince-Hall: Englewood Cliffs, NJ, USA, 1999; 663p. [Google Scholar]
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Chugunova, N.I. Guidebook for the Study of Fish Age and Growth; Izd. Akademii nauk SSSR: Moscow, Russia, 1959; 164p. [Google Scholar]
- Pravdin, I.F. Guide to the Study of Fish; Pischevaya Promyshlennost: Moscow, Russia, 1966; 376p. [Google Scholar]
- Panfili, J.; de Pontual, H.; Troadec, H.; Wright, P.J. (Eds.) Manual of Fish Sclerochronology; Ifremer-lRD Coedition: Brest, France, 2002; 464p. [Google Scholar]
- Magurran, A.E. Measuring Biological Diversity; Blackwell Science: Hoboken, NJ, USA, 2004; 256p. [Google Scholar]
- Whittaker, R.H. Evolution of species diversity in land communities. Evol. Biol. 1977, 10, 1–67. [Google Scholar]
- Butler, V.L. Resource depression on the Northwest Coast of North America. Antiquity 2000, 74, 649–661. [Google Scholar] [CrossRef]
- Sokolov, L.I.; Tsepkin, E.A. Sterlet Acipenser ruthenus L. in middle and late Holocene. Bull. Moscow Soc. Nat. Biol. Ser. 1971, 76, 137–145. [Google Scholar]
- Borzenko, M.P. Caspian stellate sturgeon (systematics, biology and fishing). Izv. Azerbaijan Sci. Res. Fish. Stn. 1942, 7, 3–114. [Google Scholar]
- Berg, L.S. Chapter 1. In Freshwater Fish of the USSR and Neighboring Countries; Izd. Akademii nauk SSSR: Moscow-Leningrad, Russia, 1948; 466p. [Google Scholar]
- Shmidtov, A.I. Sterlet (Acipenser ruthenus L.). Materials on the Biology and Fishing of Sterlet in the Lower Reaches of the Kama River; Scientific notes of Kazan University; Zoology; Volume 99; Book 4; Kazan University: Kazan, Russia, 1939; pp. 13–279. [Google Scholar]
- Derzhavin, A.N. Volga Salmon (Based on Historical Materials); Collection Articles in Honor of N.M. Knipovich; Pishchepromizdat: Moscow, Russia; Leningrad, Russia, 1939; pp. 187–206. [Google Scholar]
- Klimenko, V.; Solomina, O. Climatic variations in the East European Plain during the last Millennium: State of the art. In The Polish Climate in the European Context: An Historical Overview; Przybylak, R., Ed.; Springer: Dordrecht, The Netherlands, 2010; pp. 71–101. [Google Scholar] [CrossRef]
- Bolgov, M.V.; Krasnozhon, G.F.; Lyubushin, A.A. Caspian Sea: Extreme Hydrological Events; Nauka: Moscow, Russia, 2007; 381p. [Google Scholar]
- Pobedintseva, M.A.; Makunin, A.I.; Kichigin, I.G.; Kulemzina, A.I.; Serdyukova, N.A.; Romanenko, S.A.; Vorobieva, N.V.; Interesova, E.A.; Korentovich, M.A.; Zaytsev, V.F.; et al. Population genetic structure and phylogeography of sterlet (Acipenser ruthenus, Acipenseridae) in the Ob and Yenisei river basins. Mitochondrial DNA Part A 2019, 30, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.Y.; Eng, B.; Waye, J.S.; Dudar, J.C.; Saunders, S.R. Technical Note: Improved DNA Extraction From Ancient Bones Using Silica-Based Spin Columns. Am. J. Phys. Anthropol. 1998, 105, 539–543. [Google Scholar] [CrossRef]
- Sanderson, C.; Radley, K.; Mayton, L. Ethylenediaminetetraacetic acid in ammonium hydroxide for reducing decalcification time. Biotech. Histochem. 1995, 70, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Cheng, L.; Li, J.-T.; Lu, C.Y.; Wang, Y.; Sun, X.W. Complete mitochondrial genome of sterlet (Acipenser ruthenus). Mitochondrial DNA 2013, 26, 259–260. [Google Scholar] [CrossRef]
- Dabney, J.; Meyer, M.; Pääbo, S. Ancient DNA damage. Cold Spring Harb Perspect Biol. 2013, 5, a012567. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Fu, L.; Niu, B.; Zhu, Z.; Wu, S.; Li, W. CD-HIT: Accelerated for clustering the next-generation sequencing data. Bioinformatics 2012, 28, 150–3152. [Google Scholar] [CrossRef] [PubMed]
- Okonechnikov, K.; Golosova, O.; Fursov, M.; Ugene Team. Unipro UGENE: A unified bioinformatics toolkit. Bioinformatics 2012, 28, 1166–1167. [Google Scholar] [CrossRef] [PubMed]
- Clement, M.; Snell, Q.; Walker, P.; Posada, D.; Crandall, K. TCS: Estimating gene genealogies. Parallel Distributed Process. Symp. Int. 2002, 3, 184–190. [Google Scholar]
- Leigh, J.W.; Bryant, D. POPART: Full-feature software for haplotype network construction. Methods. Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Kessler, K.F. Ichthyofauna of the Volga River. Proc. St. Petersburg Soc. Nat. 1870, 1, 236–311. [Google Scholar]
- Butorin, N.V. Volga and Its Life; Nauka: Leningrad, Russia, 1978; 348p. [Google Scholar]
- Schletterer, M.; Shaporenko, S.I.; Kuzovlev, V.V.; Minin, A.E.; Van Geest, G.J.; Middelkoop, H.; Górski, K. The Volga: Management issues in the largest river basin in Europe. River Res. Appl. 2019, 35, 510–519. [Google Scholar] [CrossRef]
- Pallas, P.S. Travels through Various Provinces of the Russian Empire, 3 Volumes (Vol. 1, 504 pp., Vol. 2, 744 pp., Vol. 3, 760 pp. + 118 plates) 1771–1776; Akademische Druck und Verlagsanstalt: Graz, Austria, 1967. [Google Scholar]
- Lepekhin, I.I. Day Notes of a Trip to Different Provinces of the Russian State. 1768 and 1769; Imperatorskaya Akademiya Nauk: St. Petersburg, Russia, 1771; 538p. [Google Scholar]
- van de Wolfshaar, K.E.; Middelkoop, H.; Addink, E.; Winter, H.V.; Nagelkerke, L.A.J. Linking Flow Regime, Floodplain Lake Connectivity and Fish Catch in a Large River-Floodplain System, the Volga–Akhtuba Floodplain (Russian Federation). Ecosystems 2011, 14, 920–934. [Google Scholar] [CrossRef]
- Górski, K.; van den Bosch, L.V.; van de Wolfshaar, K.E.; Middelkoop, H.; Nagelkerke, L.A.J.; Filippov, O.V.; Zolotarev, D.V.; Yakovlev, S.V.; Minin, A.E.; Winter, H.V.; et al. Post-damming flow regime development in a large lowland river (Volga, Russian Federation): Implications for floodplain inundation and fisheries. River Res. Appl. 2012, 28, 1121–1134. [Google Scholar] [CrossRef]
- Samoylenko, V.V. Dynamics, structure and drivers of fish catch in Russian Federation (2005–2017). Izv. TINRO 2019, 196, 204–218. [Google Scholar] [CrossRef]
- Ponomareva, E.N.; Balykin, P.A.; Startsev, A.V.; Korchunov, A.A.; Savitskaya, S.S. Current state of fisheries in the Volga-Caspian subarea. Vestnik Astrakhan State Tech. Univ. Series: Fish. Ind. 2022, 7–15. [Google Scholar] [CrossRef]
- Maltsev, S.A. Conservation of the Sturgeon Fish in Lower Volga. In Biology, Conservation and Sustainable Development of Sturgeons; Carmona, R., Domezain, A., García-Gallego, M., Hernando, J.A., Rodríguez, F., Ruiz-Rejón, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 265–273. [Google Scholar]
- Altukhov, Y.P.; Evsyukov, A.N. Juvenile Overproduction at Fish Hatcheries May Explain the Degradation of the Volga Stock of the Russian Sturgeon. Dokl. Biol. Sci. 2001, 380, 454–456. [Google Scholar] [CrossRef]
- Górski, K.; Winter, H.V.; DE Leeuw, J.J.; Minin, A.E.; Nagelkerke, L.A.J. Fish spawning in a large temperate floodplain: The role of flooding and temperature. Freshw. Biol. 2010, 55, 1509–1519. [Google Scholar] [CrossRef]
- Górski, K.; Buijse, A.D.; Winter, H.V.; De Leeuw, J.J.; Compton, T.J.; Vekhov, D.A.; Zolotarev, D.V.; Verreth, J.A.J.; Nagelkerke, L.A.J. Geomorphology and Flooding Shape Fish Distribution in A Large-Scale Temperate Floodplain. River Res. Appl. 2012, 29, 1226–1236. [Google Scholar] [CrossRef]
- Artaev, O.; Ruchin, A.; Ivanchev, V.; Ivancheva, E.; Sarychev, V.; Moreva, O.; Mikheev, V.; Medvedev, D.; Klevakin, A. Fish occurrence in the middle Volga and upper Don regions (Russia). Biodivers. Data J. 2020, 8, e54959. [Google Scholar] [CrossRef] [PubMed]
- Askeyev, O.V.; Monakhov, S.P.; Askeyev, I.V.; Askeyev, A.O.; Sparks, T.H. Fish assemblages in lakes along environmental gradients at the eastern edge of Europe. Environ. Biol. Fish. 2023, 106, 1265–1276. [Google Scholar] [CrossRef]
- Askeyev, O.; Askeyev, I.; Askeyev, A.; Monakhov, S.; Yanybaev, N. River fish assemblages in relation to environmental factors in the eastern extremity of Europe (Tatarstan Republic, Russia). Environ. Biol. Fishes 2014, 98, 1277–1293. [Google Scholar] [CrossRef]
- Askeyev, A.O.; Askeyev, O.V.; Yanybaev, N.M.; Askeyev, I.V.; Monakhov, S.P.; Marić, S.; Hulsman, K. River fish assemblages along an elevation gradient in the eastern extremity of Europe. Environ. Biol. Fishes 2017, 100, 585–596. [Google Scholar] [CrossRef]
- Askeyev, A.O.; Askeyev, O.V.; Askeyev, I.V.; Monakhov, S.P. Predatory fish species as indicators of biodiversity: Their distribution in environmental gradients in small and mid-sized rivers in Eastern Europe. Environ. Biol. Fishes 2021, 104, 767–778. [Google Scholar] [CrossRef]
- Marić, S.; Askeyev, O.V.; Askeyev, A.O.; Monakhov, S.P.; Yanybaev, N.M.; Askeyev, I.V.; Galimova, D.N.; Snoj, A. Lack of mtDNA variation among remote middle Volga and upper Ural brown trout suggests recent and rapid recolonization. J. Appl. Ichthyol. 2016, 32, 948–953. [Google Scholar] [CrossRef]
- Marić, S.; Alekseyev, S.; Snoj, A.; Askeyev, O.; Askeyev, I.; Weiss, S. First mtDNA sequencing of Volga and Ob basin taimen Hucho taimen: European populations stem from a late Pleistocene expansion of H. taimen out of western Siberia and are not intermediate to Hucho hucho. J. Fish Biol. 2014, 85, 530–539. [Google Scholar] [CrossRef]
- Lecaudey, L.A.; Schletterer, M.; Kuzovlev, V.V.; Hahn, C.; Weiss, S.J. Fish diversity in the headwaters of the Volga River using environmental DNA metabarcoding. Aquat. Conserv. Mar. Freshw. Ecosyst. 2019, 29, 1785–1800. [Google Scholar] [CrossRef]
- Schenekar, T.; Schletterer, M.; Lecaudey, L.A.; Weiss, S.J. Reference databases, primer choice, and assay sensitivity for environmental metabarcoding: Lessons learnt from a re-evaluation of an eDNA fish assessment in the Volga headwaters. River Res. Appl. 2020, 36, 1004–1013. [Google Scholar] [CrossRef]
- Marić, S.; Askeyev, I.V.; Askeyev, O.V.; Monakhov, S.P.; Bravničar, J.; Snoj, A. Phylogenetic and population genetic analysis of Thymallus thymallus (Actinopterygii, Salmonidae) from the middle Volga and upper Ural drainages. Hydrobiologia 2014, 740, 167–176. [Google Scholar] [CrossRef]
- Haponski, A.E.; Stepien, C.A. Phylogenetic and biogeographical relationships of the Sander pikeperches (Percidae: Perciformes): Pat terns across North America and Eurasia. Biol. J. Linn. Soc. 2013, 110, 156–179. [Google Scholar] [CrossRef]
- Triantafyllidis, A.; Krieg, F.; Cottin, C.; Abatzopoulos, T.J.; Triantaphyllidis, C.; Guyomard, R. Genetic structure and phylogeography of European catfish (Silurus glanis) populations. Mol. Ecol. 2002, 11, 1039–1055. [Google Scholar] [CrossRef] [PubMed]
- Van Houdt, J.K.J.; De Cleyn, L.; Perretti, A.; Volckaert, F.A.M. A mitogenic view on the evolutionary history of the Holarctic freshwater gadoid, burbot (Lota lota). Mol. Ecol. 2005, 14, 2445–2457. [Google Scholar] [CrossRef] [PubMed]
- Rastorguev, S.M.; Nedoluzhko, A.V.; Mazur, A.M.; Gruzdeva, N.M.; Volkov, A.A.; Barmintseva, A.E.; Mugue, N.S.; Prokhortchouk, E.B. High-throughput SNP-genotyping analysis of the relationships among Ponto-Caspian sturgeon species. Ecol. Evol. 2013, 3, 2612–2618. [Google Scholar] [CrossRef] [PubMed]
- Perdices, A.; Vasil’eva, E.; Vasil’ev, V. From Asia to Europe across Siberia: Phylogeography of the Siberian spined loach (Teleostei, Cobitidae). Zool. Scr. 2015, 44, 29–40. [Google Scholar] [CrossRef]
- Levin, B.A.; Simonov, E.P.; Ermakov, O.A.; Levina, M.A.; Interesova, E.A.; Kovalchuk, O.M.; Malinina, Y.A.; Mamilov, N.S.; Mustafayev, N.J.; Pilin, D.V.; et al. Phylogeny and phylogeography of the roaches, genus Rutilus (Cyprinidae), at the Eastern part of its range as inferred from mtDNA analysis. Hydrobiologia 2016, 788, 33–46. [Google Scholar] [CrossRef]
- Artaev, O.N.; Ermakov, O.A.; Vekhov, D.A.; Konovalov, A.F.; Levina, M.A.; Pozdeev, I.V.; Ruchin, A.B.; Alyushin, I.V.; Iljin, V.Y.; Levin, B.A. Genetic Screening of Distribution Pattern of Roaches Rutilus rutilus and R. lacustris (Cyprinidae) in Broad Range of Secondary Contact (Volga Basin). Inland Water Biol. 2021, 14, 205–214. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).