In Silico Investigation on the Interaction of Chiral Phytochemicals from Opuntia ficus-indica with SARS-CoV-2 Mpro
Abstract
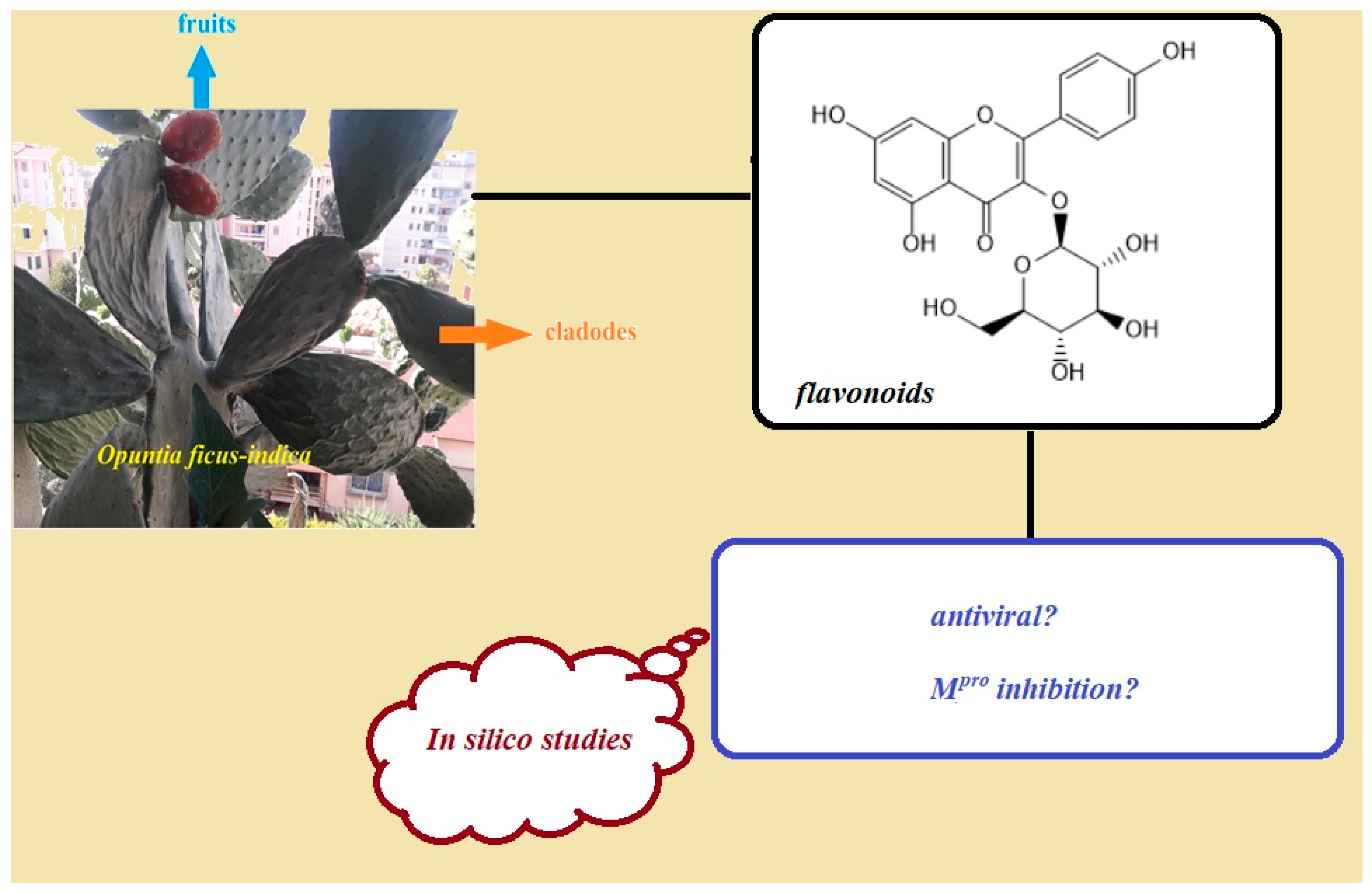
:1. Introduction
2. Methods
2.1. Molecular Docking Studies
2.2. COVID-19 Docking Server Studies
2.3. LogP, Druglikeness, PAINS, and Toxicity Predictions
3. Results and Discussion

| Compound Name | Mpro BE of Top Scoring Pose | Mpro BE-Average Score | Standard Deviation | Reference on Compound Detection in Opuntia spp. |
|---|---|---|---|---|
| sinapic acid | −6.1 | −5.7 | 0.2 | Elkadi et al. [32] |
| ferulic acid | −5.7 | −5.65 | 0.05 | Guevara-Figueroa et al. [63] |
| ferulic acid hexoside | −6.8 | −6.625 | 0.178 | Elkadi et al. [32] |
| 3-O-caffeoyl quinic acid | −7.1 | −6.925 | 0.249 | Elkadi et al. [32] |
| quinic acid | −5.7 | −5.25 | 0.36 | Ammar et al. [64] |
| caffeic acid | −5.7 | −5.625 | 0.083 | Ammar et al. [64] |
| isorhamnetin | −7.3 | −6.625 | 0.536 | Elkadi et al. [32] |
| isorhamnetin 3-O-glucoside | −7.5 | −6.75 | 0.46 | Guevara-Figueroa et al. [63] |
| quercetin 5,4′-dimethyl ether | −7.3 | −6.375 | 0.683 | Elkadi et al. [32] |
| astragalin | −7.9 | −7.2 | 0.4 | Elkadi et al. [32] |
| N3 inhibitor [61] | −7.6 | Bharadwaj et al. [61] |



| Pose n. | RF-Score (pKd) | Score Value (Kcal/mol) |
|---|---|---|
| 1 | 6.03 | −8.70 |
| 2 | 5.95 | −8.40 |
| 3 | 5.51 | −7.60 |
| 4 | 5.40 | −7.40 |
| 5 | 5.93 | −7.40 |
| 6 | 5.68 | −7.30 |
| 7 | 5.57 | −7.00 |
| 8 | 5.52 | −6.90 |
| 9 | 5.34 | −6.90 |
| 10 | 5.48 | −6.80 |


| Human Hepatotoxicity (H-HT) | Skin Sensitization | Ames Mutagenicity (Ames) | |
|---|---|---|---|
| Category * | 0 | 0 | 0 |
| Probability | 0.032 | 0.26 | 0.48 |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Makhdoom, R.; El-Sherif, G.; Hijazi, M.A. Effect of Enzymatic Treatment on Polyphenolics Content in Prickly Pear (Opuntia ficus indica) Juice. Life Sci. J. 2016, 13, 88–93. [Google Scholar]
- Erre, P.; Chessa, I.; Nieddu, G.; Jones, P.G. Diversity and spatial distribution of Opuntia spp. in the Mediterranean Basin. J. Arid Environ. 2009, 73, 1058–1066. [Google Scholar] [CrossRef]
- Kim, J.-H.; Park, S.-M.; Ha, H.-J.; Moon, C.-J.; Shin, T.-K.; Kim, J.-M.; Lee, N.-H.; Kim, H.-C.; Jang, K.-J.; Wie, M.-B. Opuntia ficus-indica attenuates neuronal injury in in vitro and in vivo models of cerebral ischemia. J. Ethnopharmacol. 2006, 104, 257–262. [Google Scholar] [CrossRef]
- El-Mostafa, K.; El Kharrassi, Y.; Badreddine, A.; Andreoletti, P.; Vamecq, J.; El Kebbaj, M.; Latruffe, N.; Lizard, G.; Nasser, B.; Cherkaoui-Malki, M. Nopal cactus (Opuntia ficus-indica) as a source of bioactive compounds for nutrition, health and disease. Molecules 2014, 19, 14879–14901. [Google Scholar] [CrossRef] [Green Version]
- Aragona, M.; Lauriano, E.; Pergolizzi, S.; Faggio, C. Opuntia ficus-indica (L.) Miller as a source of bioactivity compounds for health and nutrition. Nat. Prod. Res. 2018, 32, 2037–2049. [Google Scholar] [CrossRef]
- Hernández-Urbiola, M.I.; Pérez-Torrero, E.; Rodríguez-García, M.E. Chemical analysis of nutritional content of prickly pads (Opuntia ficus indica) at varied ages in an organic harvest. Int. J. Environ. Res. Public Health 2011, 8, 1287–1295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inglese, P.; Barbera, G.; La Mantia, T. Research strategies for the improvement of cactuspear (Opuntia ficus-indica) fruit quality and production. J. Arid Environ. 1995, 29, 455–468. [Google Scholar] [CrossRef]
- Snyman, H.A. Growth rate and water-use efficiency of cactus pears Opuntia ficus-indica and O. robusta. Arid Land Res. Manag. 2013, 27, 337–348. [Google Scholar] [CrossRef]
- Comparettia, A.; Feboa, P.; Grecoa, C.; Mammanob, M.M.; Orlandoa, S. Potential production of biogas from prinkly pear (Opuntia ficus-indica L.) in Sicilian uncultivated areas. Chem. Eng. 2017, 58. [Google Scholar] [CrossRef]
- do Nascimento Santos, T.; Dutra, E.D.; do Prado, A.G.; Leite, F.C.B.; de Souza, R.d.F.R.; dos Santos, D.C.; de Abreu, C.A.M.; Simões, D.A.; de Morais, M.A., Jr.; Menezes, R.S.C. Potential for biofuels from the biomass of prickly pear cladodes: Challenges for bioethanol and biogas production in dry areas. Biomass Bioenergy 2016, 85, 215–222. [Google Scholar] [CrossRef]
- Neffar, S.; Chenchouni, H.; Beddiar, A.; Redjel, N. Rehabilitation of degraded rangeland in drylands by prickly pear (Opuntia ficus-indica L.) plantations: Effect on soil and spontaneous vegetation. Ecol. Balk. 2013, 5, 63–76. [Google Scholar]
- Nefzaoui, A.; Louhaichi, M.; Ben Salem, H. Cactus as a tool to mitigate drought and to combat desertification. J. Arid Land Stud. 2014, 24, 121–124. [Google Scholar]
- Bautista-Cruz, A.; Leyva-Pablo, T.; de León-González, F.; Zornoza, R.; Martínez-Gallegos, V.; Fuentes-Ponce, M.; Rodríguez-Sánchez, L. Cultivation of Opuntia ficus-indica under different soil management practices: A possible sustainable agricultural system to promote soil carbon sequestration and increase soil microbial biomass and activity. Land Degrad. Dev. 2018, 29, 38–46. [Google Scholar] [CrossRef] [Green Version]
- Ayadi, M.; Abdelmaksoud, W.; Ennouri, M.; Attia, H. Cladodes from Opuntia ficus indica as a source of dietary fiber: Effect on dough characteristics and cake making. Ind. Crop. Prod. 2009, 30, 40–47. [Google Scholar] [CrossRef]
- Barbera, G.; Carimi, F.; Inglese, P. Past and present role of the Indian-fig prickly-pear (Opuntia ficus-indica (L.) Miller, Cactaceae) in the agriculture of Sicily. Econ. Bot. 1992, 46, 10–20. [Google Scholar] [CrossRef]
- Barba, F.J.; Garcia, C.; Fessard, A.; Munekata, P.E.; Lorenzo, J.M.; Aboudia, A.; Ouadia, A.; Remize, F. Opuntia Ficus Indica Edible Parts: A Food and Nutritional Security Perspective. Food Rev. Int. 2020, 1–23. [Google Scholar] [CrossRef]
- Costa, R.G.; Beltrão Filho, E.M.; de Medeiros, A.N.; Givisiez, P.E.N.; do Egypto, R.d.C.R.; Melo, A.A.S. Effects of increasing levels of cactus pear (Opuntia ficus-indica L. Miller) in the diet of dairy goats and its contribution as a source of water. Small Rumin. Res. 2009, 82, 62–65. [Google Scholar] [CrossRef]
- Gebretsadik, G.; Animut, G.; Tegegne, F. Assessment of the potential of cactus pear (Opuntia ficus indica) as livestock feed in Northern Ethiopia. Livest. Res. Rural Dev. 2013, 25, 1–10. [Google Scholar]
- Kaur, M.; Kaur, A.; Sharma, R. Pharmacological actions of Opuntia ficus indica: A Review. J. Appl. Pharm. Sci. 2012, 2, 15–18. [Google Scholar] [CrossRef] [Green Version]
- Andreu, L.; Nuncio-Jáuregui, N.; Carbonell-Barrachina, Á.A.; Legua, P.; Hernández, F. Antioxidant properties and chemical characterization of Spanish Opuntia ficus—Indica Mill. cladodes and fruits. J. Sci. Food Agric. 2018, 98, 1566–1573. [Google Scholar] [CrossRef]
- Butera, D.; Tesoriere, L.; Di Gaudio, F.; Bongiorno, A.; Allegra, M.; Pintaudi, A.M.; Kohen, R.; Livrea, M.A. Antioxidant activities of Sicilian prickly pear (Opuntia ficus indica) fruit extracts and reducing properties of its betalains: Betanin and indicaxanthin. J. Agric. Food Chem. 2002, 50, 6895–6901. [Google Scholar] [CrossRef] [Green Version]
- Benayad, Z.; Martinez-Villaluenga, C.; Frias, J.; Gomez-Cordoves, C.; Es-Safi, N.E. Phenolic composition, antioxidant and anti-inflammatory activities of extracts from Moroccan Opuntia ficus-indica flowers obtained by different extraction methods. Ind. Crop. Prod. 2014, 62, 412–420. [Google Scholar] [CrossRef] [Green Version]
- Antunes-Ricardo, M.; Gutiérrez-Uribe, J.A.; Martínez-Vitela, C.; Serna-Saldívar, S.O. Topical anti-inflammatory effects of isorhamnetin glycosides isolated from Opuntia ficus-indica. BioMed Res. Int. 2015, 2015, 847320. [Google Scholar] [CrossRef] [Green Version]
- Ammar, I.; Salem, M.B.; Harrabi, B.; Mzid, M.; Bardaa, S.; Sahnoun, Z.; Attia, H.; Ennouri, M. Anti-inflammatory activity and phenolic composition of prickly pear (Opuntia ficus-indica) flowers. Ind. Crop. Prod. 2018, 112, 313–319. [Google Scholar] [CrossRef]
- Frati, A.C.; Jiménez, E.; Ariza, C.R. Hypoglycemic effect of Opuntia ficus indica in non insulin—Dependent diabetes mellitus patients. Phytother. Res. 1990, 4, 195–197. [Google Scholar] [CrossRef]
- Leem, K.-H.; Kim, M.-G.; Hahm, Y.-T.; Kim, H.K. Hypoglycemic effect of Opuntia ficus-indica var. saboten is due to enhanced peripheral glucose uptake through activation of AMPK/p38 MAPK pathway. Nutrients 2016, 8, 800. [Google Scholar] [CrossRef]
- Elshehy, H.; Salah, S.; Abdel-Mawla, E.; Agamy, N. Protective Effect of Opuntia ficus-indica against Diabetes in Alloxan-induced Diabetic Rats. Can. J. Clin. Nutr. 2020, 8, 20–34. [Google Scholar] [CrossRef]
- Yao, X.; Hu, H.; Qin, Y.; Liu, J. Development of antioxidant, antimicrobial and ammonia-sensitive films based on quaternary ammonium chitosan, polyvinyl alcohol and betalains-rich cactus pears (Opuntia ficus-indica) extract. Food Hydrocoll. 2020, 106, 105896. [Google Scholar] [CrossRef]
- El-Hawary, S.S.; Sobeh, M.; Badr, W.K.; Abdelfattah, M.A.; Ali, Z.Y.; El-Tantawy, M.E.; Rabeh, M.A.; Wink, M. HPLC-PDA-MS/MS profiling of secondary metabolites from Opuntia ficus-indica cladode, peel and fruit pulp extracts and their antioxidant, neuroprotective effect in rats with aluminum chloride induced neurotoxicity. Saudi J. Biol. Sci. 2020, 27, 2829–2838. [Google Scholar] [CrossRef]
- Rasoulpour, R.; Izadpanah, K.; Afsharifar, A. Opuntin B, the antiviral protein isolated from prickly pear (Opuntia ficus-indica (L.) Miller) cladode exhibits ribonuclease activity. Microb. Pathog. 2020, 140, 103929. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, S.; Sheth, N.; Jivani, N.; Rathod, I.; Shah, P. Biological actions of Opuntia species. Syst. Rev. Pharm. 2010, 1, 146. [Google Scholar] [CrossRef]
- Elkady, W.M.; Bishr, M.M.; Abdel-Aziz, M.M.; Salama, O.M. Identification and isolation of anti-pneumonia bioactive compounds from Opuntia ficus-indica fruit waste peels. Food Funct. 2020, 11, 5275–5283. [Google Scholar] [CrossRef]
- Roviello, V.; Roviello, G.N. Lower COVID-19 mortality in Italian forested areas suggests immunoprotection by Mediterranean plants. Environ. Chem. Lett. 2020. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, M.; De Giglio, M.A.R.; Roviello, G.N. Anti-Coronavirus Vaccines: Past Investigations on SARS-CoV-1 and MERS-CoV, the Approved Vaccines from BioNTech/Pfizer, Moderna, Oxford/AstraZeneca and others under Development Against SARS-CoV-2 Infection. Curr. Med. Chem. 2021, 28. [Google Scholar] [CrossRef]
- Costanzo, M.; De Giglio, M.A.R.; Roviello, G.N. SARS CoV-2: Recent Reports on Antiviral Therapies Based on Lopinavir/Ritonavir, Darunavir/Umifenovir, Hydroxychloroquine, Remdesivir, Favipiravir and Other Drugs for the Treatment of the New Coronavirus. Curr. Med. Chem. 2020, 27. [Google Scholar] [CrossRef] [PubMed]
- Borbone, N.; Piccialli, G.; Roviello, G.N.; Oliviero, G. Nucleoside Analogs and Nucleoside Precursors as Drugs in the Fight against SARS-CoV-2 and Other Coronaviruses. Molecules 2021, 26, 986. [Google Scholar] [CrossRef]
- Yang, Y.; Islam, M.S.; Wang, J.; Li, Y.; Chen, X. Traditional Chinese medicine in the treatment of patients infected with 2019-new coronavirus (SARS-CoV-2): A review and perspective. Int. J. Biol. Sci. 2020, 16, 1708. [Google Scholar] [CrossRef]
- Vicidomini, C.; Roviello, V.; Roviello, G.N. Molecular Basis of the Therapeutical Potential of Clove (Syzygium aromaticum L.) and Clues to Its Anti-COVID-19 Utility. Molecules 2021, 26, 1880. [Google Scholar] [CrossRef]
- Bacha, U.; Barrila, J.; Velazquez-Campoy, A.; Leavitt, S.A.; Freire, E. Identification of novel inhibitors of the SARS coronavirus main protease 3CLpro. Biochemistry 2004, 43, 4906–4912. [Google Scholar] [CrossRef]
- Berman, H.M.; Battistuz, T.; Bhat, T.N.; Bluhm, W.F.; Bourne, P.E.; Burkhardt, K.; Feng, Z.; Gilliland, G.L.; Iype, L.; Jain, S.; et al. The Protein Data Bank. Acta Crystallogr. Sect. D Biol. Crystallogr. 2002, 58, 899–907. [Google Scholar] [CrossRef]
- Potemkin, V.; Potemkin, A.; Grishina, M. Internet Resources for Drug Discovery and Design. Curr. Top. Med. Chem. 2019, 18, 1955–1975. [Google Scholar] [CrossRef] [PubMed]
- Fik-Jaskółka, M.A.; Mkrtchyan, A.F.; Saghyan, A.S.; Palumbo, R.; Belter, A.; Hayriyan, L.A.; Simonyan, H.; Roviello, V.; Roviello, G.N. Spectroscopic and SEM evidences for G4-DNA binding by a synthetic alkyne-containing amino acid with anticancer activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 229, 117884. [Google Scholar] [CrossRef]
- Fik-Jaskółka, M.A.; Mkrtchyan, A.F.; Saghyan, A.S.; Palumbo, R.; Belter, A.; Hayriyan, L.A.; Simonyan, H.; Roviello, V.; Roviello, G.N. Biological macromolecule binding and anticancer activity of synthetic alkyne-containing l-phenylalanine derivatives. Amino Acids 2020, 52, 755–769. [Google Scholar] [CrossRef]
- Kiss, R.; Sandor, M.; Szalai, F.A. http://Mcule.com: A public web service for drug discovery. J. Cheminform. 2012, 4. [Google Scholar] [CrossRef] [Green Version]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2009. [Google Scholar] [CrossRef] [Green Version]
- Umesh, K.D.; Selvaraj, C.; Singh, S.K.; Dubey, V.K. Identification of new anti-nCoV drug chemical compounds from Indian spices exploiting SARS-CoV-2 main protease as target. J. Biomol. Struct. Dyn. 2020, 1–9. [Google Scholar] [CrossRef]
- Roviello, V.; Musumeci, D.; Mokhir, A.; Roviello, G.N. Evidence of protein binding by a nucleopeptide based on a thymine-decorated L-diaminopropanoic acid through CD and in silico studies. Curr. Med. Chem. 2021, 28. [Google Scholar] [CrossRef]
- Chakraborti, S.; Bheemireddy, S.; Srinivasan, N. Repurposing drugs against main protease of SARS-CoV-2: Mechanism-based insights supported by available laboratory and clinical data. Mol. Omics 2020. [Google Scholar] [CrossRef]
- Rarey, M.; Steinegger, R.; Nittinger, E.; Meyder, A.; Flachsenberg, F.; Fährrolfes, R.; Diedrich, K.; Schöning-Stierand, K. ProteinsPlus: Interactive analysis of protein–ligand binding interfaces. Nucleic Acids Res. 2020. [Google Scholar] [CrossRef] [Green Version]
- Adasme, M.F.; Linnemann, K.L.; Bolz, S.N.; Kaiser, F.; Salentin, S.; Haupt, V.J.; Schroeder, M. PLIP 2021: Expanding the scope of the protein–ligand interaction profiler to DNA and RNA. Nucleic Acids Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera?A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [Green Version]
- Kong, R.; Yang, G.; Xue, R.; Liu, M.; Wang, F.; Hu, J.; Guo, X.; Chang, S.; Ponty, Y. COVID-19 Docking Server: A meta server for docking small molecules, peptides and antibodies against potential targets of COVID-19. Bioinformatics 2020. [Google Scholar] [CrossRef]
- Kong, R.; Wang, F.; Zhang, J.; Wang, F.; Chang, S. CoDockPP: A Multistage Approach for Global and Site-Specific Protein–Protein Docking. J. Chem. Inf. Modeling 2019, 59, 3556–3564. [Google Scholar] [CrossRef]
- Li, H.; Leung, K.-S.; Wong, M.-H.; Ballester, P.J. Correcting the impact of docking pose generation error on binding affinity prediction. BMC Bioinform. 2016, 17. [Google Scholar] [CrossRef] [Green Version]
- Rivero-Segura, N.A.; Gomez-Verjan, J.C. In Silico Screening of Natural Products Isolated from Mexican Herbal Medicines against COVID-19. Biomolecules 2021, 11, 216. [Google Scholar] [CrossRef]
- Jin, Z.; Du, X.; Xu, Y.; Deng, Y.; Liu, M.; Zhao, Y.; Zhang, B.; Li, X.; Zhang, L.; Peng, C.; et al. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature 2020. [Google Scholar] [CrossRef] [Green Version]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Baell, J.B.; Holloway, G.A. New substructure filters for removal of pan assay interference compounds (PAINS) from screening libraries and for their exclusion in bioassays. J. Med. Chem. 2010, 53, 2719–2740. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Lin, D.; Sun, X.; Curth, U.; Drosten, C.; Sauerhering, L.; Becker, S.; Rox, K.; Hilgenfeld, R. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science 2020, eabb3405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mangum, E.M.; Graham, K.K. Lopinavir-Ritonavir: A New Protease Inhibitor. Pharmacotherapy 2001, 21, 1352–1363. [Google Scholar] [CrossRef]
- Bharadwaj, S.; Azhar, E.I.; Kamal, M.A.; Bajrai, L.H.; Dubey, A.; Jha, K.; Yadava, U.; Kang, S.G.; Dwivedi, V.D. SARS-CoV-2 Mpro inhibitors: Identification of anti-SARS-CoV-2 Mpro compounds from FDA approved drugs. J. Biomol. Struct. Dyn. 2020, 1–16. [Google Scholar] [CrossRef]
- Pojskic, L. Screening of preferential binding affinity of selected natural compounds to SARS-CoV-2 proteins using in silico methods. Eurasian J. Med. Oncol. 2020. [Google Scholar] [CrossRef]
- Guevara-Figueroa, T.; Jiménez-Islas, H.; Reyes-Escogido, M.L.; Mortensen, A.G.; Laursen, B.B.; Lin, L.-W.; De León-Rodríguez, A.; Fomsgaard, I.S.; de la Rosa, A.P.B. Proximate composition, phenolic acids, and flavonoids characterization of commercial and wild nopal (Opuntia spp.). J. Food Compos. Anal. 2010, 23, 525–532. [Google Scholar] [CrossRef]
- Ammar, I.; Ennouri, M.; Bouaziz, M.; Amira, A.B.; Attia, H. Phenolic profiles, phytchemicals and mineral content of decoction and infusion of Opuntia ficus-indica flowers. Plant Foods Hum. Nutr. 2015, 70, 388–394. [Google Scholar] [CrossRef]
- Banerjee, R.; Perera, L.; Tillekeratne, L.M.V. Potential SARS-CoV-2 main protease inhibitors. Drug Discov. Today 2020. [Google Scholar] [CrossRef]
- Singh, E.; Khan, R.J.; Jha, R.K.; Amera, G.M.; Jain, M.; Singh, R.P.; Muthukumaran, J.; Singh, A.K. A comprehensive review on promising anti-viral therapeutic candidates identified against main protease from SARS-CoV-2 through various computational methods. J. Genet. Eng. Biotechnol. 2020, 18, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sajid Jamal, O.M.; Alharbi, A.H.; Ahmad, V. Identification of doxorubicin as a potential therapeutic against SARS-CoV-2 (COVID-19) protease: A molecular docking and dynamics simulation studies. J. Biomol. Struct. Dyn. 2021, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Tariq, A.; Mateen, R.M.; Afzal, M.S.; Saleem, M. Paromomycin: A potential dual targeted drug effectively inhibits both spike (S1) and main protease of COVID-19. Int. J. Infect. Dis. 2020, 98, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Glaser, J.; Holzgrabe, U. Focus on PAINS: False friends in the quest for selective anti-protozoal lead structures from Nature? MedChemComm 2016, 7, 214–223. [Google Scholar] [CrossRef]
- Singab, A.N.B.; Youssef, D.T.; Noaman, E.; Kotb, S. Hepatoprotective effect of flavonol glycosides rich fraction from egyptianVicia calcarata desf. Against CCI 4-induced liver damage in rats. Arch. Pharmacal Res. 2005, 28, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.-K.; Lin, W.-N. Quercetin 5, 4′-dimethyl Ether from Rhododendron ellipticum. Phytochemistry 1995, 39, 463–464. [Google Scholar]
- The China Medicinal Materials Group. Main Record of Resource of Chinese Material Medicine in China; Science Press: Beijing, Chain, 1994; p. 892. [Google Scholar]
- Lambert, H.; Mohan, N.; Lee, T.-C. Ultrahigh binding affinity of a hydrocarbon guest inside cucurbit [7] uril enhanced by strong host–guest charge matching. Phys. Chem. Chem. Phys. 2019, 21, 14521–14529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vicidomini, C.; Roviello, V.; Roviello, G.N. In Silico Investigation on the Interaction of Chiral Phytochemicals from Opuntia ficus-indica with SARS-CoV-2 Mpro. Symmetry 2021, 13, 1041. https://doi.org/10.3390/sym13061041
Vicidomini C, Roviello V, Roviello GN. In Silico Investigation on the Interaction of Chiral Phytochemicals from Opuntia ficus-indica with SARS-CoV-2 Mpro. Symmetry. 2021; 13(6):1041. https://doi.org/10.3390/sym13061041
Chicago/Turabian StyleVicidomini, Caterina, Valentina Roviello, and Giovanni N. Roviello. 2021. "In Silico Investigation on the Interaction of Chiral Phytochemicals from Opuntia ficus-indica with SARS-CoV-2 Mpro" Symmetry 13, no. 6: 1041. https://doi.org/10.3390/sym13061041
APA StyleVicidomini, C., Roviello, V., & Roviello, G. N. (2021). In Silico Investigation on the Interaction of Chiral Phytochemicals from Opuntia ficus-indica with SARS-CoV-2 Mpro. Symmetry, 13(6), 1041. https://doi.org/10.3390/sym13061041








