Tree Diversity and Its Ecological Importance Value in Silvopastoral Systems: A Study along Elevational Gradients in the Sumaco Biosphere Reserve, Ecuadorian Amazon
Abstract
1. Introduction
2. Materials and Methods
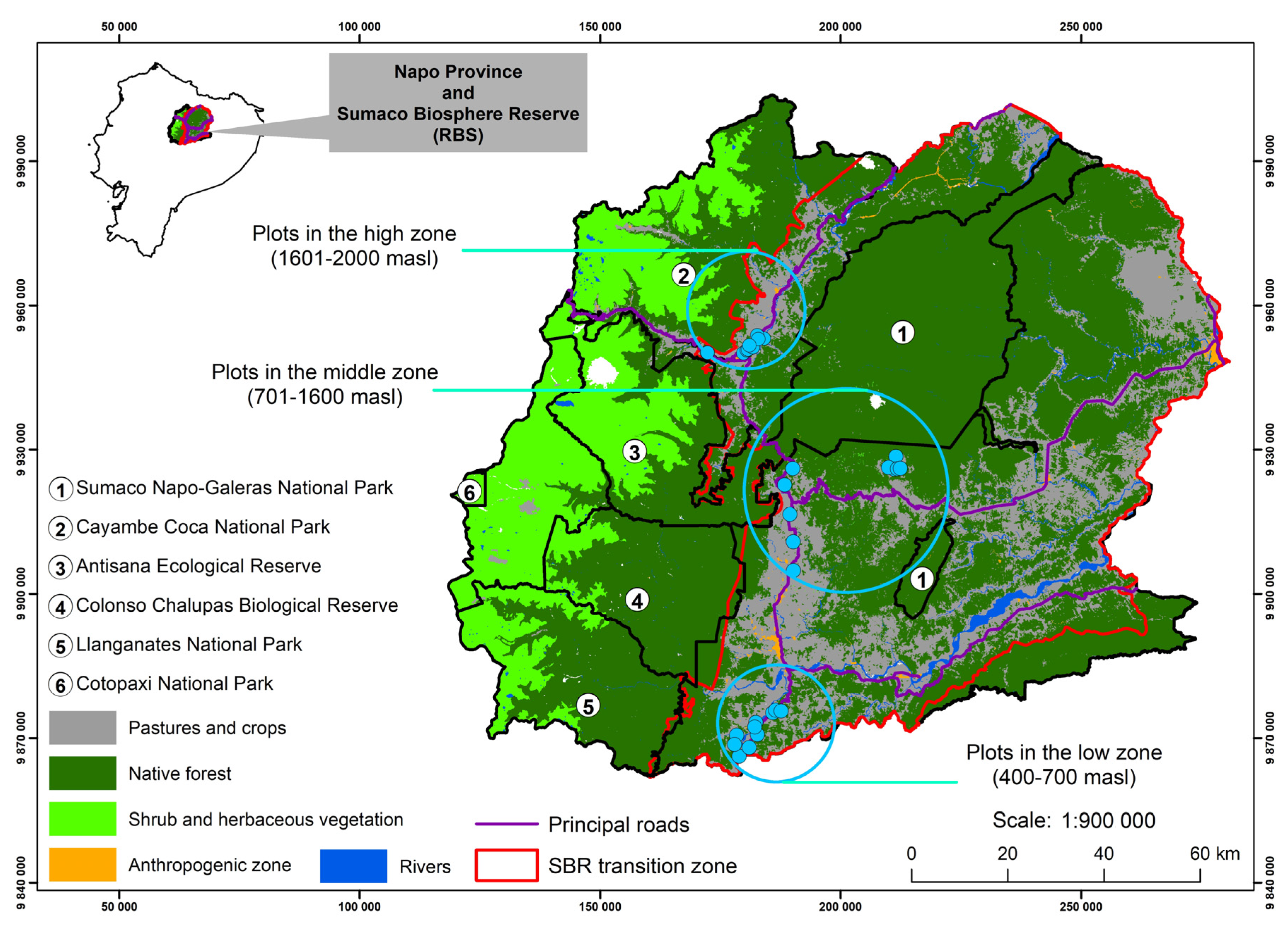
2.1. Study Area
2.2. Sampling and Data Collection
2.3. Data Analysis
- Relative abundance R.A. = percentage of number of individuals ha−1;
- Relative dominance R.D. = percentage of basal area (m2 ha−1);
- Relative frequency R.F. = percentage of plots in which a species is present.
3. Results and Discussion
3.1. Richness, Diversity Index and Structural Parameters of Silvopastoral System
3.2. Abundance of Tree Species and Ecological Importance Value
3.3. Tree Density, Conservation Status in Ecuador and IUCN
3.4. Main Reported Uses of Tree and Palm Species
3.5. Contributions to Landscape Conservation Planning
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lőrincz, Á.; Hábenczyus, A.A.; Kelemen, A.; Ratkai, B.; Tölgyesi, C.; Lőrinczi, G.; Bátori, Z.; Maák, I.E. Wood-Pastures Promote Environmental and Ecological Heterogeneity on a Small Spatial Scale. Sci. Total Environ. 2023, 906, 167510. [Google Scholar] [CrossRef]
- Álvarez, F.; Casanoves, F.; Suárez, J.C.; Rusch, G.M.; Ngo Bieng, M.A. An Assessment of Silvopastoral Systems Condition and Their Capacity to Generate Ecosystem Services in the Colombian Amazon. Ecosyst. People 2023, 19, 2213784. [Google Scholar] [CrossRef]
- Aryal, D.R.; Gómez-González, R.R.; Hernández-Nuriasmú, R.; Morales-Ruiz, D.E. Carbon Stocks and Tree Diversity in Scattered Tree Silvopastoral Systems in Chiapas, Mexico. Agrofor. Syst. 2019, 93, 213–227. [Google Scholar] [CrossRef]
- Brook, R.; Forster, E.; Styles, D.; Mazzetto, A.M.; Arndt, C.; Esquivel, M.J.; Chadwick, D. Silvopastoral Systems for Offsetting Livestock Emissions in the Tropics: A Case Study of a Dairy Farm in Costa Rica. Agron. Sustain. Dev. 2022, 42, 101. [Google Scholar] [CrossRef] [PubMed]
- Flores-Coello, G.; Hernández-Medrano, J.H.; Ku-Vera, J.; Diaz, D.; Solorio-Sánchez, F.J.; Sarabia-Salgado, L.; Galindo, F. Intensive Silvopastoral Systems Mitigate Enteric Methane Emissions from Cattle. Atmosphere 2023, 14, 863. [Google Scholar] [CrossRef]
- Ortiz, J.; Neira, P.; Panichini, M.; Curaqueo, G.; Stolpe, N.B.; Zagal, E.; Dube, F.; Gupta, S.R. Silvopastoral Systems on Degraded Lands for Soil Carbon Sequestration and Climate Change Mitigation. In Agroforestry for Sustainable Intensification of Agriculture in Asia and Africa; Springer: Berlin/Heidelberg, Germany, 2023; pp. 207–242. [Google Scholar]
- Torres, B.; Bravo, C.; Torres, A.; Tipán-Torres, C.; Vargas, J.C.; Herrera-Feijoo, R.J.; Heredia-R, M.; Barba, C.; García, A. Carbon Stock Assessment in Silvopastoral Systems along an Elevational Gradient: A Study from Cattle Producers in the Sumaco Biosphere Reserve, Ecuadorian Amazon. Sustainability 2023, 15, 449. [Google Scholar] [CrossRef]
- Sandoval, D.F.; Florez, J.F.; Valencia, K.J.E.; Cabrera, M.E.S.; Stefan, B. Economic-Environmental Assessment of Silvo-Pastoral Systems in Colombia: An Ecosystem Service Perspective. Heliyon 2023, 9, e19082. [Google Scholar] [CrossRef]
- Alvarado Sandino, C.O.; Barnes, A.P.; Sepúlveda, I.; Garratt, M.P.D.; Thompson, J.; Escobar-Tello, M.P. Examining Factors for the Adoption of Silvopastoral Agroforestry in the Colombian Amazon. Sci. Rep. 2023, 13, 12252. [Google Scholar] [CrossRef]
- Torres, B.; Andrade, V.; Heredia-R, M.; Toulkeridis, T.; Estupiñán, K.; Luna, M.; Bravo, C.; García, A. Productive Livestock Characterization and Recommendations for Good Practices Focused on the Achievement of the SDGs in the Ecuadorian Amazon. Sustainability 2022, 14, 10738. [Google Scholar] [CrossRef]
- Mauricio, R.M.; Ribeiro, R.S.; Paciullo, D.S.C.; Cangussú, M.A.; Murgueitio, E.; Chará, J.; Estrada, M.X.F. Silvopastoral Systems in Latin America for Biodiversity, Environmental, and Socioeconomic Improvements. In Agroecosystem Diversity; Elsevier: Amsterdam, The Netherlands, 2019; pp. 287–297. [Google Scholar]
- Bravo, C.; Goyes-Vera, F.; Arteaga-Crespo, Y.; García-Quintana, Y.; Changoluisa, D. A Soil Quality Index for Seven Productive Landscapes in the Andean-Amazonian Foothills of Ecuador. Land Degrad. Dev. 2021, 32, 2226–2241. [Google Scholar] [CrossRef]
- McGroddy, M.E.; Lerner, A.M.; Burbano, D.V.; Schneider, L.C.; Rudel, T.K. Carbon Stocks in Silvopastoral Systems: A Study from Four Communities in Southeastern Ecuador. Biotropica 2015, 47, 407–415. [Google Scholar] [CrossRef]
- Sandoval-Pelcastre, A.A.; Ramírez-Mella, M.; Rodríguez-Ávila, N.L.; Candelaria-Martínez, B. Tropical Trees and Shrubs with Potential to Reduce the Production of Methane in Ruminants [Árboles y Arbustos Tropicales Con Potencial Para Disminuir La Producción de Metano En Rumiantes]. Trop. Subtrop. Agroecosyst. 2020, 23, 1–16. [Google Scholar] [CrossRef]
- Montagnini, F.; Ibrahim, M.; Murgueitio Restrepo, E. Silvopastoral Systems and Climate Change Mitigation in Latin America. Bois Forets Trop. 2013, 67, 3–16. [Google Scholar] [CrossRef]
- Schinato, F.; Munka, M.C.; Olmos, V.M.; Bussoni, A.T. Microclimate, Forage Production and Carbon Storage in a Eucalypt-Based Silvopastoral System. Agric. Ecosyst. Environ. 2023, 344, 108290. [Google Scholar] [CrossRef]
- Schmitt Filho, A.L.; Kretzer, S.G.; Farley, J.; Kazama, D.C.; Sinisgalli, P.A.; Deniz, M. Applied Nucleation under High Biodiversity Silvopastoral System as an Adaptive Strategy against Microclimate Extremes in Pasture Areas. Int. J. Biometeorol. 2023, 67, 1199–1212. [Google Scholar] [CrossRef]
- Ortiz Timoteo, J.; Kainer, K.A.; Luna Cavazos, M.; García Moya, E.; Sánchez Sánchez, O.; Vibrans, H. Trees in Pastures: Local Knowledge, Management, and Motives in Tropical Veracruz, Mexico. Agrofor. Syst. 2023, 97, 687–698. [Google Scholar] [CrossRef]
- Kumar, S.; Prasad, R.; Kumar, A.; Dhyani, S.K. Integration of Fruit Trees in Agroforestry for Sustainability and Profitability of Farming Systems in Arid and Semi-Arid Regions. Indian J. Agrofor. 2019, 21, 95–99. [Google Scholar]
- Paut, R.; Dufils, A.; Derbez, F.; Dossin, A.-L.; Penvern, S. Orchard Grazing in France: Multiple Forms of Fruit Tree–Livestock Integration in Line with Farmers’ Objectives and Constraints. Forests 2021, 12, 1339. [Google Scholar] [CrossRef]
- Lugo, A.E. The Apparent Paradox of Reestablishing Species Richness on Degraded Lands with Tree Monocultures. For. Ecol. Manag. 1997, 99, 9–19. [Google Scholar] [CrossRef]
- Trujillo-Miranda, A.L.; Toledo-Aceves, T.; López-Barrera, F.; Gerez-Fernández, P. Active versus Passive Restoration: Recovery of Cloud Forest Structure, Diversity and Soil Condition in Abandoned Pastures. Ecol. Eng. 2018, 117, 50–61. [Google Scholar] [CrossRef]
- Montagnini, F.; del Fierro, S. Functions of Agroforestry Systems as Biodiversity Islands in Productive Landscapes. In Biodiversity Islands: Strategies for Conservation in Human-Dominated Environments; Springer: Berlin/Heidelberg, Germany, 2022; pp. 89–116. [Google Scholar]
- Chará, J.; Rivera, J.; Barahona, R.; Murgueitio, R.E.; Deblitz, C.; Reyes, E.; Mauricio, R.M.; Molina, J.J.; Flores, M.; Zuluaga, A. Intensive Silvopastoral Systems: Economics and Contribution to Climate Change Mitigation and Public Policies. In Integrating Landscapes: Agroforestry for Biodiversity Conservation and Food Sovereignty; Springer: Berlin/Heidelberg, Germany, 2017; pp. 395–416. [Google Scholar]
- Myers, N.; Mittermeler, R.A.; Mittermeler, C.G.; Da Fonseca, G.A.B.; Kent, J. Biodiversity Hotspots for Conservation Priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Mittermeier, R.A.; Myers, N.; Thomsen, J.B.; da Fonseca, G.A.B.; Olivieri, S. Biodiversity Hotspots and Major Tropical Wilderness Areas: Approaches to Setting Conservation Priorities. Conserv. Biol. 1998, 12, 516–520. [Google Scholar] [CrossRef]
- Myers, N. Threatened Biotas: “Hot Spots” in Tropical Forests. Environmentalist 1988, 8, 187–208. [Google Scholar] [CrossRef] [PubMed]
- Torres, B.; Günter, S.; Acevedo-Cabra, R.; Knoke, T. Livelihood Strategies, Ethnicity and Rural Income: The Case of Migrant Settlers and Indigenous Populations in the Ecuadorian Amazon. For. Policy Econ. 2018, 86, 22–34. [Google Scholar] [CrossRef]
- MAATE Sistema Nacional de Indicadores Ambientales y Sostenibilidad (SINIAS). Available online: http://sinias.ambiente.gob.ec:8099/proyecto-sinias-web/estadisticasAmbientales.jsf?menu=01 (accessed on 5 January 2020).
- Cook, J.G.; Stutzman, T.W.; Bowers, C.W.; Brenner, K.A.; Irwin, L.L. Spherical Densiometers Produce Biased Estimates of Forest Canopy Cover. Wildl. Soc. Bull. 1995, 23, 711–717. [Google Scholar]
- Castellanos-Bolaños, J.F.; Treviño-Garza, E.J.; Aguirre-Calderón, Ó.A.; Jiménez-Pérez, J.; Musalem-Santiago, M.; López-Aguillón, R. Estructura de Bosques de Pino Pátula Bajo Manejo En Ixtlán de Juárez, Oaxaca, México. Madera y Bosques 2008, 14, 51–63. [Google Scholar] [CrossRef]
- Shannon, C.E. A Mathematical Theory of Communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Jost, L.; González-Oreja, J. Midiendo La Diversidad Biológica: Más Allá Del Índice de Shannon. Acta Zool. Lilloana 2012, 56, 3–14. [Google Scholar]
- Jost, L. Entropy and Diversity. Oikos 2006, 113, 363–375. [Google Scholar] [CrossRef]
- Lamprecht, H. Ensayo Sobre Unos Métodos Para El Análisis Estructural de Los Bosques Tropicales. Acta Cient. Venez. 1962, 13, 57. [Google Scholar]
- Colwell, R.K.; Elsensohn, J.E. EstimateS Turns 20: Statistical Estimation of Species Richness and Shared Species from Samples, with Non-parametric Extrapolation. Ecography 2014, 37, 609–613. [Google Scholar] [CrossRef]
- Longino, J.T.; Coddington, J.; Colwell, R.K. The Ant Fauna of a Tropical Rain Forest: Estimating Species Richness Three Different Ways. Ecology 2002, 83, 689–702. [Google Scholar] [CrossRef]
- Curtis, J.T.; McIntosh, R.P. An Upland Forest Continuum in the Prairie-Forest Border Region of Wisconsin. Ecology 1951, 32, 476–496. [Google Scholar] [CrossRef]
- Chave, J.; Coomes, D.; Jansen, S.; Lewis, S.L.; Swenson, N.G.; Zanne, A.E. Towards a Worldwide Wood Economics Spectrum. Ecol. Lett. 2009, 12, 351–366. [Google Scholar] [CrossRef]
- Zanne, A.E.; Lopez-Gonzalez, G.; Coomes, D.A.; Ilic, J.; Jansen, S.; Lewis, S.L.; Miller, R.B.; Swenson, N.G.; Wiemann, M.C.; Chave, J. Data from: Towards a Worldwide Wood Economics Spectrum; Dryad: Washington, DC, USA, 2020. [Google Scholar]
- Nabais, C.; Hansen, J.K.; David-Schwartz, R.; Klisz, M.; López, R.; Rozenberg, P. The Effect of Climate on Wood Density: What Provenance Trials Tell Us? For. Ecol. Manag. 2018, 408, 148–156.31. [Google Scholar] [CrossRef]
- Flores, O.; Coomes, D.A. Estimating the Wood Density of Species for Carbon Stock Assessments. Methods Ecol. Evol. 2011, 2, 214–220. [Google Scholar] [CrossRef]
- Ketterings, Q.M.; Coe, R.; van Noordwijk, M.; Palm, C.A. Reducing Uncertainty in the Use of Allometric Biomass Equations for Predicting Above-Ground Tree Biomass in Mixed Secondary Forests. For. Ecol. Manag. 2001, 146, 199–209. [Google Scholar] [CrossRef]
- De la Torre, L.; Navarrete, H.; Muriel, P.; Macía, M.J.; Balslev, H. Enciclopedia de Las Plantas Útiles Del Ecuador (Con Extracto de Datos); Herbario QCA de la Escuela de Ciencias Biológicas de la Pontificia: Quito, Ecuador, 2008; ISBN 9978771352. [Google Scholar]
- Chamberlain, S.A.; Szöcs, E. Taxize: Taxonomic Search and Retrieval in R. F1000Research 2013, 2, 191. [Google Scholar] [CrossRef] [PubMed]
- Graefe, S.; Rodrigo, R.; Cueva, E.; Butz, P.; Werner, F.A.; Homeier, J. Impact of Disturbance on Forest Structure and Tree Species Composition in a Tropical Dry Forest of South Ecuador. Ecotropica 2020, 22, 202002. [Google Scholar]
- Whittaker, R.J.; Willis, K.J.; Field, R. Scale and Species Richness: Towards a General, Hierarchical Theory of Species Diversity. J. Biogeogr. 2001, 28, 453–470. [Google Scholar] [CrossRef]
- Willis, K.J.; Whittaker, R.J. Species Diversity—Scale Matters. Science 2002, 295, 1245–1248. [Google Scholar] [CrossRef]
- Deng, L.; Shangguan, Z. Species Composition, Richness and Aboveground Biomass of Natural Grassland in Hilly-Gully Regions of the Loess Plateau, China. J. Integr. Agric. 2014, 13, 2527–2536. [Google Scholar] [CrossRef][Green Version]
- Villanueva, C.; Tobar López, D.; Ibrahim, M.A.; Casasola Coto, F.; Barrantes, J.; Arguedas, R. Árboles Dispersos En Potreros En Fincas Ganaderas Del Pacífico Central de Costa Rica. Agrofor. Am. 2013, 45, 12–20. [Google Scholar]
- Esquivel, M.J.; Harvey, C.A.; Finegan, B.; Casanoves, F.; Skarpe, C.; Nieuwenhuyse, A. Regeneración Natural de Árboles y Arbustos En Potreros Activos de Nicaragua. Agrofor. Am. 2009, 47, 76–84. [Google Scholar]
- Mejía, E.; Pacheco, P.; Muzo, A.; Torres, B. Smallholders and Timber Extraction in the Ecuadorian Amazon: Amidst Market Opportunities and Regulatory Constraints. Int. For. Rev. 2015, 17, 38–50. [Google Scholar] [CrossRef]
- Vasco, C.; Torres, B.; Pacheco, P.; Griess, V. The Socioeconomic Determinants of Legal and Illegal Smallholder Logging: Evidence from the Ecuadorian Amazon. For. Policy Econ. 2017, 78, 133–140. [Google Scholar] [CrossRef]
- Argote, K.; Rodríguez-Sánchez, B.; Quintero, M.; Francesconi, W. One Tree at a Time: Restoring Landscape Connectivity through Silvopastoral Systems in Transformed Amazon Landscapes. Diversity 2022, 14, 846. [Google Scholar] [CrossRef]
- López-Tobar, R.; Herrera-Feijoo, R.J.; Mateo, R.G.; García-Robredo, F.; Torres, B. Botanical Collection Patterns and Conservation Categories of the Most Traded Timber Species from the Ecuadorian Amazon: The Role of Protected Areas. Plants 2023, 12, 3327. [Google Scholar] [CrossRef] [PubMed]
- Mejía, E.; Pacheco, P. Forest Use and Timber Markets in the Ecuadorian Amazon; CIFOR: Bogor, Indonesia, 2014; Volume 111, ISBN 6021504143. [Google Scholar]
- Giro, A.; Pezzopane, J.R.M.; Barioni Junior, W.; Pedroso, A.D.F.; Lemes, A.P.; Botta, D.; Romanello, N.; Barreto, A.D.N.; Garcia, A.R. Behavior and Body Surface Temperature of Beef Cattle in Integrated Crop-Livestock Systems with or without Tree Shading. Sci. Total Environ. 2019, 684, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Congo Yépez, C.; Velástegui Lara, F.; Caicedo Vargas, C.; Rodríguez Iturralde, L.; Vera Zambrano, A.; Montero Cruz, O. Árboles Dispersos y Su Efecto En La Productividad de Los Potreros En La Amazonía Ecuatoriana. Granja Rev. Cienc. L Vida 2018, 27, 64–76. [Google Scholar] [CrossRef]
- Chará-Serna, A.M.; Chará, J. Effect of Silvopastoral Systems on Biodiversity and the Provision of Ecosystem Services in Tropical Agro-Landscapes [Efecto de Los Sistemas Silvopastoriles Sobre La Biodiversidad y La Provisión de Servicios Ecosistémicos En Agropaisajes Tropicales]. Livest. Res. Rural Dev. 2020, 32, 85–104. [Google Scholar]
- Lojka, B.; Dumas, L.; Preininger, D.; Polesny, Z.; Banout, J. The Use and Integration of Inga Edulis in Agroforestry Systems in the Amazon-Review Article. Agric. Trop. Subtrop. 2010, 43, 352–359. [Google Scholar]
- Barron, A.R.; Purves, D.W.; Hedin, L.O. Facultative Nitrogen Fixation by Canopy Legumes in a Lowland Tropical Forest. Oecologia 2011, 165, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Li, L.; Zhao, H.; Zhang, S.; Tu, Y.; Liu, M.; Zhao, Y.; Jiang, L. Dietary Citrus Flavonoid Extract Improves Lactational Performance through Modulating Rumen Microbiome and Metabolites in Dairy Cows. Food Funct. 2023, 14, 94–111. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Li, L.; Zhao, H.; Liu, M.; Jiang, L.; Zhao, Y. Citrus Flavonoid Extracts Alter the Profiling of Rumen Antibiotic Resistance Genes and Virulence Factors of Dairy Cows. Front. Microbiol. 2023, 14, 1201262. [Google Scholar] [CrossRef] [PubMed]
- Palacios, W.A.; de Lourdes Torres, M.; Quintana, M.A.; Asadobay, P.; Iglesias, J.; Quillupangui, R.; Rojas, E.; Santiana, J.; Sola, A.; Rivas-Torres, G. A New Species and a New Record for Cedrela (Meliaceae, Sapindales) in Ecuador: Morphological, Molecular, and Distribution Evidence. Phytotaxa 2023, 595, 127–138. [Google Scholar] [CrossRef]
- Llerena, S.A.; Salinas, N.; Oliveira, O.L.; Jadán-Guerrero, M.; Segovia-Salcedo, C. Distribution of the Genus Cedrela in Ecuador. Rudn J. Ecol. Life Saf. 2018, 26, 125–133. [Google Scholar] [CrossRef]
- Guevara Andino, J.E.; Pitman, N.C.A.; Ulloa Ulloa, C.; Romoleroux, K.; Fernández-Fernández, D.; Ceron, C.; Palacios, W.; Neill, D.A.; Oleas, N.; Altamirano, P. Trees of Amazonian Ecuador: A Taxonomically Verified Species List with Data on Abundance and Distribution. Ecology 2019, 100, e02894. [Google Scholar] [CrossRef]
- Sánchez-Capa, M.; Corell González, M.; Mestanza-Ramón, C. Edible Fruits from the Ecuadorian Amazon: Ethnobotany, Physicochemical Characteristics, and Bioactive Components. Plants 2023, 12, 3635. [Google Scholar] [CrossRef]
- Gachet, M.S.; Schühly, W. Jacaranda—An Ethnopharmacological and Phytochemical Review. J. Ethnopharmacol. 2009, 121, 14–27. [Google Scholar] [CrossRef]
- Richa, R.; Kohli, D.; Vishwakarma, D.; Mishra, A.; Kabdal, B.; Kothakota, A.; Richa, S.; Sirohi, R.; Kumar, R.; Naik, B. Citrus Fruit: Classification, Value Addition, Nutritional and Medicinal Values, and Relation with Pandemic and Hidden Hunger. J. Agric. Food Res. 2023, 14, 100718. [Google Scholar] [CrossRef]
- Mark, J.; Newton, A.; Oldfield, S.; Rivers, M. A Working List of Commercial Timber Tree Species. 2014. [Google Scholar]
- Leite, D.O.D.; de FA Nonato, C.; Camilo, C.J.; de Carvalho, N.K.G.; da Nobrega, M.G.L.A.; Pereira, R.C.; da Costa, J.G.M. Annona Genus: Traditional Uses, Phytochemistry and Biological Activities. Curr. Pharm. Des. 2020, 26, 4056–4091. [Google Scholar] [CrossRef] [PubMed]
- Valarezo, E.; Ojeda-Riascos, S.; Cartuche, L.; Andrade-González, N.; González-Sánchez, I.; Meneses, M.A. Extraction and Study of the Essential Oil of Copal (Dacryodes Peruviana), an Amazonian Fruit with the Highest Yield Worldwide. Plants 2020, 9, 1658. [Google Scholar] [CrossRef] [PubMed]
- Wani, A.M.; Sahoo, G. Forest Ecosystem Services and Biodiversity. In Spatial Modeling in Forest Resources Management; Springer: Berlin/Heidelberg, Germany, 2021; pp. 529–552. [Google Scholar]
- Peredo Parada, S.; Barrera Salas, C. Multifunctional Plants: Ecosystem Services and Undervalued Knowledge of Biocultural Diversity in Rural Communities—Local Initiatives for Agroecological Transition in Chile. Land 2023, 13, 39. [Google Scholar] [CrossRef]
- Ministerio del Ambiente del Ecuador Bosques Para El Buen Vivir—Plan de Acción REDD+ Ecuador (2016–2025); MAATE: Quito, Ecuador, 2016.
- Jadán, O.; Cifuentes Jara, M.; Torres, B.; Selesi, D.; Veintimilla Ramos, D.A.; Günter, S. Influence of Tree Cover on Diversity, Carbon Sequestration and Productivity of Cocoa Systems in the Ecuadorian Amazon. Programa Cambio Climático y Cuencas 2015. [Google Scholar] [CrossRef]
- Sierra, R. Patrones y Factores de Deforestación en el Ecuador Continental, 1990–2010. Y un Acercamiento a los Próximos 10 Años; Conservación Internacional Ecuador y Forest Trends: Quito, Ecuador, 2013; 57p. [Google Scholar]
- Castro, M.; Sierra, R.; Calva, O.; Camacho, J.; López, F.; Lozano, P. Zonas de Procesos Homogéneos de Deforestación del Ecuador: Factores Promotores y Tendencias al 2020; Programa GESOREN-GIZ y Ministerio de Ambiente del Ecuador: Quito, Ecuador, 2013. [Google Scholar]
- Harvey, C.A.; Villanueva, C.; Esquivel, H.; Gómez, R.; Ibrahim, M.; Lopez, M.; Martinez, J.; Muñoz, D.; Restrepo, C.; Saénz, J.C.; et al. Conservation Value of Dispersed Tree Cover Threatened by Pasture Management. For. Ecol. Manag. 2011, 261, 1664–1674. [Google Scholar] [CrossRef]
- Santos-Gally, R.; Boege, K. Biodiversity Islands: The Role of Native Tree Islands within Silvopastoral Systems in a Neotropical Region. In Biodiversity Islands: Strategies for Conservation in Human-Dominated Environments; Springer: Berlin/Heidelberg, Germany, 2022; pp. 117–138. [Google Scholar]
- Sales-Baptista, E.; Ferraz-de-Oliveira, M.I. Grazing in Silvopastoral Systems: Multiple Solutions for Diversified Benefits. Agrofor. Syst. 2021, 95, 1–6. [Google Scholar] [CrossRef]
- Calle, D.Z.; Giraldo, S.A.M.; Cardozo, A.; Galindo, A.; Murgueitio, R.E. Enhancing Biodiversity in Neotropical Silvopastoral Systems: Use of Indigenous Trees and Palms. In Integrating Landscapes: Agroforestry for Biodiversity Conservation and Food Sovereignty; Springer: Berlin/Heidelberg, Germany, 2017; pp. 417–438. [Google Scholar]
- Garbach, K.; Lubell, M.; DeClerck, F.A.J. Payment for Ecosystem Services: The Roles of Positive Incentives and Information Sharing in Stimulating Adoption of Silvopastoral Conservation Practices. Agric. Ecosyst. Environ. 2012, 156, 27–36. [Google Scholar] [CrossRef]
- Torres, B.; Eche, D.; Torres, Y.; Bravo, C.; Velasco, C.; García, A. Identification and Assessment of Livestock Best Management Practices (BMPs) Using the REDD+ Approach in the Ecuadorian Amazon. Agronomy 2021, 11, 1336. [Google Scholar] [CrossRef]
- Torres, B.; Espinoza, Í.; Torres, A.; Herrera-Feijoo, R.; Luna, M.; García, A. Livelihood Capitals and Opportunity Cost for Grazing Areas’ Restoration: A Sustainable Intensification Strategy in the Ecuadorian Amazon. Animals 2023, 13, 714. [Google Scholar] [CrossRef]
- Rivera, J.E.; Serna, L.; Arango, J.; Barahona, R.; Murgueitio, E.; Torres, C.F.; Chará, J. Silvopastoral Systems and Their Role in Climate Change Mitigation and Nationally Determined Contributions in Latin America. In Silvopastoral Systems of Meso America and Northern South America; Springer: Berlin/Heidelberg, Germany, 2023; pp. 25–53. [Google Scholar]
- Huertas, S.M.; Bobadilla, P.E.; Alcántara, I.; Akkermans, E.; van Eerdenburg, F.J.C.M. Benefits of Silvopastoral Systems for Keeping Beef Cattle. Animals 2021, 11, 992. [Google Scholar] [CrossRef] [PubMed]



| Variable | Amazon Altitudinal Gradient (Zone) | ||
|---|---|---|---|
| Low | Middle | High | |
| Elevation range (masl) | 400–700 | 701–1600 | 1601–2000 |
| Number of plots | 12 | 8 | 6 |
| Total area sampled (ha) | 3.39 | 2.2 | 1.69 |
| Average elevation (masl) | 543.1 | 1114.1 | 1778.0 |
| Year of settlement | 1975 | 1984 | 1952 |
| Ethnicity of head of household (% Kichwa) | 0.0 | 56.1 | 0.0 |
| Variable | Amazon Altitudinal Gradient (Zone) | Average | p-Value 1 | ||
|---|---|---|---|---|---|
| Low n = 12 | Middle n = 8 | High n = 6 | |||
| Richness (species) | 10.17 ± 3.21 a | 6.63 ± 2.72 ab | 5.53 ± 2.51 b | 7.96 ± 3.49 | *** |
| Richness (family) | 8.50 ± 2.23 a | 6.13 ± 2.58 ab | 4.67 ± 1.63 b | 6.88 ± 2.68 | *** |
| Exponential Shannon (Hexp Shannon) | 5.62 ± 2.42 | 5.04 ± 2.83 | 3.77 ± 1.42 | 5.02 ± 2.40 | n/s |
| Inverse Simpson (Dinv Simpson) | 4.06 ± 1.82 | 4.18 ± 2.67 | 3.09 ± 1.06 | 3.87 ± 1.97 | n/s |
| Equity (E) | 0.57 ± 0.19 | 0.73 ± 0.09 | 0.71 ± 0.12 | 0.65 ± 0.16 | n/s |
| Tree density (ha−1) | 193 ± 97.23 a | 83.25 ± 38.33 b | 101 ± 41.54 b | 138 ± 87.50 | *** |
| Basal area m2 (ha−1) | 8.67 ± 4.23 | 4.19 ± 3.65 | 6.03 ± 4.97 | 6.68 ± 4.53 | n/s |
| Average DBH (cm) | 20.32 ± 5.39 | 22.37 ± 11.82 | 22.85 ± 12.69 | 21.53 ± 9.24 | n/s |
| Maximum DBH (cm) | 27.78 | 40.67 | 41.54 | 41.54 | - |
| Family | Species | RA (%) | RF (%) | RD (%) | IVIs |
|---|---|---|---|---|---|
| Silvopasture low zone (400–700 masl) | |||||
| Cordiaceae | Cordia alliodora | 27.37 | 7.38 | 16.97 | 17.24 |
| Bignoniaceae | Jacaranda copaia | 12.84 | 4.92 | 21.12 | 12.96 |
| Myrtaceae | Psidium guajava | 17.58 | 8.20 | 3.94 | 9.91 |
| Vochysiaceae | Vochysia braceliniae | 4.43 | 4.92 | 10.92 | 6.76 |
| Meliaceae | Cedrela odorata | 3.21 | 5.74 | 4.68 | 4.54 |
| Fabaceae | Piptadenia pteroclada | 1.53 | 4.92 | 5.44 | 3.96 |
| Myristicaceae | Virola flexuosa | 2.60 | 1.64 | 6.99 | 3.74 |
| Lauraceae | Ocotea sp. | 1.99 | 5.74 | 3.16 | 3.63 |
| Lauraceae | Nectandra sp. | 2.45 | 4.10 | 3.23 | 3.26 |
| Asteraceae | Piptocoma discolor | 2.75 | 5.74 | 0.93 | 3.14 |
| Subtotal | 76.76 | 53.28 | 77.37 | 69.14 | |
| Silvopasture middle zone (701–1600 masl) | |||||
| Cordiaceae | Cordia alliodora | 12.23 | 5.66 | 26.39 | 14.76 |
| Asteraceae | Piptocoma discolor | 17.02 | 5.66 | 13.21 | 11.97 |
| Meliaceae | Cedrela odorata | 15.96 | 7.55 | 4.28 | 9.26 |
| Fabaceae | Inga sp. | 5.85 | 11.32 | 6.99 | 8.06 |
| Myrtaceae | Psidium guajava | 10.64 | 5.66 | 1.78 | 6.03 |
| Fabaceae | Inga edulis | 3.19 | 5.66 | 5.72 | 4.86 |
| Lecythidaceae | Grias neuberthii | 1.60 | 5.66 | 6.08 | 4.44 |
| Burseraceae | Dacryodes peruviana | 3.19 | 1.89 | 5.92 | 3.66 |
| Urticaceae | Cecropia membranacea | 1.60 | 5.66 | 3.10 | 3.45 |
| Lauraceae | Nectandra sp. | 5.32 | 3.77 | 1.25 | 3.45 |
| Subtotal | 76.60 | 58.49 | 74.72 | 69.94 | |
| Silvopasture high zone (1601–2000 masl) | |||||
| Moraceae | Ficus sp. | 5.85 | 12.50 | 53.51 | 23.95 |
| Malvaceae | Heliocarpus americanus | 25.15 | 18.75 | 21.41 | 21.77 |
| Myrtaceae | Psidium guajava | 38.01 | 9.38 | 6.38 | 17.92 |
| Lauraceae | Ocotea sp. | 11.11 | 15.63 | 5.07 | 10.60 |
| Fabaceae | Inga sp. | 9.94 | 9.38 | 5.56 | 8.29 |
| Lauraceae | Nectandra sp. | 4.68 | 9.38 | 0.99 | 5.01 |
| Rutaceae | Citrus limon | 1.75 | 6.25 | 0.33 | 2.78 |
| Meliaceae | Cedrela montana | 0.58 | 3.13 | 2.41 | 2.04 |
| Phyllanthaceae | Hieronyma oblonga | 0.58 | 3.13 | 1.80 | 1.84 |
| Euphorbiaceae | Croton lechleri | 0.58 | 3.13 | 1.55 | 1.75 |
| Subtotal | 98.25 | 90.63 | 99.00 | 95.96 | |
| Species | Tree Density (Ind ≥ 10 cm DAP/ha) | Status | * Category IUCN | Wood Density g/cm3 | ||
|---|---|---|---|---|---|---|
| Low | Middle | High | ||||
| Annona papilionella | 0 | 1 | 0 | Native | LC | 0.48 |
| Annona sp. | 9 | 1 | 0 | Native | NE | 0.47 |
| Rollinia sp. | 0 | 0 | 1 | Native | NE | 0.61 |
| Apeiba membranaceae | 1 | 0 | 0 | Native | NE | 0.27 |
| Bactris gasipaes | 9 | 0 | 0 | Native | NE | 0.43 |
| Iriartea deltoidea | 3 | 1 | 0 | Native | LC | 0.27 |
| Wettinia maynensis | 0 | 5 | 0 | Native | LC | 0.31 |
| Piptocoma discolor | 6 | 26 | 0 | Native | LC | 0.47 |
| Vernonanthura patens | 2 | 0 | 0 | Native | LC | 0.54 |
| Crescentia cujete | 1 | 0 | 0 | Native | LC | 0.70 |
| Jacaranda copaia | 84 | 3 | 0 | Native | LC | 0.60 |
| Cordia alliodora | 0 | 18 | 0 | Native | LC | 0.51 |
| Dacryodes peruviana | 0 | 6 | 0 | Native | LC | 0.61 |
| Protium nodulosum | 0 | 4 | 0 | Native | LC | 0.55 |
| Calophyllum brasiliense | 1 | 1 | 0 | Native | LC | 0.47 |
| Terminalia oblonga | 1 | 0 | 0 | Native | LC | 0.69 |
| Cordia alliodora | 122 | 0 | 0 | Native | LC | 0.51 |
| Croton lechleri | 0 | 0 | 1 | Native | NE | 0.47 |
| Sapium glandulosum | 0 | 1 | 0 | Native | LC | 0.44 |
| Dussia tessmannii | 2 | 0 | 0 | Native | LC | 0.47 |
| Erythrina poeppigiana | 1 | 0 | 0 | Native | LC | 0.47 |
| Inga edulis | 2 | 6 | 0 | Native | LC | 0.51 |
| Inga sp. | 0 | 8 | 16 | Native | NE | 0.57 |
| Piptadenia pteroclada | 9 | 0 | 0 | Native | LC | 0.76 |
| Nectandra sp. | 14 | 3 | 3 | Native | NE | 0.53 |
| Ocotea sp. | 12 | 0 | 14 | Native | NE | 0.54 |
| Persea americana | 1 | 0 | 0 | Native | LC | 0.60 |
| Grias neuberthii | 8 | 3 | 0 | Native | LC | 0.62 |
| Ceiba samauma | 2 | 0 | 0 | Native | NE | 0.57 |
| Heliocarpus americanus | 0 | 0 | 0 | Native | LC | 0.47 |
| Sterculia tessmannii | 2 | 2 | 37 | Native | LC | 0.47 |
| Miconia sp. | 7 | 0 | 0 | Native | NE | 0.63 |
| Cabralea canjerana | 5 | 0 | 0 | Native | LC | 0.53 |
| Cedrela montana | 0 | 0 | 1 | Native | VU | 0.47 |
| Cedrela odorata | 16 | 12 | 1 | Native | VU | 0.44 |
| Brosimum guianense | 2 | 0 | 0 | Native | LC | 0.47 |
| Ficus cuatrecasana | 0 | 0 | 1 | Native | NE | 0.47 |
| Ficus maxima | 2 | 1 | 0 | Native | LC | 0.47 |
| Ficus sp. | 6 | 0 | 10 | Native | NE | 0.42 |
| Virola flexuosa | 16 | 0 | 0 | Native | LC | 0.47 |
| Psidium guajava | 48 | 5 | 28 | Native | LC | 0.71 |
| Hieronyma oblonga | 0 | 0 | 1 | Native | LC | 0.47 |
| Citrus limon | 1 | 1 | 2 | No Native | NE | 0.71 |
| Citrus sinensis | 7 | 0 | 0 | No Native | NE | 0.71 |
| Pouteria caimito | 6 | 2 | 1 | Native | LC | 0.81 |
| Pouteria sp. | 0 | 0 | 0 | Native | NE | 0.77 |
| Cecropia membranacea | 0 | 3 | 0 | Native | LC | 0.33 |
| Cecropia sp. | 1 | 0 | 0 | Native | NE | 0.36 |
| Pourouma cecropiifolia | 4 | 1 | 0 | Native | LC | 0.36 |
| Vochysia braceliniae | 28 | 0 | 0 | Native | LC | 0.39 |
| Vochysia ferruginea | 6 | 0 | 0 | Native | LC | 0.36 |
| Scientific Name | Family | Use | |||
|---|---|---|---|---|---|
| Human and Livestock Feed | Medicinal Use | Craft Use | Material for Construction | ||
| Annona papilionella | Annonaceae | √ | √ | √ | √ |
| Annona sp. | √ | √ | √ | √ | |
| Rollinia sp. | √ | √ | √ | √ | |
| Apeiba membranacea | Arecaceae | √ | √ | √ | √ |
| Bactris gasipaes | √ | √ | √ | √ | |
| Iriartea deltoidea | √ | √ | √ | √ | |
| Wettinia maynensis | √ | √ | √ | √ | |
| Piptocoma discolor | Asteraceae | √ | √ | √ | |
| Vernonanthura patens | √ | √ | √ | ||
| Crescentia cujete | Bignoniaceae | √ | √ | √ | |
| Jacaranda copaia | √ | √ | |||
| Cordia alliodora | Cordiaceae | √ | √ | ||
| Dacryodes peruviana | Burseraceae | √ | √ | √ | √ |
| Protium nodulosum | √ | √ | √ | √ | |
| Calophyllum brasiliense | Calophyllaceae | √ | √ | ||
| Terminalia oblonga | Combretaceae | √ | √ | ||
| Croton lechleri | Euphorbiaceae | √ | √ | ||
| Sapium glandulosum | √ | √ | √ | ||
| Dussia tessmannii | Fabaceae | √ | √ | ||
| Erythrina poeppigiana | √ | ||||
| Inga edulis | √ | √ | √ | ||
| Inga sp. | √ | √ | √ | ||
| Piptadenia pteroclada | √ | √ | |||
| Nectandra sp. | Lauraceae | √ | |||
| Ocotea sp. | √ | √ | |||
| Persea americana | √ | √ | √ | ||
| Grias neuberthii | Lecythidaceae | √ | √ | √ | |
| Ceiba samauma | Malvaceae | √ | √ | ||
| Heliocarpus americanus | √ | √ | √ | ||
| Sterculia tessmannii | √ | √ | |||
| Miconia sp. | Melastomataceae | √ | √ | ||
| Cabralea canjerana | Meliaceae | √ | |||
| Cedrela montana | √ | ||||
| Cedrela odorata | √ | √ | |||
| Brosimum guianense | Moraceae | √ | √ | ||
| Ficus cuatrecasana | √ | ||||
| Ficus maxima | √ | √ | √ | ||
| Ficus sp. | √ | √ | √ | ||
| Virola flexuosa | Myristicaceae | √ | |||
| Psidium guajava | Myrtaceae | √ | √ | √ | |
| Hieronyma oblonga | Phyllanthaceae | √ | √ | ||
| Citrus limon | Rutaceae | √ | √ | √ | |
| Citrus sinensis | √ | √ | √ | ||
| Pouteria caimito | Sapotaceae | √ | √ | √ | |
| Pouteria sp. | √ | √ | √ | ||
| Cecropia membranacea | Urticaceae | √ | √ | √ | |
| Cecropia sp. | √ | √ | √ | ||
| Pourouma cecropiifolia | √ | √ | |||
| Vochysia braceliniae | Vochysiaceae | √ | √ | ||
| Vochysia ferruginea | √ | √ | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres, B.; Herrera-Feijoo, R.J.; Torres-Navarrete, A.; Bravo, C.; García, A. Tree Diversity and Its Ecological Importance Value in Silvopastoral Systems: A Study along Elevational Gradients in the Sumaco Biosphere Reserve, Ecuadorian Amazon. Land 2024, 13, 281. https://doi.org/10.3390/land13030281
Torres B, Herrera-Feijoo RJ, Torres-Navarrete A, Bravo C, García A. Tree Diversity and Its Ecological Importance Value in Silvopastoral Systems: A Study along Elevational Gradients in the Sumaco Biosphere Reserve, Ecuadorian Amazon. Land. 2024; 13(3):281. https://doi.org/10.3390/land13030281
Chicago/Turabian StyleTorres, Bolier, Robinson J. Herrera-Feijoo, Alexandra Torres-Navarrete, Carlos Bravo, and Antón García. 2024. "Tree Diversity and Its Ecological Importance Value in Silvopastoral Systems: A Study along Elevational Gradients in the Sumaco Biosphere Reserve, Ecuadorian Amazon" Land 13, no. 3: 281. https://doi.org/10.3390/land13030281
APA StyleTorres, B., Herrera-Feijoo, R. J., Torres-Navarrete, A., Bravo, C., & García, A. (2024). Tree Diversity and Its Ecological Importance Value in Silvopastoral Systems: A Study along Elevational Gradients in the Sumaco Biosphere Reserve, Ecuadorian Amazon. Land, 13(3), 281. https://doi.org/10.3390/land13030281










