Monitoring of Herbicide Residues in Agricultural Soils in Vojvodina Province (Northern Serbia)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Studied Area and Soil Sampling
2.2. Determination of Soil Property
2.3. Pesticide Residue Analyses
2.4. Data Analysis
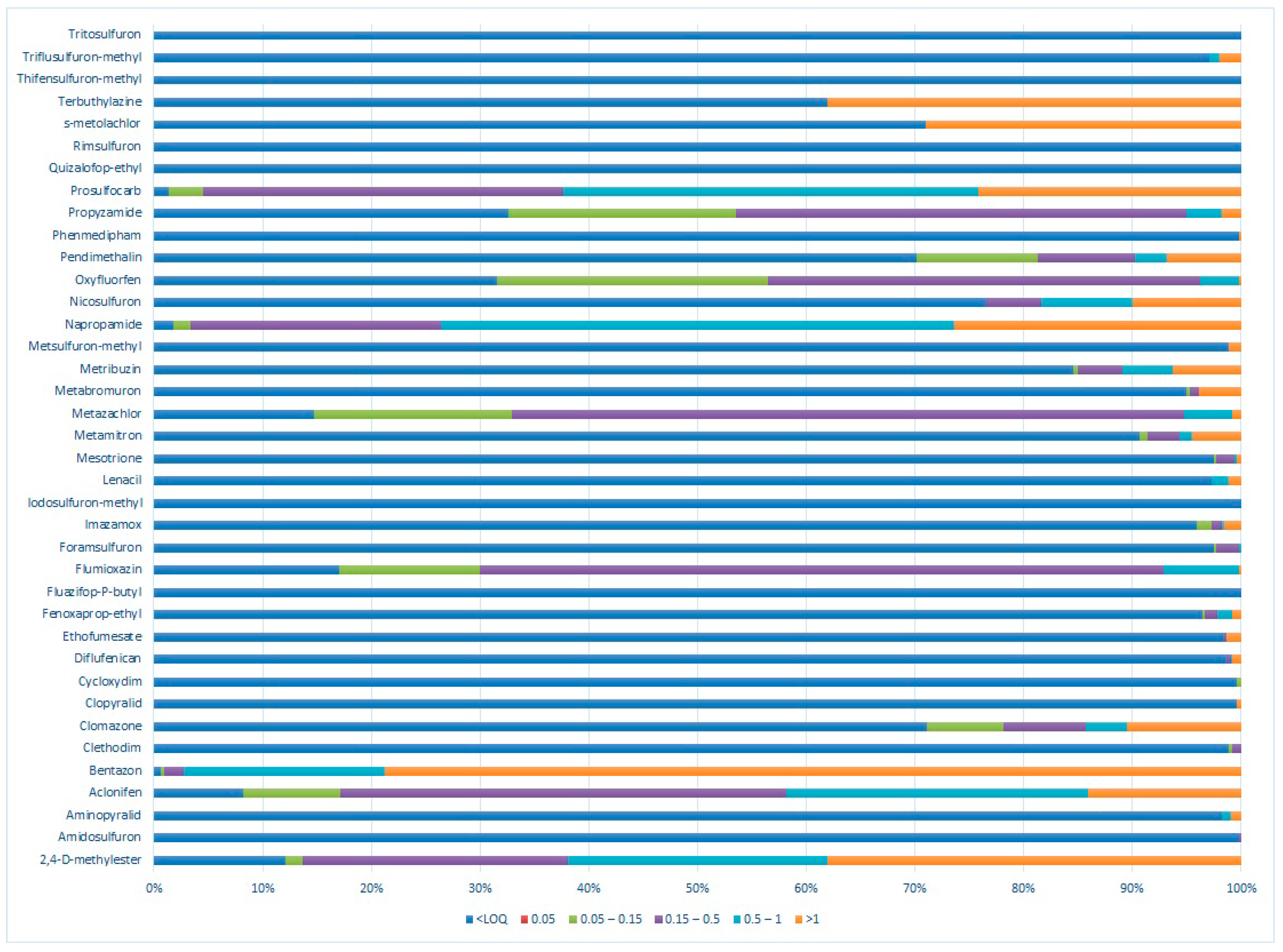
3. Results
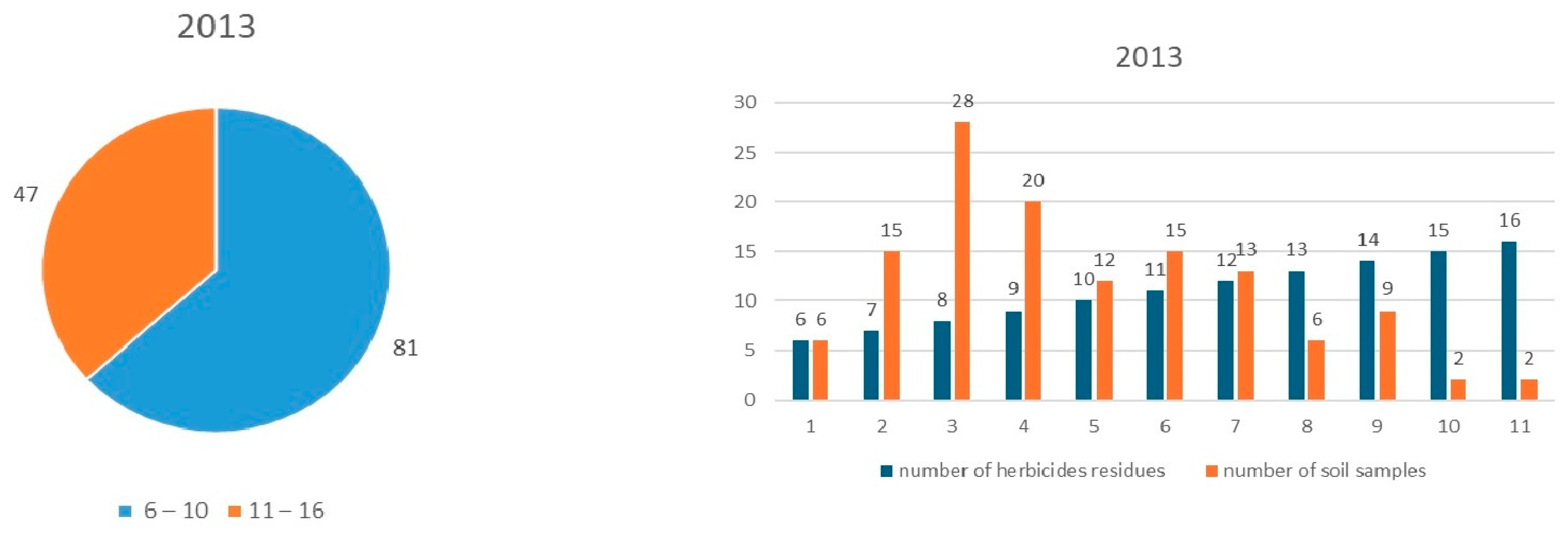
3.1. Monitoring of Herbicide Residues in 2013
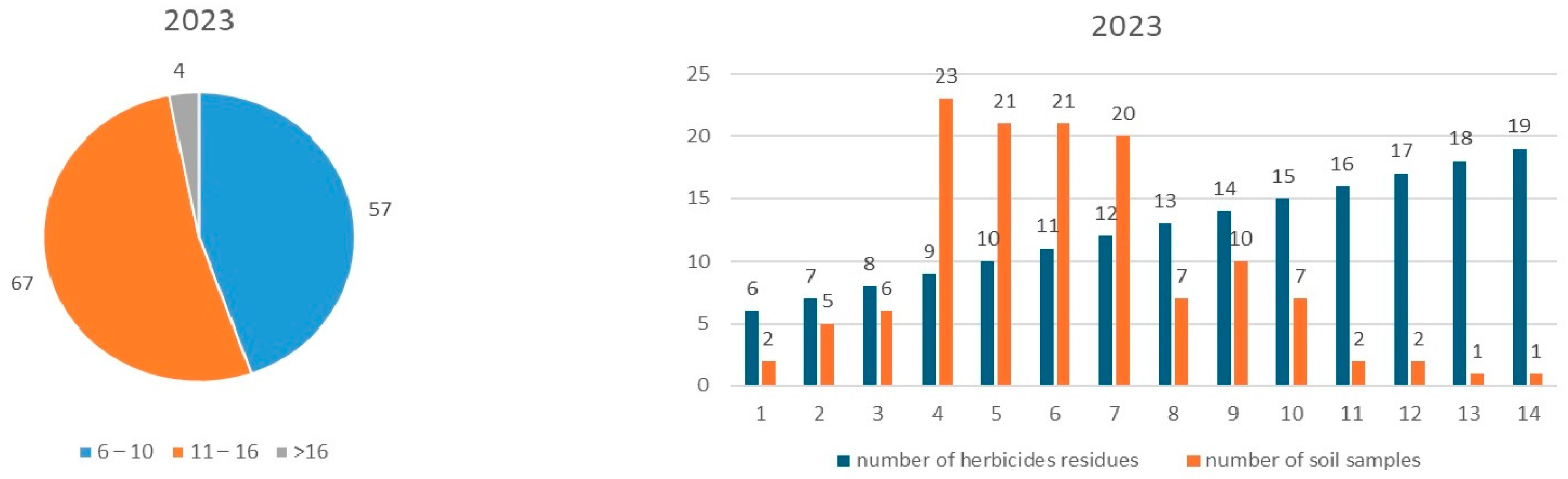
3.2. Monitoring of Herbicide Residues in 2023
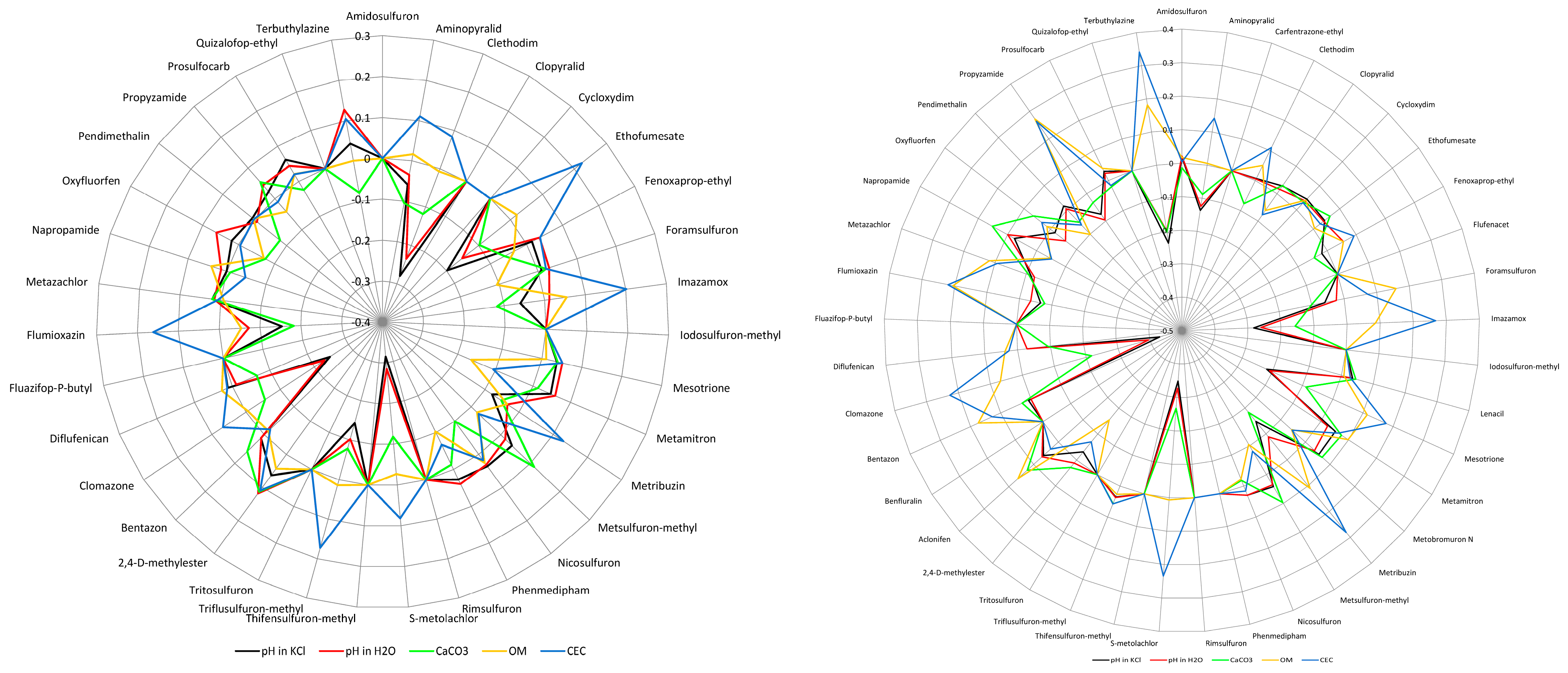
3.3. Soil Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Herbicide | Chemical Group | Application Time | Molecular Formula | Molecular Weight (g/mol) | Water Solubility (mg/L) pH 7 | log KO/W at pH 7 and 20 °C | Vapor Pressure (mPa) (at 20 °C) | Soil Degradation, DT50 in Field (day) | Bioconcentration Factor (l L/kg−) | Threshold of Toxicological Concern (Cramer Class) |
|---|---|---|---|---|---|---|---|---|---|---|
| 2,4-D-methyl ester | Phenoxy-carboxylates | post em | C9H8Cl2O | 235.064 | / | / | / | / | / | High (class III) |
| Aclonifen | Diphenyl-ether | post em | C12H9ClN2O3 | 264.66 | 1.4 | 4.37 | 0.016 | 80.4 | 2896 | High (class III) |
| Amidosulfuron | Sulfonylureas | post em | C9H15N5O7S2 | 369.4 | 5600 | −1.56 | 1.3 × 10−3 | 21 (typical) | 4.85 | High (class III) |
| Aminopyralid | 6-Chloropicolinates | post em | C6H4Cl2N2O2 | 207.02 | 2480 | −2.87 | 2.59 × 10−5 | 12.1 | 100 | High (class III) |
| Benfluralin | Dinitroanilines | post em | C13H16F3N3O4 | 335.28 | 0.064 | 5.27 | 1.8 | 39.9 | / | High (class III) |
| Bentazon | Benzothiadiazinone | post em | C10H12N2O3S | 240.3 | 7112 | −0.46 | 0.17 | 7.5 | 21 | High (class III) |
| Carfentrazone-ethyl | N-Phenyl-triazolinones | post em | C15H14Cl2F3N3O3 | 412.2 | 29.3 | 3.7 | 7.2 × 10−3 | 8.2 | 176 | High (class III) |
| Clethodim | Cyclohexanediones (DIMs) | post em | C17H26ClNO3S | 359.92 | 5450 | 1.50 | 2.68 × 10−2 | 3 | 3.5 | High (class III) |
| Clomazone | Isoxazolidinone | post em | C12H14ClNO2 | 239.7 | 1212 | 2.58 | 27 | 27.3 | 40 | High (class III) |
| Clopyralid | 6-Chloropicolinates | post em | C6H3Cl2NO2 | 192.0 | 7850 | −2.63 | 1.36 | 8.2 | 1 | High (class III) |
| Cycloxydim | Cyclohexanediones (DIMs) | post em | C17H27NO3S | 325.47 | 53 | 1.36 | 0.01 | 5 | low risk | High (class III) |
| Diflufenican | Phenyl ethers | pre/post em | C19H11F5N2O2 | 394.3 | 0.05 | 4.2 | 4.25 × 10−3 | 64.6 | 1276 | High (class III) |
| Ethofumesate | Benzofurans | pre/post em | C13H18O5S | 286.35 | 50 | 2.7 | 0.65 | 37.8 | 144 | High (class III) |
| Fenoxaprop-ethyl | Aryloxyphenoxy-propionates (FOPs) | post em | C18H16ClNO5 | 361.77 | 0.9 | 4.28 | 1.87 × 10−4 | 4 | 4.9 | High (class III) |
| Fluazifop-P-butyl | Aryloxyphenoxy-propionates | post em | C19H20F3NO4 | 383.4 | 0.93 | 4.5 | 0.12 | 8.2 | 320 | High (class III) |
| Flufenacet | α-Oxyacetamides | post em | C14H13F4N3O2S | 363.33 | 51 | 3.5 | 0.09 | 39 | 71.4 | High (class III) |
| Flumioxazin | N-Phenyl-imides | pre em | C19H15FN2O4 | 354.3 | 0.786 | 2.55 | 0.32 | 17.6 | low risk | High (class III) |
| Foramsulfuron | Sulfonylureas | post em | C17H20N6O7S | 452.4 | 3293 | −0.78 | 4.20 × 10−8 | 25.4 (typical) | low risk | High (class III) |
| Imazamox | Imidazolinones | post em | C15H19N3O4 | 305.33 | 626,000 | −2.9 | 6.3 × 10−8 | 16.7 | 0.1 | High (class III) |
| Iodosulfuron-methyl | Sulfonylureas | post em | C14H14IN5O6S | 507.26 | 25,000 | −0.7 | 2.6 × 10−6 | 3.2 | low risk | High (class III) |
| Lenacil | Uracils | post em | C13H18N2O2 | 234.29 | 2.9 | 1.69 | 1.7 × 10−6 | 39.8 | 18 | High (class III) |
| Mesotrione | Triketones | pre/post em | C14H13NO7S | 339.32 | 1500 | 0.11 | 5.7 × 10−3 | 5 | low risk | High (class III) |
| Metamitron | Triazinones | post em | C10H10N4O | 202.21 | 1770 | 0.85 | 7.44 × 10−4 | 11.1 | 75 | High (class III) |
| Metazachlor | α-Chloroacetamides | pre/post em | C14H16ClN3O | 277.75 | 450 | 2.49 | 0.089 | 6.8 | low risk | High (class III) |
| Metobromuron N | Phenylureas | post em | C9H11BrN2O2 | 259.1 | 328 | 2.48 | 0.144 | 22.4 | low risk | High (class III) |
| Metribuzin | Triazines | pre/post em | C8H14N4OS | 214.29 | 10,700 | 1.7 | 0.121 | 19 | 10 | High (class III) |
| Metsulfuron-methyl | Sulfonylureas | post em | C14H15N5O6S | 381.37 | 2790 | −1.87 | 1 × 10−6 | 13.3 | 1 | High (class III) |
| Napropamide | Acetamides | pre em | C17H21NO2 | 271.35 | 74 | 3.3 | 2.2 × 10−2 | 72 | 98 | High (class III) |
| Nicosulfuron | Sulfonylureas | post em | C15H18N6O6S | 410.4 | 90,700 | −2.16 | 6 × 10−3 | 13.5 | low risk | High (class III) |
| Oxyfluorfen | Diphenyl ethers | pre/post em | C15H11ClF3NO4 | 361.7 | 0.116 | 4.86 | 0.026 | 73 | 1637 | High (class III) |
| Pendimethalin | Dinitroanilines | pre em | C13H19N3O4 | 281.312 | 0.33 | 5.4 | 3.34 | 100.6 | 5100 | High (class III) |
| Phenmedipham | Phenlcarbamates | post em | C16H16N2O4 | 300.31 | 1.8 | 2.7 | 7 × 10−7 | 16.7 | 165 | High (class III) |
| Propyzamide | Benzamides | post em | C12H11Cl2NO | 256.12 | 9 | 3.27 | 0.058 | 50.5 (typical) | 49 | High (class III) |
| Prosulfocarb | Thiocarbamates | post em | C14H21NOS | 251.39 | 13.2 | 4.48 | 0.79 | 9.8 | 700 | High (class III) |
| Quizalofop-ethyl | Aryloxyphnoxy-propionates (FOPs)) | post em | C19H17ClN2O4 | 372.8 | 0.31 | 4.28 | 0.04 | 60 | 867 | High (class III) |
| Rimsulfuron | Sulfonylureas | post em | C14H17N5O7S2 | 431.4 | 7300 | −1.46 | 8.9 × 10−4 | 10.8 | low risk | High (class III) |
| s-metolachor | Chloroacetamide | pre em | C15H22ClNO2 | 283.79 | 480 | 3.05 | 3.7 | 23.17 | 68.8 | High (class III) |
| Thifensulfuron-methyl | Sulfonylureas | post em | C12H13N5O6S2 | 387.4 | 54.1 | −1.65 | 5.19 × 10−6 | 10 | 0.8 | High (class III) |
| Triflusulfuron-methyl | Sulfonylureas | post em | C17H19F3N6O6S | 492.43 | 260 | 0.94 | 1.01 × 10−2 | 4.5 | 1.3 | High (class III) |
| Tritosulfuron | Sulfonylureas | post em | C13H9F6N5O4S | 445.3 | 78.3 | 0.62 | 9.3 × 10−5 | 8.2 | low risk | High (class III) |
| Terbuthylazine | Triazines | pre/post em | C9H16ClN5 | 229.81 | 6.6 | 3.4 | 0.152 | 21.8 | 34 | High (class III) |
References
- Ferreira, C.S.S.; Seifollahi-Aghmiuni, S.; Destouni, G.; Ghajarnia, N.; Kalantari, Z. Soil degradation in the European Mediterranean region: Processes, status and consequences. Sci. Total Environ. 2022, 805, 150106. [Google Scholar] [CrossRef]
- Rust, N.; Lunder, O.E.; Iversen, S.; Vella, S.; Oughton, E.A.; Breland, T.A.; Glass, J.H.; Maynard, C.M.; McMorran, R.; Reed, M.S. Perceived Causes and Solutions to Soil Degradation in the UK and Norway. Land 2022, 11, 131. [Google Scholar] [CrossRef]
- Tilman, D.; Fargione, J.; Wolff, B.; D’antonio, C.; Dobson, A.; Howarth, R.; Schindler, D.; Schlesinger, W.H.; Simberloff, D.; Swackhamer, D. Forecasting Agriculturally Driven Global. Science 2001, 292, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, G.; Atreya, K.; Scheepers, P.T.J.; Geissen, V. Concentration and distribution of pesticide residues in soil: Nondietary human health risk assessment. Chemosphere 2020, 253, 126594. [Google Scholar] [CrossRef] [PubMed]
- Faostat Analytical Brief 46. Pesticides Use, Pesticides Trade and Pesticides Indicators Global, Regional and Country Trends, 1990–2020. Available online: https://openknowledge.fao.org/server/api/core/bitstreams/78705276-3455-4c70-ac77-3082095f83b3/content (accessed on 9 July 2024).
- Stolte, J.; Tesfai, M.; Øygarden, L.; Kværnø, S.; Keizer, J.; Verheijen, F.; Panagos, P.; Ballabio, C.; Hesselet, R. Soil Threats in Europe: Status, Methods, Drivers and Effects on Ecosystem Services; Publications Office of the European Union: Luxembourg, 2016. [Google Scholar]
- Hvězdová, M.; Kosubová, P.; Košíková, M.; Scherr, K.E.; Šimek, Z.; Brodský, L.; Šudoma, M.; Škulcová, L.; Sáňka, M.; Svobodová, M.; et al. Currently and recently used pesticides in Central European arable soils. Sci. Total Environ. 2018, 613–614, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Beckie, H.J.; Hall, L.M.; Busi, R.; Ashworth, M.B. Chapter 7 Evolution and Persistence of Herbicide–Resistant Weeds. In Persistence Strategies of Weeds Upadhyaya; Upadhyaya, M.K., Clements, D.R., Shrestha, A., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2022. [Google Scholar]
- Geissen, V.; Silva, V.; Lwanga, E.H.; Beriot, N.; Oostindie, K.; Bin, Z.; Pynem, E.; Busink, S.; Zomer, P.; Mol, H.; et al. Cocktails of pesticide residues in conventional and organic farming systems in Europe e Legacy of the past and turning point for the future. Environ. Pollut. 2021, 278, 116827. [Google Scholar] [CrossRef]
- Froger, C.; Jolivet, C.; Budzinski, H.; Pierdet, M.; Caria, G.; Saby, N.P.A.; Arrouays, D.; Bispo, A. Pesticide Residues in French Soils: Occurrence, Risks, and Persistence. Environ. Sci. Technol. 2023, 57, 7818–7827. [Google Scholar] [CrossRef]
- Carey, A.E.; Gowen, J.A.; Tai, H. Pesticide residue levels in soils and crops from 37 states, 1972—National soils monitoring program (IV). Pestic. Monit. J. 1979, 12, 209–229. [Google Scholar]
- Ukalska-Jaruga, A.; Smreczak, B.; Siebielec, G. Assessment of Pesticide Residue Content in Polish Agricultural Soils. Molecules 2020, 25, 587. [Google Scholar] [CrossRef]
- Kosubova, P.; Skulcov, L.; Polakov, S.; Hofman, J.; Bielsk, L. Spatial and temporal distribution of the currently-used and recently-banned pesticides in arable soils of the Czech Republic. Chemosphere 2020, 254, 126902. [Google Scholar] [CrossRef]
- Chiaia-Hernandez, A.C.; Keller, A.; Wachter, D.; Steinlin, C.; Camenzuli, L.; Hollender, J.; Krauss, M. Long–term persistence of pesticides and TPs in archived agricultural soil samples and comparison with pesticide application. Environ. Sci. Technol. 2017, 51, 10642–10651. [Google Scholar] [CrossRef]
- Riedo, J.; Wächter, D.; Gubler, A.; Wettstein, F.E.; Meuli, R.G.; Bucheli, T.D. Pesticide residues in agricultural soils in light of their on-farm application history. Environ. Pollut. 2023, 331, 121892. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.L.; Antunes, S.C.; Castro, B.B.; Marques, C.R.; Gonçalves, A.M.M.; Gonçalves, F.; Pereira, R. Toxicity evaluation of three pesticides on non–target aquatic and soil organisms: Commercial formulation versus active ingredient. Ecotoxicology 2009, 18, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lu, Z.; Miller, H.; Liu, J.; Hou, Z.; Liang, S.; Zhao, X.; Hao, Z.; Borch, T. Fungicide azoxystrobin induced changes on the soil microbiome. Appl. Soil Ecol. 2020, 145, 103343. [Google Scholar] [CrossRef]
- European Parliament; Council of the European Union. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for Community action in the field of water policy. In Official Journal of the European Communities L 327, 22 December 2000; European Parliament; Council of the European Union: Brusells, Belgium, 2000. [Google Scholar]
- Carlon, C. Derivation Methods of Soil Screening Values in Europe. A Review and Evaluation of National Procedures towards Harmonization; European Commission; Joint Research Centre: Ispra, Italy, 2007; p. 306.
- Silva, V.; Mol, H.G.J.; Zomer, P.; Tienstra, M.; Ritsema, C.J.; Geissen, V. Pesticide residues in European agricultural soils—A hidden reality unfolded. Sci. Total Environ. 2019, 653, 1532–1545. [Google Scholar] [CrossRef]
- Proposal for a Directive of the European Parliament and of the Council on Soil Monitoring and Resilience (Soil Monitoring Law). Brussels, 5.7.2023 COM(2023) 416 final 2023/0232 (COD). Available online: https://eur-lex.europa.eu/resource.html?uri=cellar:01978f53-1b4f-11ee-806b-01aa75ed71a1.0001.02/DOC_1&format=PDF (accessed on 9 July 2024).
- Available online: https://rav.org.rs/sr/key-sectors/agribusiness/ (accessed on 9 July 2024).
- Available online: https://www.srbija.gov.rs/tekst/en/130127/basic-info.php (accessed on 10 July 2024).
- Available online: https://www.un.org/geospatial/content/central-and-eastern-europe (accessed on 10 July 2024).
- HRN EN ISO 10390; Soil Quality––Determination of pH (ISO 10390:2005). HZN Glasilo 1/2005; ISO: Geneva, Switzerland, 2005.
- HRN EN ISO 14235; Soil Quality––Determination of Organic Carbon by Sulfochromic Oxidation (ISO 14235:1998). Glasilo DZNM 4–6/2004; ISO: Geneva, Switzerland, 2004.
- Gračanin, M. Pedologija: II. Dio. Fiziografija Tala; Poljoprivredni Nakladni Zavod: Zagreb, SFRJ, 1947. [Google Scholar]
- HRN ISO 10693; Soil Quality––Determination of Carbonate Content––Volumetric Method (ISO 10693:1995). Glasilo DZNM 4–6/2004; ISO: Geneva, Switzerland, 2004.
- More, S.J.; Bampidis, V.; Benford, D.; Bragard, C.; Halldorsson, T.I.; Hernández-Jerez, A.F.; Hougaard Bennekou, S.; Koutsoumanis, K.P.; Machera, K.; Naegeli, H.; et al. Guidance on the use of the Threshold of Toxicological Concern approach in food safety assessment. EFSA J. 2019, 17, e05708. [Google Scholar]
- Anastassiades, M.; Lehotay, S.J.; Stajnbaher, D.; Schenck, F.J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid–phase extraction” for the determination of pesticide residues in produce. AOAC Int. J. 2003, 86, 412–431. [Google Scholar] [CrossRef]
- Lazic, S.; Šunjka, D.; Milovanovic, I.; Manojlovic, M.; Vukovic, S.; Jovanov, P. Pesticide residues in agricultural soil of Vojvodina Province, Serbia. In Proceedings of the SETAC Europe 25th Annual Meeting, Barcelona, Spain, 3–7 May 2015; Book of Abstracts. p. 431. [Google Scholar]
- Medić Pap, S.; Popović, B.; Stojić, N.; Danojević, D.; Pucarević, M.; Červenski, J.; Šperanda, M. The environmental issue of pesticide residues in agricultural soils in Serbia. Int. J. Environ. Sci. Technol. 2023, 20, 7263–7276. [Google Scholar] [CrossRef]
- Hrouzková, S.; Matisová, E. Endocrine disrupting pesticides. In Pesticides: Advances in Chemical Botanical Pesticides; Intech Open: Rijeka, Croatia, 2012; pp. 99–126. [Google Scholar]
- European Parliament; Council of the European Union. Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013. In Official Journal of the European Union L226/1; European Parliament; Council of the European Union: Bruselles, Belgium, 2013. [Google Scholar]
- Grahovac, N.L.; Stojanović, Z.S.; Kravić, S.Ž.; Orčić, D.Z.; Suturović, Z.J.; Kondić-Špika, A.Đ.; Vasin, J.R.; Šunjka, D.B.; Jakšić, S.P.; Rajković, M.M.; et al. Determination of residues of sulfonylurea herbicides in soil by using microwave-assisted extraction and high performance liquid chromatographic method. Hem. Ind. (Chem. Ind.) 2017, 201771, 289–298. [Google Scholar] [CrossRef]
- Petrović, M.; Sekulić, J. Plant Protection Products on the Serbian Market; Plant Doctor: Ashmore, Australia, 2020; Volume 48, pp. 3–4. [Google Scholar]
- Green Deal. Available online: https://commission.europa.eu/publications/delivering-european-green-deal_en#files (accessed on 12 July 2024).
- Pelosi, C.; Bertrand, C.; Daniele, G.; Coeurdassier, M.; Benoit, P.; Nélieu, S.; Lafay, F.; Bretagnolle, V.; Gaba, S.; Vulliet, E.; et al. Residues of Currently Used Pesticides in Soils and Earthworms: A Silent Threat? Agric. Ecosyst. Environ. 2021, 305, 107167. [Google Scholar] [CrossRef]
- Pose-Juan, E.; Sánchez-Martín, M.J.; Andrades, M.S.; Rodríguez-Cruz, M.S.; Herrero-Hernández, E. Pesticide residues in vineyard soils from Spain: Spatial and temporal distributions. Sci. Total Environ. 2015, 514, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Sunulahpašić, A.; Mitrić, S.; Šunjka, D.; Žabić, M.; Predić, T.; Šipka, M.; Rodić, L. Adsorption of nicosulfuron herbicide in the agricultural soils of Bosnia and Herzegovina. Plant Soil Environ. 2020, 66, 162–166. [Google Scholar] [CrossRef]
- Orgiazzi, A.; Panagos, P.; Fernández-Ugalde, O.; Wojda, P.; Labouyrie, M.; Ballabio, C.; Franco, A.; Pistocchi, A.; Montanarella, L.; Jones, A. LUCAS Soil Biodiversity and LUCAS Soil Pesticides, new tools for research and policy development. Eur. J. Soil Sci. 2022, 73, e13299. [Google Scholar] [CrossRef]
- Available online: https://esdac.jrc.ec.europa.eu/projects/lucas (accessed on 4 August 2024).
- Katagi, T. Soil column leaching of pesticides. Rev. Environ. Contam. Toxicol. 2013, 221, 1–105. [Google Scholar]
- Scherr, K.E.; Bielská, L.; Kosubová, P.; Dinisová, P.; Hvězdová, M.; Šimek, Z.; Hofman, J. Occurrence of Chlorotriazine herbicides and their transformation products in arable soils. Environ. Pollut. 2017, 222, 283–293. [Google Scholar] [CrossRef]
- Mitrić, S.; Sunulahpašić, A.; Šunjka, D.; Vuković, S.; Žabić, M.; Hamidović, S.; Kelečević, B. Dissipation dynamic of nicosulfuron in different types of agricultural soils. Plant Soil Environ. 2024, 70, 245–251. [Google Scholar] [CrossRef]
- McBean, C. The Pesticide Manual: A World Compendium, 16th ed.; BCPC: Alton, UK, 2012. [Google Scholar]
| Active Substance | Depth (cm) | 2013 | 2023 | ||||
|---|---|---|---|---|---|---|---|
| Frequency (%) | Min (mg/kg) | Max (mg/kg) | Frequency (%) | Min (mg/kg) | Max (mg/kg) | ||
| 2.4-D-methyl ester | 0–30 | 89.06 | <LOD | 5.65 | 85.16 | <LOD | 4.37 |
| 30–60 | 88.28 | <LOD | 7.73 | 10.94 | <LOD | 6.77 | |
| Aclonifen | 0–30 | 0.00 | na | na | 91.41 | <LOD | 9.42 |
| 30–60 | 0.00 | na | na | 92.19 | <LOD | 6.83 | |
| Amidosulfuron | 0–30 | 0.00 | <LOD | <LOD | 0.78 | <LOD | 0.20 |
| 30–60 | 0.00 | <LOD | <LOD | 0.00 | <LOD | <LOD | |
| Aminopyralid | 0–30 | 0.78 | <LOD | 0.76 | 2.34 | <LOD | 1.28 |
| 30–60 | 2.34 | <LOD | 2.65 | 0.00 | <LOD | <LOD | |
| Benfluralin | 0–30 | 0.00 | na | na | 0.00 | <LOD | <LOD |
| 30–60 | 0.00 | na | na | 0.00 | <LOD | <LOD | |
| Bentazon | 0–30 | 100.00 | 0.126 | 5.27 | 99.22 | <LOD | 7.70 |
| 30–60 | 99.22 | <LOD | 5.68 | 99.22 | <LOD | 9.02 | |
| Carfentrazone-ethyl | 0–30 | 0.00 | na | na | 0.00 | <LOD | <LOD |
| 30–60 | 0.00 | na | na | 0.00 | <LOD | <LOD | |
| Clethodim | 0–30 | 2.34 | <LOD | 0.45 | 1.56 | <LOD | 0.21 |
| 30–60 | 2.34 | <LOD | 0.19 | 0.00 | <LOD | <LOD | |
| Clomazone | 0–30 | 32.03 | <LOD | 229.40 | 29.69 | <LOD | 22.62 |
| 30–60 | 27.34 | <LOD | 72.03 | 26.56 | <LOD | 25.98 | |
| Clopyralid | 0–30 | 0.00 | <LOD | 0 | 0.00 | <LOD | <LOD |
| 30–60 | 0.00 | <LOD | 0 | 1.56 | <LOD | 179.80 | |
| Cycloxydim | 0–30 | 0.00 | <LOD | 0 | 0.78 | <LOD | 0.12 |
| 30–60 | 0.00 | <LOD | 0 | 0.78 | <LOD | 0.13 | |
| Diflufenican | 0–30 | 0.78 | <LOD | 2.07 | 1.56 | <LOD | 3.81 |
| 30–60 | 1.56 | <LOD | 4.22 | 1.56 | <LOD | 3.27 | |
| Ethofumesate | 0–30 | 0.78 | <LOD | 24.34 | 2.34 | <LOD | 8.96 |
| 30–60 | 0.78 | <LOD | 6.29 | 2.34 | <LOD | 1.88 | |
| Fenoxaprop-ethyl | 0–30 | 5.47 | <LOD | 1.67 | 3.13 | <LOD | 2.09 |
| 30–60 | 2.34 | <LOD | 0.39 | 3.13 | <LOD | 3.99 | |
| Fluazifop-P-butyl | 0–30 | 0.00 | <LOD | <LOD | 0.00 | <LOD | <LOD |
| 30–60 | 0.00 | <LOD | <LOD | 0.00 | <LOD | <LOD | |
| Flufenacet | 0–30 | 0.00 | na | na | 0.00 | <LOD | <LOD |
| 30–60 | 0.00 | na | na | 0.00 | <LOD | <LOD | |
| Flumioxazin | 0–30 | 67.97 | <LOD | 1.83 | 72.66 | <LOD | 1.10 |
| 30–60 | 71.09 | <LOD | 0.86 | 79.69 | <LOD | 0.72 | |
| Foramsulfuron | 0–30 | 0.78 | <LOD | 0.11 | 4.69 | <LOD | 0.39 |
| 30–60 | 3.13 | <LOD | 0.19 | 0.00 | <LOD | <LOD | |
| Imazamox | 0–30 | 5.47 | <LOD | 3.69 | 3.91 | <LOD | 8.53 |
| 30–60 | 3.91 | <LOD | 1.89 | 0.00 | <LOD | <LOD | |
| Iodosulfuron-methyl | 0–30 | 0.00 | <LOD | <LOD | 0.00 | <LOD | <LOD |
| 30–60 | 0.00 | <LOD | <LOD | 0.00 | <LOD | <LOD | |
| Lenacil | 0–30 | 0.00 | na | na | 2.34 | <LOD | 21.84 |
| 30–60 | 0.00 | na | na | 0.00 | <LOD | <LOD | |
| Mesotrione | 0–30 | 3.13 | <LOD | 0.37 | 3.91 | <LOD | 24.06 |
| 30–60 | 0.78 | <LOD | 0.27 | 2.34 | <LOD | 1.87 | |
| Metamitron | 0–30 | 3.13 | <LOD | 0.72 | 1.56 | <LOD | 4.05 |
| 30–60 | 3.91 | <LOD | 3.94 | 1.56 | <LOD | 3.38 | |
| Metazachlor | 0–30 | 78.91 | <LOD | 1.44 | 75.78 | <LOD | 1.16 |
| 30–60 | 68.75 | <LOD | 1.29 | 78.13 | <LOD | 0.67 | |
| Metobromuron N | 0–30 | 0.00 | na | na | 8.59 | <LOD | 4.33 |
| 30–60 | 0.00 | na | na | 1.56 | <LOD | 3.21 | |
| Metribuzin | 0–30 | 12.50 | <LOD | 10.36 | 12.50 | <LOD | 14.44 |
| 30–60 | 10.94 | <LOD | 5.19 | 16.41 | <LOD | 9.74 | |
| Metsulfuron-methyl | 0–30 | 0.78 | <LOD | 26.14 | 12.8 | <LOD | 3.18 |
| 30–60 | 0.78 | <LOD | 12.64 | 3.13 | <LOD | 65.01 | |
| Napropamide | 0–30 | 99.22 | <LOD | 2.21 | 98.44 | <LOD | 2.74 |
| 30–60 | 97.66 | <LOD | 3.17 | 97.66 | <LOD | 2.72 | |
| Nicosulfuron | 0–30 | 24.22 | <LOD | 40.19 | 25.00 | <LOD | 76.57 |
| 30–60 | 22.66 | <LOD | 21.7 | 21.88 | <LOD | 46.39 | |
| Oxyfluorfen | 0–30 | 52.34 | <LOD | 0.70 | 60.16 | <LOD | 1.04 |
| 30–60 | 48.44 | <LOD | 0.69 | 53.91 | <LOD | 0.63 | |
| Pendimethalin | 0–30 | 28.13 | <LOD | 37.62 | 35.16 | <LOD | 50.78 |
| 30–60 | 27.34 | <LOD | 11.04 | 28.91 | <LOD | 30.69 | |
| Phenmedipham | 0–30 | 0.78 | <LOD | 4.66 | 0.00 | <LOD | <LOD |
| 30–60 | 0.00 | <LOD | <LOD | 0.00 | <LOD | <LOD | |
| Propyzamide | 0–30 | 61.72 | <LOD | 0.71 | 67.19 | <LOD | 3.47 |
| 30–60 | 69.53 | <LOD | 1.19 | 71.09 | <LOD | 2.34 | |
| Prosulfocarb | 0–30 | 100.00 | 0.109 | 5.58 | 98.44 | <LOD | 5.86 |
| 30–60 | 97.66 | <LOD | 2.93 | 98.44 | <LOD | 5.97 | |
| Quizalofop-ethyl | 0–30 | 0.00 | <LOD | <LOD | 0.00 | <LOD | <LOD |
| 30–60 | 0.00 | <LOD | <LOD | 0.00 | <LOD | <LOD | |
| Rimsulfuron | 0–30 | 0.00 | <LOD | <LOD | 0.00 | <LOD | <LOD |
| 30–60 | 0.00 | <LOD | <LOD | 0.00 | <LOD | <LOD | |
| s-metolachlor | 0–30 | 38.28 | <LOD | 670.20 | 35.16 | <LOD | 114.70 |
| 30–60 | 30.47 | <LOD | 329.90 | 31.25 | <LOD | 80.95 | |
| Thifensulfuron-methyl | 0–30 | 0.00 | <LOD | <LOD | 0.00 | <LOD | <LOD |
| 30–60 | 0.00 | <LOD | <LOD | 0.00 | <LOD | <LOD | |
| Triflusulfuron-methyl | 0–30 | 1.56 | <LOD | 10.07 | 3.91 | <LOD | 16.08 |
| 30–60 | 3.13 | <LOD | 2.14 | 0.00 | <LOD | <LOD | |
| Tritosulfuron | 0–30 | 0.00 | <LOD | <LOD | 0.00 | <LOD | <LOD |
| 30–60 | 0.00 | <LOD | <LOD | 0.00 | <LOD | <LOD | |
| Terbuthylazine | 0–30 | 42.97 | <LOD | 37.85 | 41.41 | <LOD | 99.03 |
| 30–60 | 35.16 | <LOD | 32.03 | 32.03 | <LOD | 36.14 | |
| Active Substances | Depth of Soil Sample | t-Test for Independent Samples | |
|---|---|---|---|
| 0–30 cm | 30–60 cm | ||
| ± SD mg a.s./kg Soil | ± SD mg a.s./kg Soil | ||
| 2,4-D-methylester | 1.305 ± 1.058 | 1.477 ± 1.351 | t = 1.065 NS; p = 0.288; d.f. = 225 |
| Amidosulfuron | – | – | – |
| Aminopyralid | 0.758 | 1.479 ± 1.014 | t = 0.615 NS; p = 0.601; d.f. = 2 |
| Bentazon | 1.939 ± 1.061 | 1.918 ± 1.081 | t = 0.160 NS; p = 0.873; d.f. = 253 |
| Clethodim | 0.453 | 0.139 ± 0.041 | t = 6.577 *; p = 0.0.22; d.f. = 2 |
| Clomazone | 11.856 ± 6.213 | 4.763 ± 2.176 | t = 1.010 NS; p = 0.316; d.f. = 74 |
| Clopyralid | – | – | – |
| Cycloxydim | – | – | – |
| Diflufenican | 2.068 | 2.315 ± 2.697 | t = 0.075 NS; p = 0.953; d.f. = 1 |
| Ethofumesate | 24.335 | 6.288 | – |
| Fenoxaprop-ethyl | 0.711 ± 0.464 | 0.234 ± 0.145 | t = 1.696 NS; p = 0.128; d.f. = 8 |
| Fluazifop-P-butyl | – | – | – |
| Flumioxazin | 0.336 ± 0.209 | 0.331 ± 0.184 | t = 0.149 NS; p = 0.882; d.f. = 177 |
| Foramsulfuron | 0.110 | 0.150 ± 0.028 | t = 1.289 NS; p = 0.288; d.f. = 3 |
| Imazamox | 1.037 ± 1.349 | 0.817 ± 0.741 | t = 0.328 NS; p = 0.750; d.f. = 10 |
| Iodosulfuron-methyl | – | – | – |
| Mesotrione | 0.251 ± 0.111 | 0.273 | t = 0.175 NS; p = 0.872; d.f. = 3 |
| Metamitron | 0.371 ± 0.266 | 1.109 ± 1.586 | t = 0.908 NS; p = 0.394; d.f. = 7 |
| Metazachlor | 0.278 ± 0.202 | 0.259 ± 0.164 | t = 0.692 NS; p = 0.489; d.f. = 187 |
| Metribuzin | 1.724 ± 2.511 | 1.074 ± 1.357 | t = 0.864 NS; p = 0.395; d.f. = 28 |
| Metsulfuron-methyl | 26.139 | 12.648 | – |
| Napropamide | 0.775 ± 0.416 | 0.811 ± 0.477 | t = 0.657 NS; p = 0.511; d.f. = 250 |
| Nicosulfuron | 4.669 ± 8.209 | 2.648 ± 4.444 | t = 1.174 NS; p = 0.245; d.f. = 58 |
| Oxyfluorfen | 0.207 ± 0.106 | 0.213 ± 0.121 | t = 0.324 NS; p = 0.746; d.f. = 127 |
| Pendimethalin | 2.658 ± 6.980 | 1.201 ± 2.558 | t = 1.161 NS; p = 0.250; d.f. = 69 |
| Phenmedipham | 4.657 ± 0.934 | – | – |
| Propyzamide | 0.215 ± 0.107 | 0.228 ± 0.202 | t = 0.522 NS; p = 0.602; d.f. = 166 |
| Prosulfocarb | 0.789 ± 0.650 | 0.772 ± 0.595 | t = 0.219 NS; p = 0.827; d.f. = 251 |
| Quizalofop-ethyl | – | – | – |
| Rimsulfuron | – | – | – |
| s-metolachlor | 29.878 ± 99.076 | 21.781 ± 54.520 | t = 0.458 NS; p = 0.648; d.f. = 86 |
| Terbuthylazine | 5.301 ± 7.459 | 4.527 ± 5.516 | t = 0.578 NS; p = 0.565; d.f. = 98 |
| Thifensulfuron-methyl | – | – | – |
| Triflusulfuron-methyl | 5.481 ± 6.488 | 1.489 ± 0.662 | t = 1.399 NS; p = 0.234; d.f. = 4 |
| Tritosulfuron | – | – | – |
| Active Substances | Depth of Soil Sample | t-Test for Independent Samples | |
|---|---|---|---|
| 0–30 cm | 30–60 cm | ||
| ± SD mg a.s./kg Soil | ± SD mg a.s./kg Soil | ||
| 2,4-D-methylester | 1.163 ± 1.114 | 0.966 ± 0.837 | t = 1.4605 NS; p = 0.146; d.f. = 221 |
| Aclonifen | 0.765 ± 1.098 | 0.588 ± 0.856 | t = 1.372 NS; p = 0.171; d.f. = 233 |
| Amidosulfuron | 0.200 | – | – |
| Aminopyralid | 0.338 ± 0.386 | – | – |
| Benfluralin | – | – | – |
| Bentazon | 2.137 ± 1.387 | 1.832 ± 1.105 | t = 1.944 NS; p = 0.053; d.f. = 252 |
| Carfentrazone-ethyl | – | – | – |
| Clethodim | 0.180 ± 0.039 | – | – |
| Clomazone | 2.672 ± 5.708 | 0.983 ± 1.260 | t = 1.616 NS; p = 0.111; d.f. = 70 |
| Clopyralid | – | 98.976 ± 114.292 | – |
| Cycloxydim | 0.129 | 0.119 | – |
| Diflufenican | 3.538 ± 0.382 | 0.208 ± 0.082 | t = 12.049 *; p = 0.0068; d.f. = 2 |
| Ethofumesate | 4.451 ± 3.370 | 4.451 ± 3.370 | t = 1.345 NS; p = 0.250; d.f. = 4 |
| Fenoxaprop-ethyl | 1.325 ± 1.302 | 1.134 | t = 0.137 NS; p = 0.895; d.f. = 6 |
| Flufenacet | – | – | – |
| Fluazifop-P-butyl | – | – | – |
| Flumioxazin | 0.361 ± 0.264 | 0.264 ± 0.176 | t = 3.924 **; p = 0.0001; d.f. = 193 |
| Foramsulfuron | 0.273 ± 0.137 | – | – |
| Imazamox | 2.292 ± 2.942 | – | – |
| Iodosulfuron-methyl | – | – | – |
| Lenacil | 4.138 ± 7.828 | – | – |
| Mesotrione | 4.549 ± 9.579 | 0.303 ± 0.127 | t = 0.595 NS; p = 0.574; d.f. = 6 |
| Metabromuron | 1.177 ± 1.345 | 3.283 ± 0.729 | t = 3.588 **; p = 0.0043; d.f. = 11 |
| Metamitron | 4.276 ± 4.126 | 0.893 ± 1.063 | t = 3.546 **; p = 0.0011 *; d.f. = 37 |
| Metazachlor | 0.264 ± 0.131 | 0.238 ± 0.140 | t = 1.317 NS; p = 0.189; d.f. = 198 |
| Metribuzin | 4.694 ± 4.169 | 0.893 ± 1.063 | t = 3.934 ***; p = 0.0004; d.f. = 35 |
| Metsulfuron-methyl | 3.182 | 25.205 ± 34.489 | t = 0.553 NS; p = 0.636; d.f. = 2 |
| Napropamide | 0.746 ± 0.462 | 0.903 ± 0.491 | t = 2.602 **; p = 0.009; d.f. = 249 |
| Nicosulfuron | 6.042 ± 14.914 | 0.429 ± 0.439 | t = 1.676 NS; p = 0.099; d.f. = 40 |
| Oxyfluorfen | 0.226 ± 0.127 | 0.258 ± 0.167 | t = 1.318 NS; p = 0.190; d.f. = 144 |
| Pendimethalin | 3.319 ± 8.637 | 4.078 ± 9.020 | t = 0.377 NS; p = 0.707; d.f. = 80 |
| Phenmedipham | – | – | – |
| Propyzamide | 0.376 ± 0.553 | 0.290 ± 0.320 | t = 1.285 NS; p = 0.200; d.f. = 175 |
| Prosulfocarb | 0.742 ± 0.484 | 0.843 ± 0.812 | t = 1.197 NS; p = 0.232; d.f. = 250 |
| Quizalofop-ethyl | – | – | – |
| Rimsulfuron | – | – | – |
| s-metolachlor | 17.928 ± 26.503 | 14.306 ± 14.539 | t = 0.511 NS; p = 0.610; d.f. = 83 |
| Terbuthylazine | 8.666 ± 14.685 | 2.981 ± 2.452 | t = 2.202 *; p = 0.030; d.f. = 93 |
| Thifensulfuron-methyl | – | – | – |
| Triflusulfuron-methyl | 4.901 ± 6.065 | – | – |
| Tritosulfuron | – | – | – |
| Soil Properties 2013 | Max | Min | Average ± SD | CoV | LQ | UQ |
|---|---|---|---|---|---|---|
| pH in KCl | 8.53 | 4.78 | 7.29 ± 0.6 | 0.09 | 7.27 | 7.57 |
| pH in water | 9.79 | 5.80 | 8.23 ± 0.51 | 0.06 | 8.13 | 8.41 |
| CaCO3 (%) | 31.98 | 0.27 | 11.86 ± 7.85 | 0.66 | 5.01 | 17.89 |
| Organic matter (%) | 8.41 | 0.29 | 3.32 ± 1.11 | 0.34 | 2.64 | 4.00 |
| CEC (T) | 68.50 | 1.50 | 24.82 ± 7.46 | 0.30 | 20.75 | 28.44 |
| Mechanical composition | ||||||
| Coarse sand | 37.0 | 0.10 | 1.99 ± 3.71 | 1.87 | 0.50 | 2.00 |
| Fine sand | 84.20 | 8.60 | 36.42 ± 11.55 | 0.32 | 28.53 | 41.78 |
| Powder | 55.60 | 5.00 | 31.73 ± 7.62 | 0.24 | 28.63 | 36.00 |
| Clay | 59.10 | 6.50 | 29.86 ± 8.52 | 0.29 | 24.53 | 35.33 |
| Soil Properties 2023 | Max | Min | Average ± SD | CoV | LQ | UQ |
| pH in KCl | 8.28 | 4.46 | 7.26 ± 0.68 | 0.09 | 7.23 | 7.62 |
| pH in water | 9.27 | 6.06 | 8.18 ± 0.54 | 0.07 | 8.14 | 8.43 |
| CaCO3 (%) | 39.86 | 0.09 | 12.37 ± 8.36 | 0.68 | 3.94 | 18.23 |
| Organic matter (%) | 7.98 | 0.84 | 3.38 ± 1.18 | 0.35 | 2.55 | 3.96 |
| CEC (T) | 56.75 | 1.00 | 24.55 ± 8.13 | 0.33 | 19.44 | 29.19 |
| Mechanical composition | ||||||
| Coarse sand | 23.20 | 0.10 | 1.72 ± 2.69 | 1.56 | 0.50 | 1.70 |
| Fine sand | 81.40 | 9.60 | 37.42 ± 12.86 | 0.34 | 29.65 | 44.88 |
| Powder | 63.20 | 6.10 | 31.86 ± 7.90 | 0.25 | 28.18 | 36.43 |
| Clay | 56.00 | 5.40 | 29.01 ± 8.93 | 0.31 | 23.40 | 33.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šunjka, D.; Pucarević, M.; Lazić, S.; Stojić, N.; Milošević, L.; El Bilali, H.; Bošković, D.; Vuković, S.; Mitrić, S.; Berjan, S.; et al. Monitoring of Herbicide Residues in Agricultural Soils in Vojvodina Province (Northern Serbia). Land 2024, 13, 1347. https://doi.org/10.3390/land13091347
Šunjka D, Pucarević M, Lazić S, Stojić N, Milošević L, El Bilali H, Bošković D, Vuković S, Mitrić S, Berjan S, et al. Monitoring of Herbicide Residues in Agricultural Soils in Vojvodina Province (Northern Serbia). Land. 2024; 13(9):1347. https://doi.org/10.3390/land13091347
Chicago/Turabian StyleŠunjka, Dragana, Mira Pucarević, Sanja Lazić, Nataša Stojić, Ljiljana Milošević, Hamid El Bilali, Dragana Bošković, Slavica Vuković, Siniša Mitrić, Siniša Berjan, and et al. 2024. "Monitoring of Herbicide Residues in Agricultural Soils in Vojvodina Province (Northern Serbia)" Land 13, no. 9: 1347. https://doi.org/10.3390/land13091347









