Disinfection of Secondary Urban Wastewater Using Hydrogen Peroxide Combined with UV/Visible Radiation: Effect of Operating Conditions and Assessment of Microorganism Competition
Abstract
1. Introduction
2. Materials and Methods
2.1. Secondary Urban Effluent
2.2. Experimental Procedure
2.3. Analytical Methods
3. Results and Discussion
3.1. Processes Screening
3.2. Parametric Study
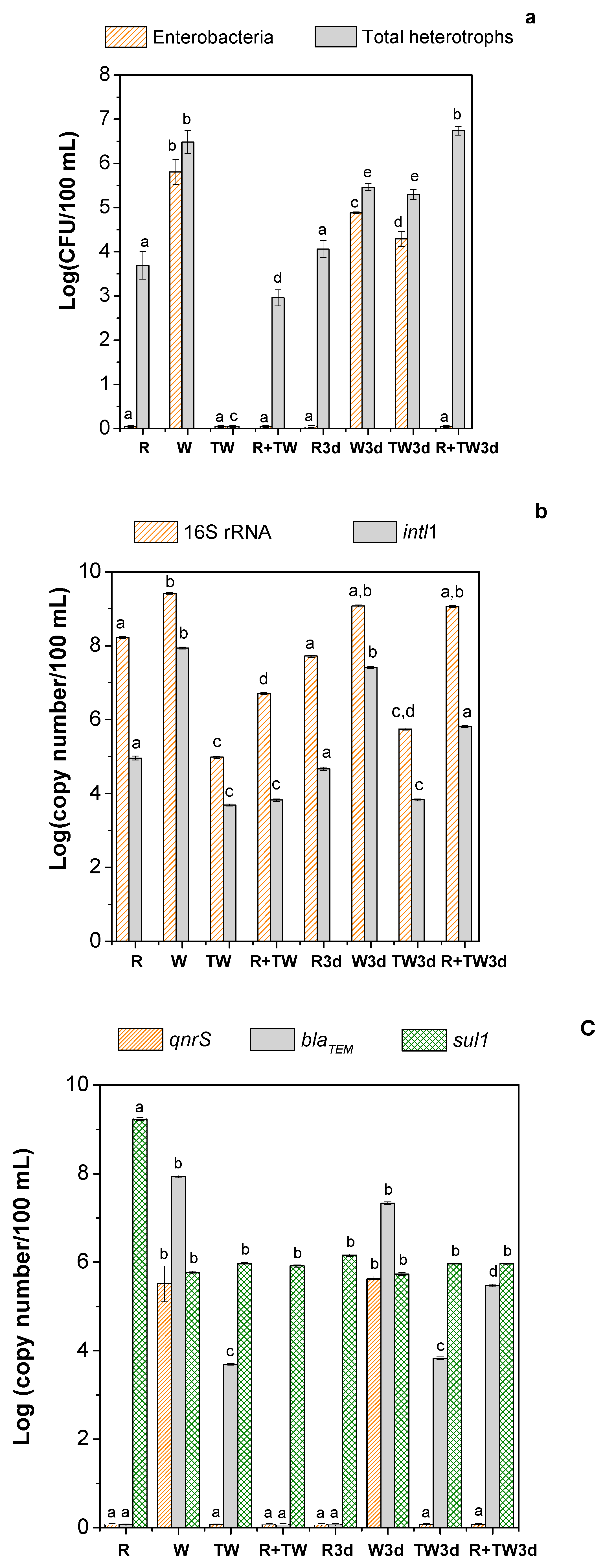
3.3. Effect of the Dilution of Treated Water with River Water on the Activation of Genes and the Regrowth of Microorganisms
3.4. Effect of the Dilution of Treated Water with River Water on Physicochemical Parameters of the Effluent
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ferreira, L.C.; Castro-Alférez, M.; Nahim-Granados, S.; Polo-López, M.I.; Lucas, M.S.; Li Puma, G.; Fernández-Ibáñez, P. Inactivation of water pathogens with solar photo-activated persulfate oxidation. Chem. Eng. J. 2020, 381, 122275. [Google Scholar] [CrossRef]
- La Manna, P.; De Carluccio, M.; Iannece, P.; Vigliotta, G.; Proto, A.; Rizzo, L. Chelating agents supported solar photo-Fenton and sunlight/H2O2 processes for pharmaceuticals removal and resistant pathogens inactivation in quaternary treatment for urban wastewater reuse. J. Hazard. Mater. 2023, 452, 131235. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K.; Dionysiou, D.D.; Fatta-Kassinos, D. Scope of the Book Wastewater Reuse and Current Challenges. In Wastewater Reuse and Current Challenges; Fatta-Kassinos, D., Dionysiou, D.D., Kümmerer, K., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–5. [Google Scholar]
- Michael, S.G.; Michael-Kordatou, I.; Nahim-Granados, S.; Polo-López, M.I.; Rocha, J.; Martínez-Piernas, A.B.; Fernández-Ibáñez, P.; Agüera, A.; Manaia, C.M.; Fatta-Kassinos, D. Investigating the impact of UV-C/H2O2 and sunlight/H2O2 on the removal of antibiotics, antibiotic resistance determinants and toxicity present in urban wastewater. Chem. Eng. J. 2020, 388, 124383. [Google Scholar] [CrossRef]
- Rizzo, L.; Manaia, C.; Merlin, C.; Schwartz, T.; Dagot, C.; Ploy, M.C.; Michael, I.; Fatta-Kassinos, D. Urban wastewater treatment plants as hotspots for antibiotic resistant bacteria and genes spread into the environment: A review. Sci. Total Environ. 2013, 447, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Godinho, O.; Lage, O.M.; Quinteira, S. Antibiotic-Resistant Bacteria across a Wastewater Treatment Plant. Appl. Microbiol. 2024, 4, 364–375. [Google Scholar] [CrossRef]
- Tree Julia, A.; Adams Martin, R.; Lees David, N. Chlorination of Indicator Bacteria and Viruses in Primary Sewage Effluent. Appl. Environ. Microbiol. 2003, 69, 2038–2043. [Google Scholar] [CrossRef]
- Huang, J.-J.; Hu, H.-Y.; Tang, F.; Li, Y.; Lu, S.-Q.; Lu, Y. Inactivation and reactivation of antibiotic-resistant bacteria by chlorination in secondary effluents of a municipal wastewater treatment plant. Water Res. 2011, 45, 2775–2781. [Google Scholar] [CrossRef]
- Hassen, A.; Heyouni, A.; Shayeb, H.; Cherif, M.; Boudabous, A. Inactivation of indicator bacteria in wastewater by chlorine—A kinetics study. Bioresour. Technol. 2000, 72, 85–93. [Google Scholar] [CrossRef]
- Xu, P.; Janex, M.-L.; Savoye, P.; Cockx, A.; Lazarova, V. Wastewater disinfection by ozone: Main parameters for process design. Water Res. 2002, 36, 1043–1055. [Google Scholar] [CrossRef]
- Iakovides, I.C.; Michael-Kordatou, I.; Moreira, N.F.F.; Ribeiro, A.R.; Fernandes, T.; Pereira, M.F.R.; Nunes, O.C.; Manaia, C.M.; Silva, A.M.T.; Fatta-Kassinos, D. Continuous ozonation of urban wastewater: Removal of antibiotics, antibiotic-resistant Escherichia coli and antibiotic resistance genes and phytotoxicity. Water Res. 2019, 159, 333–347. [Google Scholar] [CrossRef]
- Janex, M.L.; Savoye, P.; Roustan, M.; Do-Quang, Z.; Laîné, J.M.; Lazarova, V. Wastewater Disinfection by Ozone: Influence of Water Quality and Kinetics Modeling. Ozone Sci. Eng. 2000, 22, 113–121. [Google Scholar] [CrossRef]
- Koivunen, J.; Heinonen-Tanski, H. Inactivation of enteric microorganisms with chemical disinfectants, UV irradiation and combined chemical/UV treatments. Water Res. 2005, 39, 1519–1526. [Google Scholar] [CrossRef] [PubMed]
- Hassen, A.; Mahrouk, M.; Ouzari, H.; Cherif, M.; Boudabous, A.; Damelincourt, J.J. UV disinfection of treated wastewater in a large-scale pilot plant and inactivation of selected bacteria in a laboratory UV device. Bioresour. Technol. 2000, 74, 141–150. [Google Scholar] [CrossRef]
- Hallmich, C.; Gehr, R. Effect of pre- and post-UV disinfection conditions on photoreactivation of fecal coliforms in wastewater effluents. Water Res. 2010, 44, 2885–2893. [Google Scholar] [CrossRef] [PubMed]
- Sichel, C.; Blanco, J.; Malato, S.; Fernández-Ibáñez, P. Effects of experimental conditions on E. coli survival during solar photocatalytic water disinfection. J. Photochem. Photobiol. A Chem. 2007, 189, 239–246. [Google Scholar] [CrossRef]
- Topac, B.; Alkan, U. Comparison of solar/H2O2 and solar photo-fenton processes for the disinfection of domestic wastewaters. KSCE J. Civ. Eng. 2016, 20, 2632–2639. [Google Scholar] [CrossRef]
- Wang, W.; Wang, H.; Li, G.; An, T.; Zhao, H.; Wong, P.K. Catalyst-free activation of persulfate by visible light for water disinfection: Efficiency and mechanisms. Water Res. 2019, 157, 106–118. [Google Scholar] [CrossRef]
- Sánchez-Montes, I.; Salmerón García, I.; Rivas Ibañez, G.; Aquino, J.M.; Polo-López, M.I.; Malato, S.; Oller, I. UVC-based advanced oxidation processes for simultaneous removal of microcontaminants and pathogens from simulated municipal wastewater at pilot plant scale. Environ. Sci. Water Res. Technol. 2020, 6, 2553–2566. [Google Scholar] [CrossRef]
- Kokkinos, P.; Venieri, D.; Mantzavinos, D. Advanced Oxidation Processes for Water and Wastewater Viral Disinfection. A Systematic Review. Food Environ. Virol. 2021, 13, 283–302. [Google Scholar] [CrossRef]
- Hernandez, R.; Zappi, M.; Colucci, J.; Jones, R. Comparing the performance of various advanced oxidation processes for treatment of acetone contaminated water. J. Hazard. Mater. 2002, 92, 33–50. [Google Scholar] [CrossRef]
- Muruganandham, M.; Suri, R.; Jafari, S.; Sillanpää, M.; Lee, G.-J.; Wu, J.; Swaminathan, M. Recent developments in homogeneous advanced oxidation processes for water and wastewater treatment. Int. J. Photoenergy 2014, 2014, 821674. [Google Scholar] [CrossRef]
- Imlay, J.A.; Linn, S. DNA Damage and Oxygen Radical Toxicity. Science 1988, 240, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- Menacho, C.; Marez, C.; Chueca, P.; Goñi, P.; Ormad, M.P. Inactivation of Acanthamoeba and its endosymbiont bacteria by the combination of solar light with H2O2. Catal. Today 2024, 431, 114562. [Google Scholar] [CrossRef]
- Malvestiti, J.A.; Dantas, R.F. Disinfection of secondary effluents by O3, O3/H2O2 and UV/H2O2: Influence of carbonate, nitrate, industrial contaminants and regrowth. J. Environ. Chem. Eng. 2018, 6, 560–567. [Google Scholar] [CrossRef]
- Maniakova, G.; Salmerón, I.; Polo-López, M.I.; Oller, I.; Rizzo, L.; Malato, S. Simultaneous removal of contaminants of emerging concern and pathogens from urban wastewater by homogeneous solar driven advanced oxidation processes. Sci. Total Environ. 2021, 766, 144320. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Tyree, C.; Huang, C.-H. Inactivation of Escherichia coli, Bacteriophage MS2, and Bacillus Spores under UV/H2O2 and UV/Peroxydisulfate Advanced Disinfection Conditions. Environ. Sci. Technol. 2016, 50, 4448–4458. [Google Scholar] [CrossRef]
- Miralles-Cuevas, S.; De la Obra, I.; Gualda-Alonso, E.; Soriano-Molina, P.; Casas López, J.L.; Sánchez Pérez, J.A. Simultaneous Disinfection and Organic Microcontaminant Removal by UVC-LED-Driven Advanced Oxidation Processes. Water 2021, 13, 1507. [Google Scholar] [CrossRef]
- Bichai, F.; Polo-López, M.I.; Fernández Ibañez, P. Solar disinfection of wastewater to reduce contamination of lettuce crops by Escherichia coli in reclaimed water irrigation. Water Res. 2012, 46, 6040–6050. [Google Scholar] [CrossRef]
- Ferro, G.; Fiorentino, A.; Alferez, M.C.; Polo-López, M.I.; Rizzo, L.; Fernández-Ibáñez, P. Urban wastewater disinfection for agricultural reuse: Effect of solar driven AOPs in the inactivation of a multidrug resistant E. coli strain. Appl. Catal. B Environ. 2015, 178, 65–73. [Google Scholar] [CrossRef]
- Ferro, G.; Polo-López, M.I.; Martínez-Piernas, A.B.; Fernández-Ibáñez, P.; Agüera, A.; Rizzo, L. Cross-Contamination of Residual Emerging Contaminants and Antibiotic Resistant Bacteria in Lettuce Crops and Soil Irrigated with Wastewater Treated by Sunlight/H2O2. Environ. Sci. Technol. 2015, 49, 11096–11104. [Google Scholar] [CrossRef]
- Adeel, M.; Granata, V.; Carapella, G.; Rizzo, L. Effect of microplastics on urban wastewater disinfection and impact on effluent reuse: Sunlight/H2O2 vs solar photo-Fenton at neutral pH. J. Hazard. Mater. 2024, 465, 133102. [Google Scholar] [CrossRef] [PubMed]
- Souza, B.; Dantas, R.; Agulló-Barceló, M.; Lucena, F.; Sans, C.; Esplugas, S.; Dezotti, M. Evaluation of UV/H2O2 on the disinfection and treatment of municipal secondary effluents for water reuse. J. Chem. Technol. Biotechnol. 2013, 88, 1697–1706. [Google Scholar] [CrossRef]
- Moreira, N.F.F.; Sousa, J.M.; Macedo, G.; Ribeiro, A.R.; Barreiros, L.; Pedrosa, M.; Faria, J.L.; Pereira, M.F.R.; Castro-Silva, S.; Segundo, M.A.; et al. Photocatalytic ozonation of urban wastewater and surface water using immobilized TiO2 with LEDs: Micropollutants, antibiotic resistance genes and estrogenic activity. Water Res. 2016, 94, 10–22. [Google Scholar] [CrossRef]
- Gillings, M.R.; Gaze, W.H.; Pruden, A.; Smalla, K.; Tiedje, J.M.; Zhu, Y.G. Using the class 1 integron-integrase gene as a proxy for anthropogenic pollution. ISME J. 2015, 9, 1269–1279. [Google Scholar] [CrossRef] [PubMed]
- Denman, S.E.; McSweeney, C.S. Development of a real-time PCR assay for monitoring anaerobic fungal and cellulolytic bacterial populations within the rumen. FEMS Microbiol. Ecol. 2006, 58, 572–582. [Google Scholar] [CrossRef]
- Narciso-da-Rocha, C.; Rocha, J.; Vaz-Moreira, I.; Lira, F.; Tamames, J.; Henriques, I.; Martinez, J.L.; Manaia, C.M. Bacterial lineages putatively associated with the dissemination of antibiotic resistance genes in a full-scale urban wastewater treatment plant. Environ. Int. 2018, 118, 179–188. [Google Scholar] [CrossRef]
- Ribeirinho-Soares, S.; Moreira, N.F.F.; Graça, C.; Pereira, M.F.R.; Silva, A.M.T.; Nunes, O.C. Overgrowth control of potentially hazardous bacteria during storage of ozone treated wastewater through natural competition. Water Res. 2022, 209, 117932. [Google Scholar] [CrossRef]
- Barraud, O.; Baclet, M.C.; Denis, F.; Ploy, M.C. Quantitative multiplex real-time PCR for detecting class 1, 2 and 3 integrons. J. Antimicrob. Chemother. 2010, 65, 1642–1645. [Google Scholar] [CrossRef]
- Pei, R.; Kim, S.C.; Carlson, K.H.; Pruden, A. Effect of river landscape on the sediment concentrations of antibiotics and corresponding antibiotic resistance genes (ARG). Water Res. 2006, 40, 2427–2435. [Google Scholar] [CrossRef]
- Marti, E.; Balcázar, J.L. Real-Time PCR assays for quantification of qnr genes in environmental water samples and chicken feces. Appl. Environ. Microbiol. 2013, 79, 1743–1745. [Google Scholar] [CrossRef]
- Bibbal, D.; Dupouy, V.; Ferré, J.P.; Toutain, P.L.; Fayet, O.; Prère, M.F.; Bousquet-Mélou, A. Impact of three ampicillin dosage regimens on selection of ampicillin resistance in Enterobacteriaceae and excretion of blaTEM genes in swine feces. Appl. Environ. Microbiol. 2007, 73, 4785–4790. [Google Scholar] [CrossRef] [PubMed]
- Rice, E.W.; Baird, R.B.; Eato, A.D. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC, USA, 2017. [Google Scholar]
- ASTM. Annual Book of ASTM Standards, Part 23: Water; Atmospheric Analysis; American Society for Testing and Materials: Philadelphia, PA, USA, 1973. [Google Scholar]
- Hammer, O.; Harper, D.; Ryan, P. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1–9. Available online: https://palaeo-electronica.org/2001_1/past/past.pdf (accessed on 9 February 2025).
- Legrini, O.; Oliveros, E.; Braun, A.M. Photochemical processes for water treatment. Chem. Rev. 1993, 93, 671–698. [Google Scholar] [CrossRef]
- EUR-Lex. Regulation (EU) 2020/741 of the European Parliament and the Council of 25 May 2020 on Minimum Requirements for Water Reuse. 2020, pp. 32–55. Available online: https://eur-lex.europa.eu/eli/reg/2020/741/oj/eng (accessed on 9 February 2025).
- FAOLEX. Decree-Law No. 119/2019 Estalishing the Legal Scheme of the Production of Water for Reuse. Repub. Diary No. 159/2019, Ser. I 2019–08-21. 2019, pp. 21–44. Available online: https://diariodarepublica.pt/dr/detalhe/decreto-lei/119-2019-124097549 (accessed on 9 February 2025).
- Ksibi, M. Chemical oxidation with hydrogen peroxide for domestic wastewater treatment. Chem. Eng. J. 2006, 119, 161–165. [Google Scholar] [CrossRef]
- Mugikura, S.; Nishikawa, M.; Igarashi, K.; Kobayashi, H. Maintenance of a neutral cytoplasmic pH is not obligatory for growth of Escherichia coli and Streptococcus faecalis at an alkaline pH. J. Biochem. 1990, 108, 86–91. [Google Scholar] [CrossRef]
- Bearson, S.; Bearson, B.; Foster, J.W. Acid stress responses in enterobacteria. FEMS Microbiol. Lett. 1997, 147, 173–180. [Google Scholar] [CrossRef]
- Pignatello, J.J.; Oliveros, E.; MacKay, A. Advanced Oxidation Processes for Organic Contaminant Destruction Based on the Fenton Reaction and Related Chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- Rodrigues, C.; Silva, R.; Carabineiro, S.; Maldonado-Hódar, F.J.; Madeira, L. Dye-containing Wastewater Treatment by Photo-Assisted Wet Peroxidation using Au Nanosized Catalysts. J. Chem. Technol. Biotechnol. 2018, 93, 3223–3232. [Google Scholar] [CrossRef]
- Calaixo, M.R.C.; Ribeirinho-Soares, S.; Madeira, L.M.; Nunes, O.C.; Rodrigues, C.S.D. Catalyst-free persulfate activation by UV/visible radiation for secondary urban wastewater disinfection. J. Environ. Manag. 2023, 348, 119486. [Google Scholar] [CrossRef]
- Rodríguez-Chueca, J.; Polo-López, M.I.; Mosteo, R.; Ormad, M.P.; Fernández-Ibáñez, P. Disinfection of real and simulated urban wastewater effluents using a mild solar photo-Fenton. Appl. Catal. B Environ. 2014, 150–151, 619–629. [Google Scholar] [CrossRef]
- Mamma, P.L.; Carluccio, M.D.; Oliva, G.; Vigliotta, G.; Rizzo, L. Urban wastewater disinfection by iron chelates mediated solar photo-Fenton: Effects on seven pathogens and antibiotic resistance transfer potential. Water Res. 2024, 249, 120966. [Google Scholar] [CrossRef]
- Rodríguez-Chueca, J.; Mosteo, R.; Ormad, M.P.; Ovelleiro, J.L. Factorial experimental design applied to Escherichia coli disinfection by Fenton and photo-Fenton processes. Sol. Energy 2012, 11, 3260–3267. [Google Scholar] [CrossRef]
- Venâncio, J.P.F.; Rodrigues, C.S.D.; Nunes, O.C.; Madeira, L.M. Application of iron-activated persulfate for municipal wastewater disinfection. J. Hazard. Mater. 2022, 426, 127989. [Google Scholar] [CrossRef]
- Venâncio, J.P.F.; Ribeirinho-Soares, S.; Lopes, L.C.; Madeira, L.M.; Nunes, O.C.; Rodrigues, C.S.D. Disinfection of treated urban effluents for reuse by combination of coagulation/flocculation and Fenton processes. Environ. Reseracher. 2023, 218, 115028. [Google Scholar] [CrossRef]
- Marrero, J.A.; Ribeiro, R.S.; Ribeirinho-Soares, S.; Pedrosa, M.; Silva, A.M.T.; Nunes, O.C. Water disinfection by persulfate activation using a nitrogen-doped reduced graphene oxide–PVDF membrane. J. Environ. Chem. Eng. 2023, 11, 109839. [Google Scholar] [CrossRef]

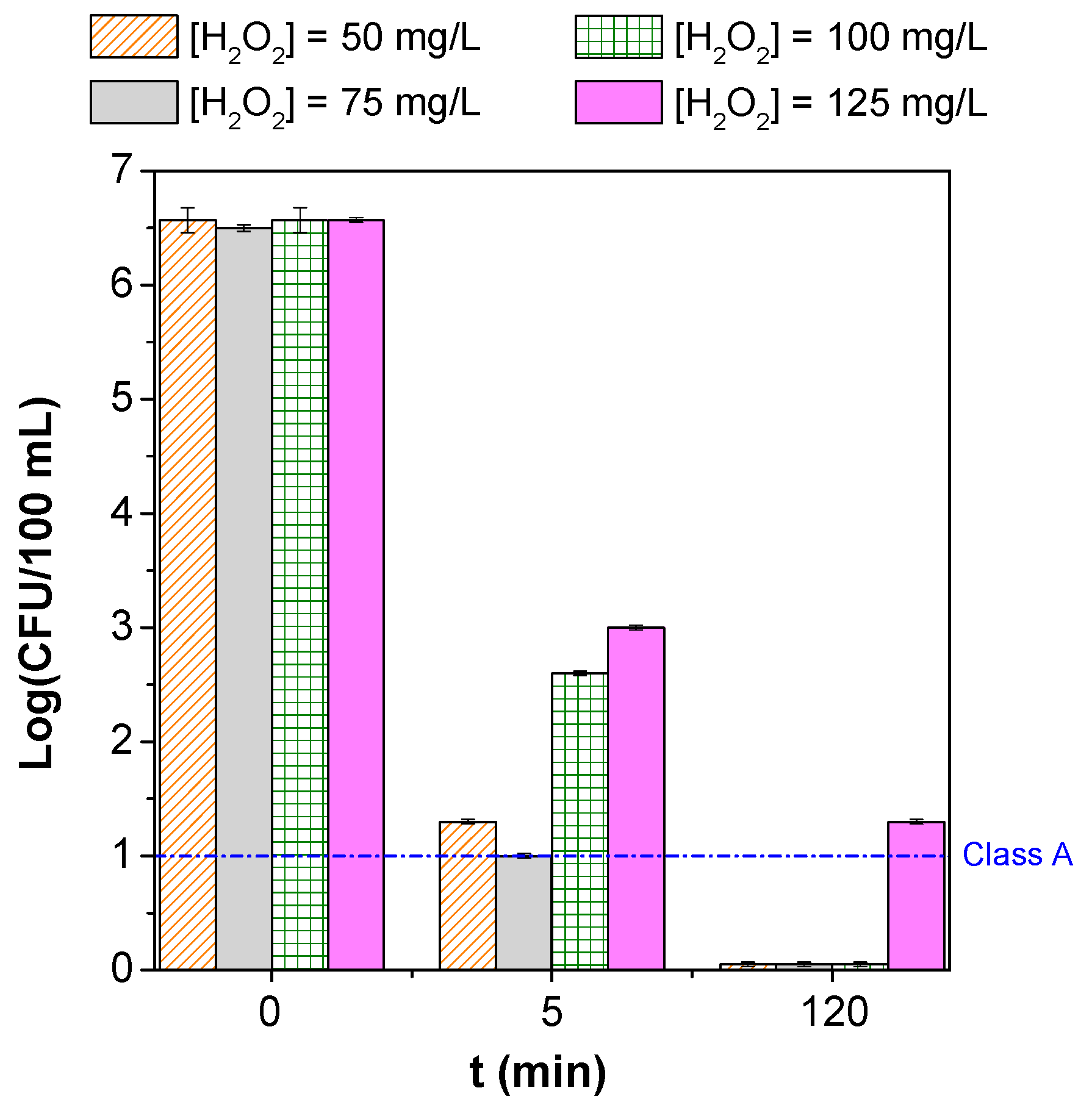
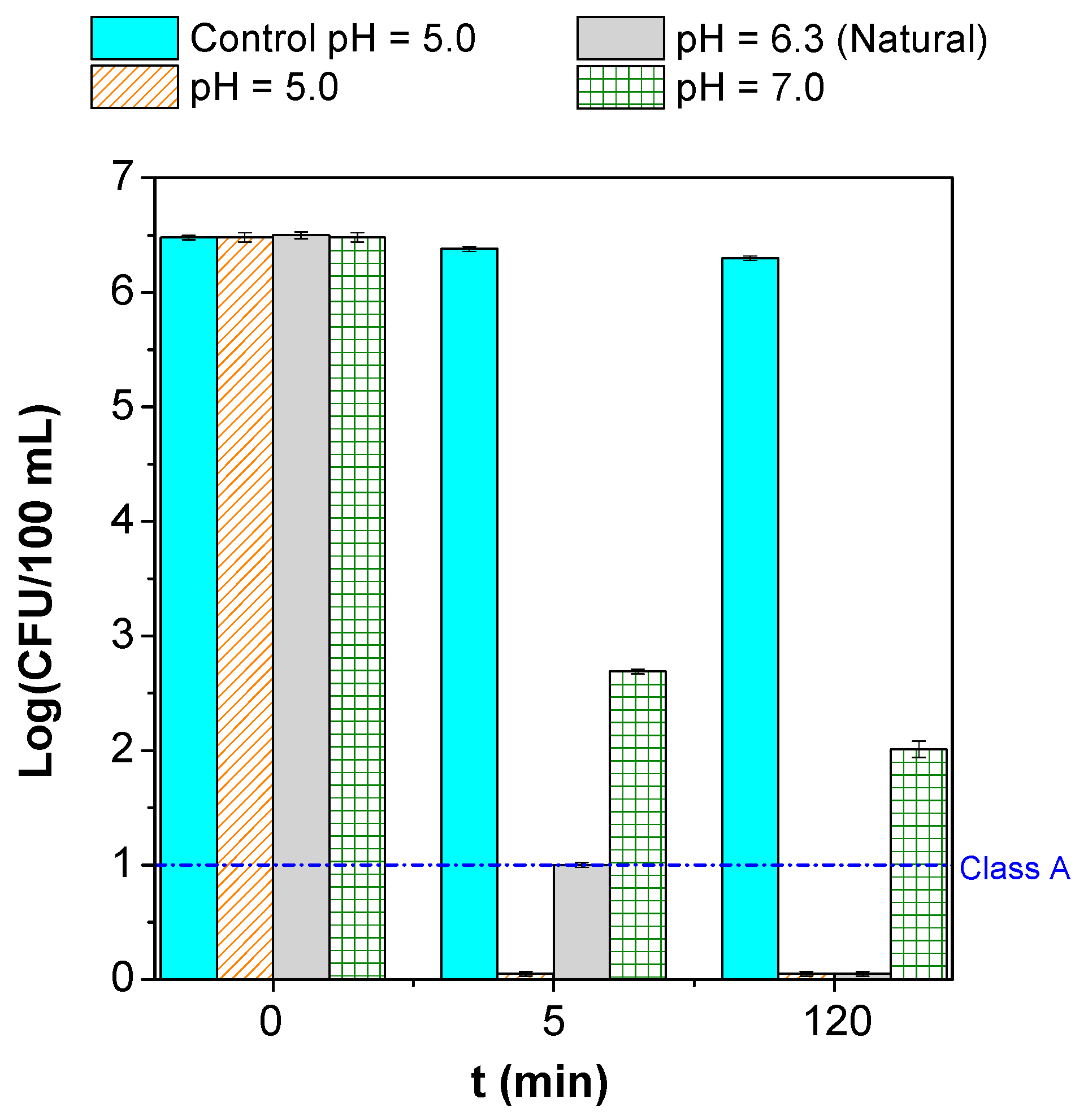
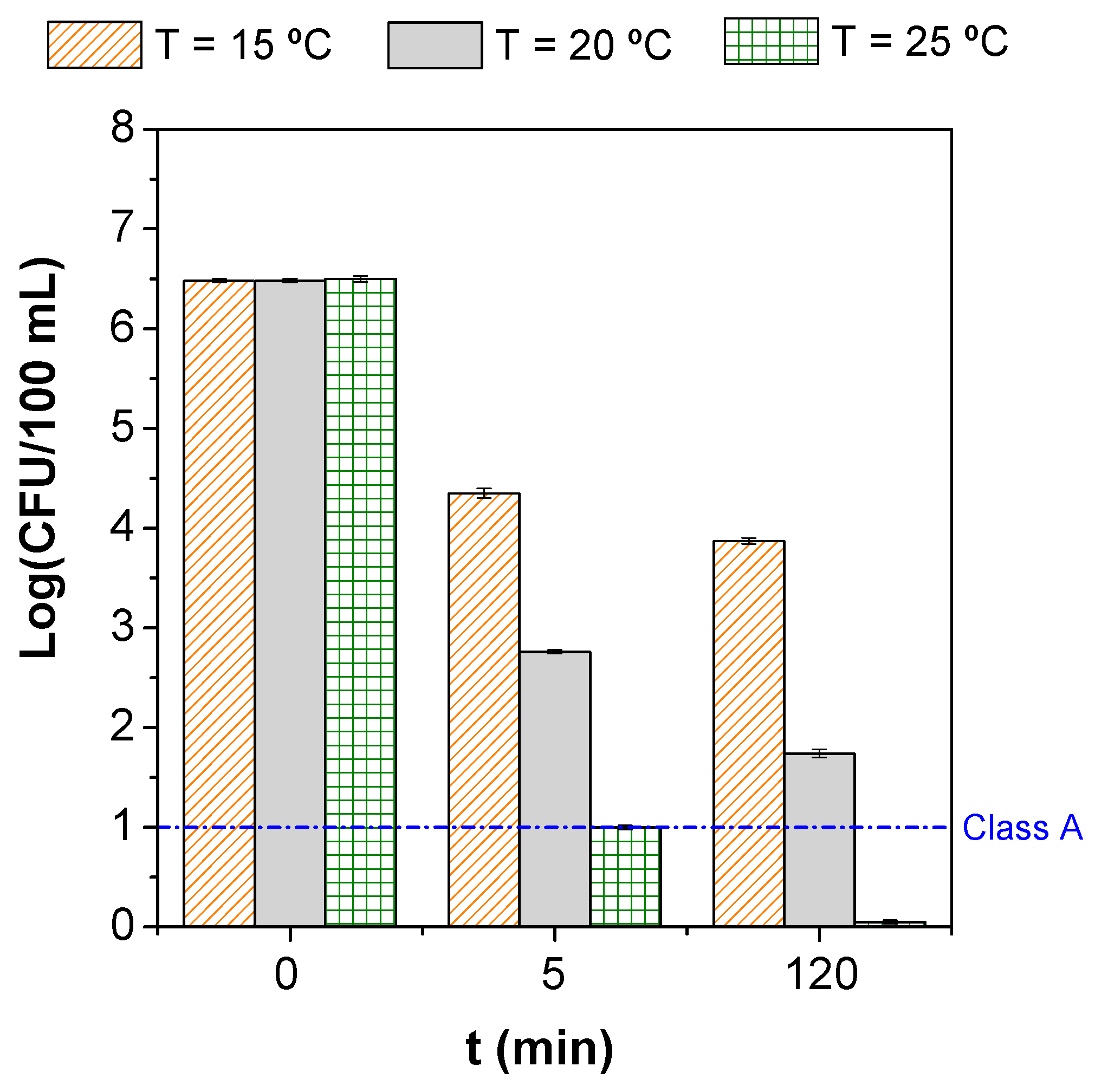
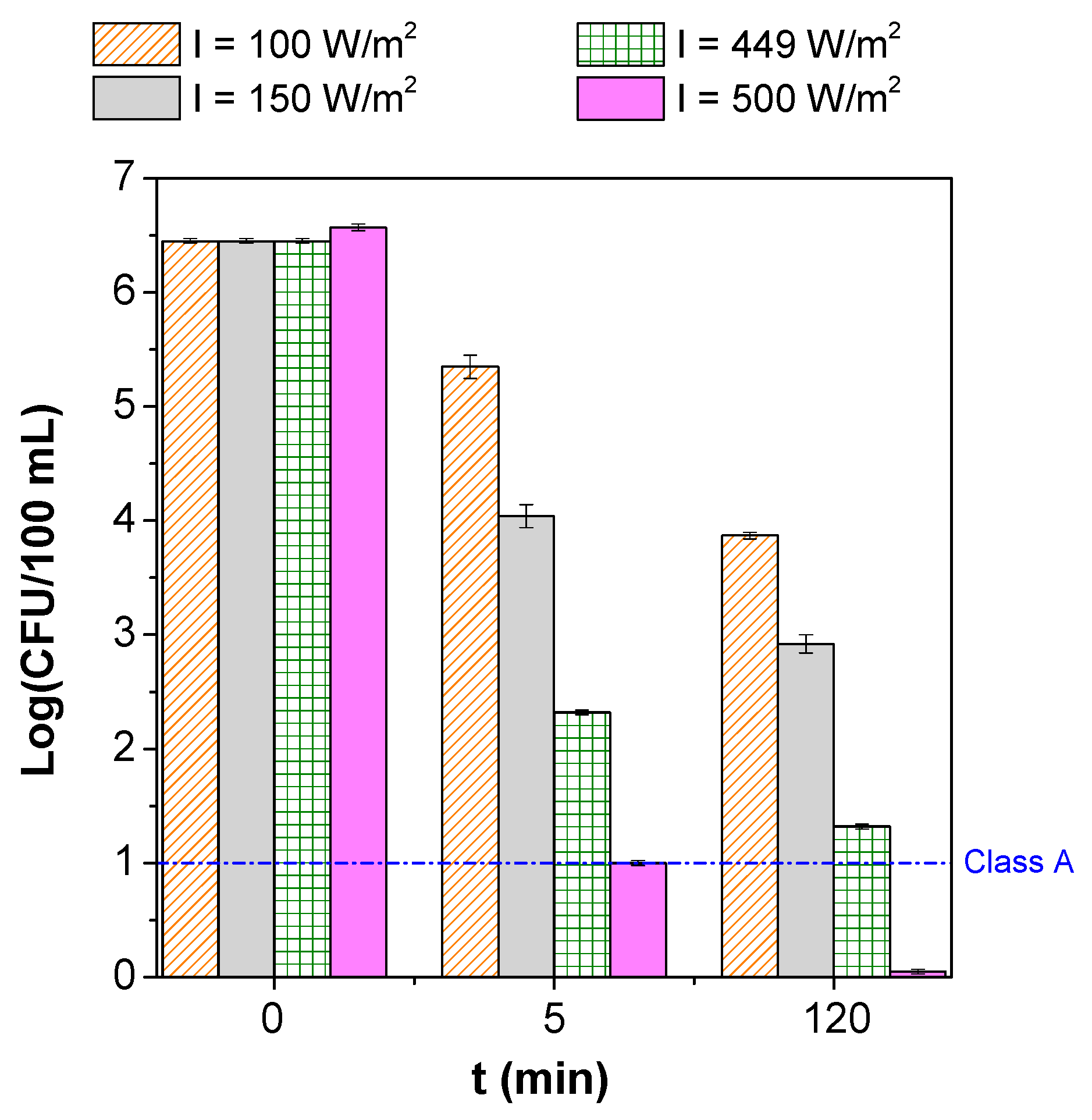
| Microorganism(s) | Water/Wastewater Type | Operating Conditions | Process’ Efficiency | References |
|---|---|---|---|---|
| Escherichia coli (E. coli) K-12 and Pseudomonas aeruginosa (P. aeruginosa) | Inoculated saline solution (ISS) or urban wastewater (UWW) | UV-C or solar radiation [H2O2]UV-C = 5 mg/L [H2O2]solar = 30 mg/L QISS UV-C = 0.003 kJ/L QISS solar = 0.04–0.06 kJ/L QUWW UV-C = 4–9 kJ/L QUWW solar = 15–20 kJ/L | CFU/mL reduced from 103 to 1 | [4] |
| E. coli, Enterococcus faecalis (E. faecalis) and Salmonella enteritidis | Inoculated solution | [H2O2] = 150 mg/L UV radiation Radiation dose = 500 mW s/cm2 | Reduction of ~1.2–1.7 log | [13] |
| [H2O2] = 35–50 mg/L UV-C radiation p = 230 W t = 60 min | CFU/100 mL reduce from 105 to 1 | [19] | ||
| [H2O2] = 50 mg/L Solar radiation I = 30 W/m2 T = 25 °C t = 15–60 min | CFU/mL reduce from 103 to 2 | [26] | ||
| Acanthamoeba P31 and C1-211 | Bacteria isolated from swimming pool and freshwater | [H2O2] = 170 mg/L Solar radiation I = 500 W/m2 t = 5 min | Reduction of 3 log | [24] |
| E. coli and total coliforms | Secondary wastewater generated in a pilot plant | [H2O2] = 90 mg/L Recirculation rate = 500 mL/min UV-C radiation Photonic flux = 9.5 × 10−7 Einstein/s p = 5 W t = 50 min | Reduction of 5–6 log | [25] |
| E. coli, bacteriophage MS2 and Bacillus spores | Inoculated water and wastewater | [H2O2] = 34–68 mg/L UV radiation Fluence rate = 2.2 × 10−7 Einstein/Ls | Log of inactivation/UV dose = 0.25 to 0.3 cm2/mJ for E. coli, ~0.1 cm2/mJ for bacteriophage MS2 and 0.65–0.9 cm2/mJ for Bacillus spores | [27] |
| E. coli | Simulated urban wastewater | [H2O2] = 150 mg/L UV radiation I = 500 W/m2 t = ~10 min pH = 7.0 | Reduction of 4 log | [17,28] |
| [H2O2] = 100 mg/L UV-C radiation I = 2.02 W/m2 Photonic flux = 0.1 μEinstein/s t = 10 min pH = 7.0–7.5 | Reduction of 6 log | [17,28] | ||
| E. coli K-12 | Distilled water (DW) Natural well water (NW) Simulated wastewater (SWW) | [H2O2] = 340 mg/L Solar radiation tDW = 0.5 h tNW = 1 h tSWW = 1.5 h QDW = 60 kJ/m2 QNW = 130 kJ/m2 QSWW = 190 kJ/m2 | Reduction of 6 log | [29] |
| E. coli s | Inoculated urban wastewater, previously sterilized | [H2O2] = 50 mg/L Solar radiation Recirculation flow rate = 16 L/min t = 120 min QUV = 6.75 kJ/L | CFU/mL reduce from 105 to 2 | [30] |
| Antibiotic resistant E. coli and E. faecalis | Inoculated urban wastewater, previously sterilized | [H2O2] = 20 mg/L Solar radiation QUV = 6.29 kJ/L for E. coli t = 120 min for E. coli QUV = 14.86 kJ/L for E. faecalis t = 240 min for E. faecalis | CFU/mL reduce from 106 to 2 | [31] |
| Target Gene | qPCR Standard | Primers | Primers (Sequence)Reference | Conditions | Efficiency (%) | Limit of Quantification (no. of Copies) | Reference |
|---|---|---|---|---|---|---|---|
| 16S rRNA | Escherichia coli ATCC 25922 | 1114F | CGGCAACGAGCGCAACCC | 95 °C for 10 min (1 cycle); 95 °C for 15 s, 55 °C for 20 s and 72 °C for 10 s (35 cycles) | 94.0 | 144 | [36] |
| 1275R | CCATTGTAGCACGTGTGTAGCC | ||||||
| intl1 | clone intI1 (pNORM) | intI1-LC | GCCTTGATGTTACCCGAGAG | 95 °C 10 min (1 cycle), 95 °C 15 s and 60 °C 1 min (40 cycles) | 90.1 | 44 | [39] |
| intI1-LC5 | GATCGGTCGAATGCGTGT | ||||||
| sul1 | clone sul1(pNORM) | sul1-FW | CGCACCGGAAACATCGCTGCAC | 95 °C for 5 min (1 cycle); 95 °C for 15 s and 60 °C for 1 min (35 cycles) | 95.0 | 11 | [40] |
| sul1-RV | TGAAGTTCCGCCGCAAGGCTCG | ||||||
| qnrS | clone qnrS(pNORM) | qnrSrtF11 | GACGTGCTAACTTGCGTGAT | 95 °C for 5 min (1 cycle); 95 °C for 15 s and 60 °C for 1 min (40 cycles) | 97.7 | 36 | [41] |
| qnrSrtR11 | TGGCATTGTTGGAAACTTG | ||||||
| blaTEM | clone blaTEM(pNORM) | blaTEM-F | TTCCTGTTTTTGCTCACCCAG | 95 °C for 10 min (1 cycle); 95 °C for 15 s, 60 °C for 1 min (40 cycles) | 95.2 | 44 | [42] |
| blaTEM-R | CTCAAGGATCTTACCGCTGTTG |
| Parameter | R3d | W3d | TW3d | R+TW3d | Limits Imposed [47,48] | |
|---|---|---|---|---|---|---|
| Irrigation (1) | Urban Utilities | |||||
| TOC (mgC/L) | 3.1 ± 0.03 a | 12.2 ± 0.1 b | 9.5 ± 0.1 c | 4.6 ± 0.1 d | - | |
| COD (mgO2/L) | 4.1 ± 0.3 a | 30.5 ± 0.4 b | 22.1 ± 1.0 c | 10.5 ± 0.5 d | - | - |
| BOD5 (mgO2/L) | 1.0 ± 0.1 a | 8.7 ± 0.6 b | 5.3 ± 0.6 c | 2.2 ± 1.1 a | <10 | <25 |
| Turbidity (NTU) | 1.3 ± 0.1 a | 12.0 ± 0.9 b | 5.9 ± 0.1 c | 4.2 ± 0.1 d | <5 | <5 |
| TSS (mg/L) | 5.0 ± 0.5 a | 24.0 ± 1.0 b | 12.0 ± 0.9 c | 8.3 ± 1.2 d | <10 | - |
| NTotal (mgN/L) | 1.7 ± 0.1 a | 33.2 ± 0.1 b | 32.5 ± 0.4 c | 19.1 ± 0.7 d | <15 (2) | - |
| NH3 (mgNH4+/L) | <0.1 ± 0.1 a | 15.6 ± 1.3 b | 10.7 ± 1.2 c | 6.7 ± 0.7 d | <10 (2) | <5 (3) |
| PTotal (mgP/L) | <0.05 ± 0.1 a | 4.8 ± 0.4 b | 3.3 ± 0.2 c | 2.1 ± 0.1 d | <5 | <2 |
| pH | 7.0 ± 0.1 a | 6.7 ± 0.2 b | 6.8 ± 0.1 c | 6.8 ± 0.1 c | - | 6.0–9.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, A.L.R.; Ribeirinho-Soares, S.; Madeira, L.M.; Nunes, O.C.; Rodrigues, C.S.D. Disinfection of Secondary Urban Wastewater Using Hydrogen Peroxide Combined with UV/Visible Radiation: Effect of Operating Conditions and Assessment of Microorganism Competition. Water 2025, 17, 596. https://doi.org/10.3390/w17040596
Gomes ALR, Ribeirinho-Soares S, Madeira LM, Nunes OC, Rodrigues CSD. Disinfection of Secondary Urban Wastewater Using Hydrogen Peroxide Combined with UV/Visible Radiation: Effect of Operating Conditions and Assessment of Microorganism Competition. Water. 2025; 17(4):596. https://doi.org/10.3390/w17040596
Chicago/Turabian StyleGomes, Ana L. R., Sara Ribeirinho-Soares, Luis M. Madeira, Olga C. Nunes, and Carmen S. D. Rodrigues. 2025. "Disinfection of Secondary Urban Wastewater Using Hydrogen Peroxide Combined with UV/Visible Radiation: Effect of Operating Conditions and Assessment of Microorganism Competition" Water 17, no. 4: 596. https://doi.org/10.3390/w17040596
APA StyleGomes, A. L. R., Ribeirinho-Soares, S., Madeira, L. M., Nunes, O. C., & Rodrigues, C. S. D. (2025). Disinfection of Secondary Urban Wastewater Using Hydrogen Peroxide Combined with UV/Visible Radiation: Effect of Operating Conditions and Assessment of Microorganism Competition. Water, 17(4), 596. https://doi.org/10.3390/w17040596









