New Species Can Broaden Myelin Research: Suitability of Little Skate, Leucoraja erinacea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tissue Preparation
2.2. Light and Electron Microscopy
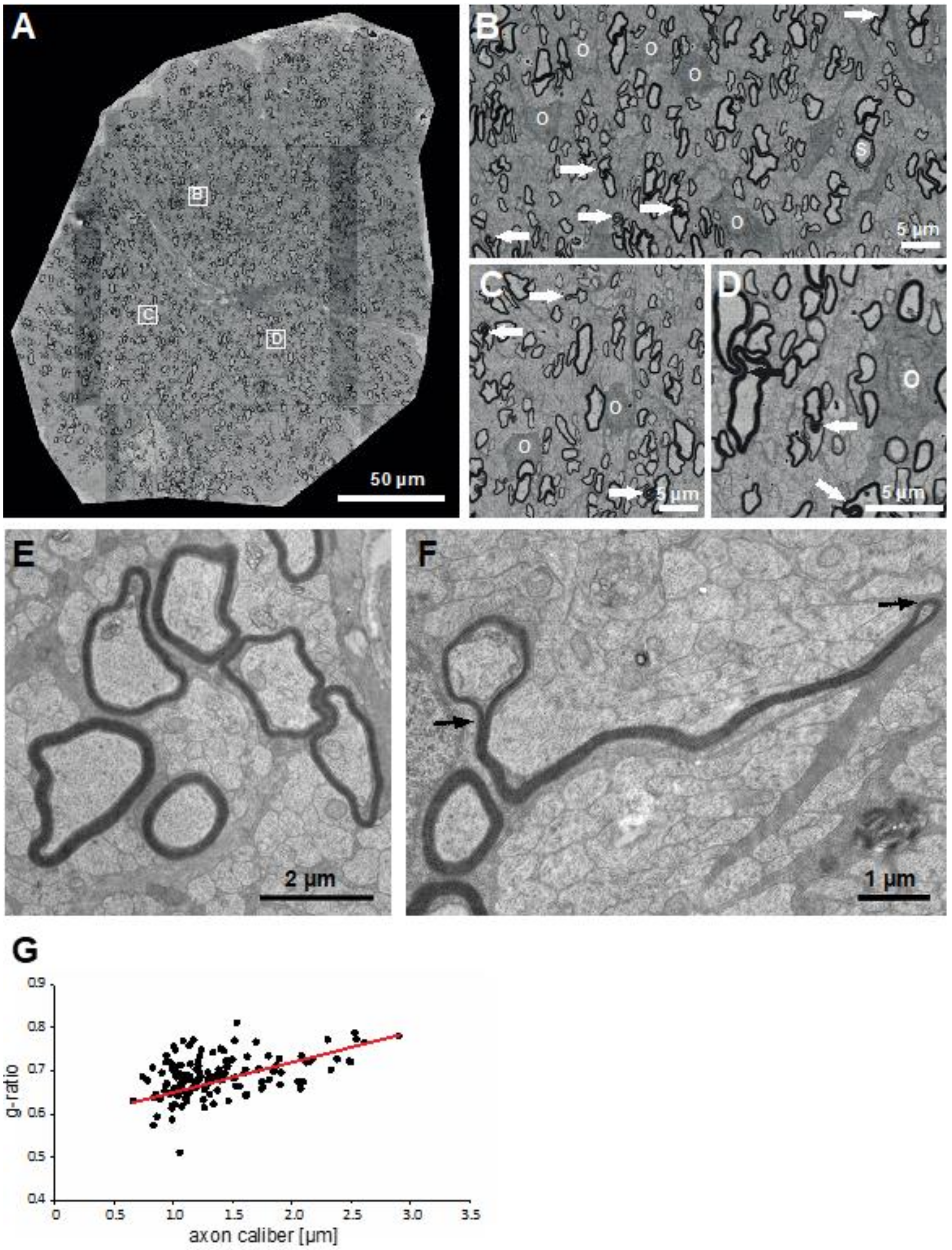
2.3. G-ratio Measurements
3. Results
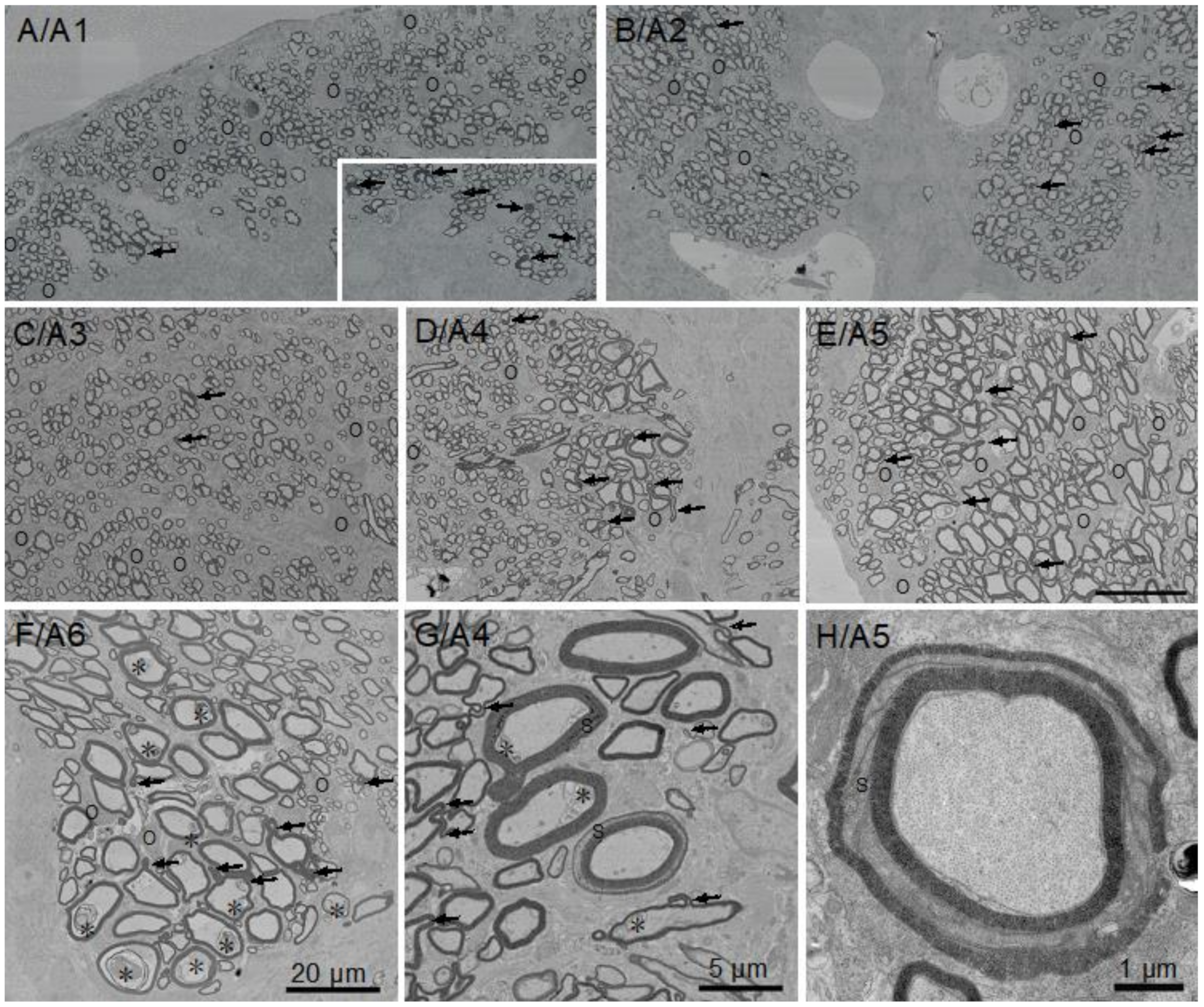
3.1. Little Skate Hatchling Spinal Cord
3.2. Myelin Thickness in Fibers in Little Skate Hatchling Spinal Cord
3.3. Ultrastructural Features of Little Skate Spinal Cord
3.4. Structural and Ultrastructural Properties of Little Skate Optic Nerve
3.5. Structural Features of a Ventral Root
4. Discussion
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Preston, M.A.; Macklin, W.B. Zebrafish as a model to investigate CNS myelination. Glia 2015, 63, 177–193. [Google Scholar] [CrossRef] [Green Version]
- Baraban, M.; Mensch, S.; Lyons, D.A. Adaptive myelination from fish to man. Brain Res. 2015. [Google Scholar] [CrossRef] [Green Version]
- Lyons, D.A.; Talbot, W.S. Glial cell development and function in zebrafish. Cold Spring Harb. Perspect. Biol. 2015, 7, a020586. [Google Scholar] [CrossRef]
- Ackerman, S.D.; Monk, K.R. The scales and tales of myelination: Using zebrafish and mouse to study myelinating glia. Brain Res. 2015. [Google Scholar] [CrossRef] [Green Version]
- Wyffels, J.; King, B.L.; Vincent, J.; Chen, C.; Wu, C.H.; Polson, S.W. SkateBase, an elasmobranch genome project and collection of molecular resources for chondrichthyan fishes. F1000Research 2014, 3, 191. [Google Scholar] [CrossRef]
- Hara, Y.; Yamaguchi, K.; Onimaru, K.; Kadota, M.; Koyanagi, M.; Keeley, S.D.; Tatsumi, K.; Tanaka, K.; Motone, F.; Kageyama, Y.; et al. Shark genomes provide insights into elasmobranch evolution and the origin of vertebrates. Nat. Ecol. Evol. 2018, 2, 1761–1771. [Google Scholar] [CrossRef] [Green Version]
- Venkatesh, B.; Lee, A.P.; Ravi, V.; Maurya, A.K.; Lian, M.M.; Swann, J.B.; Ohta, Y.; Flajnik, M.F.; Sutoh, Y.; Kasahara, M.; et al. Elephant shark genome provides unique insights into gnathostome evolution. Nature 2014, 505, 174–179. [Google Scholar] [CrossRef] [Green Version]
- Read, T.D.; Petit, R.A., 3rd; Joseph, S.J.; Alam, M.T.; Weil, M.R.; Ahmad, M.; Bhimani, R.; Vuong, J.S.; Haase, C.P.; Webb, D.H.; et al. Draft sequencing and assembly of the genome of the world’s largest fish, the whale shark: Rhincodon typus Smith 1828. BMC Genom. 2017, 18, 532. [Google Scholar] [CrossRef]
- Braasch, I.; Gehrke, A.R.; Smith, J.J.; Kawasaki, K.; Manousaki, T.; Pasquier, J.; Amores, A.; Desvignes, T.; Batzel, P.; Catchen, J.; et al. The spotted gar genome illuminates vertebrate evolution and facilitates human-teleost comparisons. Nat. Genet. 2016, 48, 427–437. [Google Scholar] [CrossRef] [Green Version]
- Amemiya, C.T.; Alfoldi, J.; Lee, A.P.; Fan, S.; Philippe, H.; Maccallum, I.; Braasch, I.; Manousaki, T.; Schneider, I.; Rohner, N.; et al. The African coelacanth genome provides insights into tetrapod evolution. Nature 2013, 496, 311–316. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.J.; Kuraku, S.; Holt, C.; Sauka-Spengler, T.; Jiang, N.; Campbell, M.S.; Yandell, M.D.; Manousaki, T.; Meyer, A.; Bloom, O.E.; et al. Sequencing of the sea lamprey (Petromyzon marinus) genome provides insights into vertebrate evolution. Nat. Genet. 2013, 45, 415–421. [Google Scholar] [CrossRef]
- Smith, J.J.; Timoshevskaya, N.; Ye, C.; Holt, C.; Keinath, M.C.; Parker, H.J.; Cook, M.E.; Hess, J.E.; Narum, S.R.; Lamanna, F.; et al. The sea lamprey germline genome provides insights into programmed genome rearrangement and vertebrate evolution. Nat. Genet. 2018, 50, 270–277. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Hara, Y.; Kaori, T.; Nishimura, O.; Smith, J.; Kadota, M.; Kuraku, S. Inference of a genome-wide protein-coding gene set of the inshore hagfish Eptatretus burgeri. bioRxiv 2020. [Google Scholar] [CrossRef]
- Martin, A.P.; Naylor, G.J.P.; Palumbi, S.R. Rates of mitochondrial DNA evolution in sharks are slow compared with mammals. Nature 1992, 357, 153–155. [Google Scholar] [CrossRef]
- Naylor, G.J.; Ryburn, J.; Fedrigo, O.; Lopez, J. Phylogenetic relationships among the major lineages of modern elasmobranchs. Reprod. Biol. Phylogeny 2005, 3, 25. [Google Scholar]
- Renz, A.J.; Meyer, A.; Kuraku, S. Revealing less derived nature of cartilaginous fish genomes with their evolutionary time scale inferred with nuclear genes. PLoS ONE 2013, 8, e66400. [Google Scholar] [CrossRef] [Green Version]
- Criswell, K.E.; Coates, M.I.; Gillis, J.A. Embryonic development of the axial column in the little skate, Leucoraja erinacea. J. Morphol. 2017, 278, 300–320. [Google Scholar] [CrossRef]
- Criswell, K.E.; Gillis, J.A. Resegmentation is an ancestral feature of the gnathostome vertebral skeleton. eLife 2020, 9. [Google Scholar] [CrossRef]
- Gillis, J.A.; Modrell, M.S.; Baker, C.V. Developmental evidence for serial homology of the vertebrate jaw and gill arch skeleton. Nat. Commun. 2013, 4, 1436. [Google Scholar] [CrossRef] [Green Version]
- Marconi, A.; Hancock-Ronemus, A.; Gillis, J.A. Adult chondrogenesis and spontaneous cartilage repair in the skate, Leucoraja erinacea. eLife 2020, 9. [Google Scholar] [CrossRef]
- Onimaru, K. The evolutionary origin of developmental enhancers in vertebrates: Insights from non-model species. Dev. Growth Differ. 2020, 62, 326–333. [Google Scholar] [CrossRef]
- Onimaru, K.; Marcon, L. Systems biology approach to the origin of the tetrapod limb. arXiv 2019, arXiv:1907.02730. [Google Scholar]
- Onimaru, K.; Tatsumi, K.; Tanegashima, C.; Kadota, M.; Nishimura, O.; Kuraku, S. Developmental hourglass and heterochronic shifts in fin and limb development. bioRxiv 2020. [Google Scholar] [CrossRef]
- Enny, A.; Flaherty, K.; Mori, S.; Turner, N.; Nakamura, T. Developmental constraints on fin diversity. Dev. Growth Differ. 2020, 62, 311–325. [Google Scholar] [CrossRef]
- Perks, K.E.; Krotinger, A.; Bodznick, D. A cerebellum-like circuit in the lateral line system of fish cancels mechanosensory input associated with its own movements. J. Exp. Biol. 2020, 223, jeb204438. [Google Scholar] [CrossRef]
- Jung, H.; Baek, M.; D’Elia, K.P.; Boisvert, C.; Currie, P.D.; Tay, B.H.; Venkatesh, B.; Brown, S.M.; Heguy, A.; Schoppik, D.; et al. The Ancient Origins of Neural Substrates for Land Walking. Cell 2018, 172, 667–682.e15. [Google Scholar] [CrossRef] [Green Version]
- Schweigreiter, R.; Roots, B.I.; Bandtlow, C.E.; Gould, R.M. Understanding myelination through studying its evolution. Int. Rev. Neurobiol. 2006, 73, 219–273. [Google Scholar]
- Coggeshall, R.E.; Leonard, R.B.; Applebaum, M.L.; Willis, W.D. Organization of peripheral nerves and spinal roots of the Atlantic stingray, Dasyatis sabina. J. Neurophysiol. 1978, 41, 97–107. [Google Scholar] [CrossRef]
- Roots, B.; Gould, R. Evolution of Myelinated Nervous Systems. In Evolution of Nervous Systems; Kaas, N.S., Ed.; Academic Press: Cambridge, MA, USA, 2007; pp. 469–484. [Google Scholar]
- Gould, R.M.; Fannon, A.M.; Moorman, S.J. Neural cells from dogfish embryos express the same subtype-specific antigens as mammalian neural cells in vivo and in vitro. Glia 1995, 15, 401–418. [Google Scholar] [CrossRef]
- Rotenstein, L.; Milanes, A.; Juarez, M.; Reyes, M.; de Bellard, M.E. Embryonic development of glial cells and myelin in the shark, Chiloscyllium punctatum. Gene Expr. Patterns 2009, 9, 572–585. [Google Scholar] [CrossRef] [Green Version]
- Gillis, J.A.; Shubin, N.H. The evolution of gnathostome development: Insight from chondrichthyan embryology. Genesis 2009, 47, 825–841. [Google Scholar] [CrossRef]
- Suriano, C.M.; Bodznick, D. Morphological development of the dorsal hindbrain in an elasmobranch fish (Leucoraja erinacea). Zool. Lett. 2018, 4, 28. [Google Scholar] [CrossRef]
- Gillis, J.A.; Dahn, R.D.; Shubin, N.H. Chondrogenesis and homology of the visceral skeleton in the little skate, Leucoraja erinacea (Chondrichthyes: Batoidea). J. Morphol. 2009, 270, 628–643. [Google Scholar] [CrossRef] [PubMed]
- Gillis, J.A.; Hall, B.K. A shared role for sonic hedgehog signalling in patterning chondrichthyan gill arch appendages and tetrapod limbs. Development 2016, 143, 1313–1317. [Google Scholar] [CrossRef] [Green Version]
- Venkatesh, B.; Kirkness, E.F.; Loh, Y.H.; Halpern, A.L.; Lee, A.P.; Johnson, J.; Dandona, N.; Viswanathan, L.D.; Tay, A.; Venter, J.C.; et al. Survey sequencing and comparative analysis of the elephant shark (Callorhinchus milii) genome. PLoS Biol. 2007, 5, e101. [Google Scholar] [CrossRef]
- Marra, N.J.; Stanhope, M.J.; Jue, N.K.; Wang, M.; Sun, Q.; Pavinski Bitar, P.; Richards, V.P.; Komissarov, A.; Rayko, M.; Kliver, S.; et al. White shark genome reveals ancient elasmobranch adaptations associated with wound healing and the maintenance of genome stability. Proc. Natl. Acad. Sci. USA 2019, 116, 4446–4455. [Google Scholar] [CrossRef] [Green Version]
- Chana-Munoz, A.; Jendroszek, A.; Sonnichsen, M.; Kristiansen, R.; Jensen, J.K.; Andreasen, P.A.; Bendixen, C.; Panitz, F. Multi-tissue RNA-seq and transcriptome characterisation of the spiny dogfish shark (Squalus acanthias) provides a molecular tool for biological research and reveals new genes involved in osmoregulation. PLoS ONE 2017, 12, e0182756. [Google Scholar] [CrossRef]
- Tanegashima, C.; Nishimura, O.; Motone, F.; Tatsumi, K.; Kadota, M.; Kuraku, S. Embryonic transcriptome sequencing of the ocellate spot skate Okamejei kenojei. Sci. Data 2018, 5, 180200. [Google Scholar] [CrossRef]
- Brunken, W.J.; Witkovsky, P.; Karten, H.J. Retinal neurochemistry of three elasmobranch species: An immunohistochemical approach. J. Comp. Neurol. 1986, 243, 1–12. [Google Scholar] [CrossRef]
- Weil, M.T.; Ruhwedel, T.; Meschkat, M.; Sadowski, B.; Mobius, W. Transmission Electron Microscopy of Oligodendrocytes and Myelin. Methods Mol. Biol. 2019, 1936, 343–375. [Google Scholar] [CrossRef] [Green Version]
- Webb, R.I.; Schieber, N.L. Volume Scanning Electron Microscopy. Serial Block-Face Scanning Electron Microscopy Focussed Ion Beam Scanning Electron Microscopy. In Cellular Imaging: Electron Tomography and Related Techniques; Hanssen, E., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 117–148. [Google Scholar] [CrossRef]
- Smeets, W.J.A.J.; Nieuwenhuys, R.; Roberts, B.L. The Central Nervous System of Cartilagenous Fishes; Springer: New York, NY, USA, 1983; pp. 1–256. [Google Scholar]
- Nieuwenhuys, R. Comparative anatomy of the spinal cord. Prog. Brain Res. 1969, 59, 1–56. [Google Scholar]
- Smeets, W.J.A.J.; Timerick, S.J.B. Cells of origin of pathways descending to the spinal cord in two chondrichthyans, the shark Scyliorhinus canicula and the rar Raja. J. Comp. Neurol. 1981, 202, 473–491. [Google Scholar] [CrossRef]
- Chomiak, T.; Hu, B. What is the optimal value of the g-ratio for myelinated fibers in the rat CNS? A theoretical approach. PLoS ONE 2009, 4, e7754. [Google Scholar] [CrossRef]
- Rushton, W.A. A theory of the effects of fibre size in medullated nerve. J. Physiol. 1951, 115, 101–122. [Google Scholar] [CrossRef]
- Gould, R.M.; Oakley, T.; Goldstone, J.V.; Dugas, J.C.; Brady, S.T.; Gow, A. Myelin sheaths are formed with proteins that originated in vertebrate lineages. Neuron Glia Biol. 2008, 4, 137–152. [Google Scholar] [CrossRef] [Green Version]
- Mobius, W.; Patzig, J.; Nave, K.A.; Werner, H.B. Phylogeny of proteolipid proteins: Divergence, constraints, and the evolution of novel functions in myelination and neuroprotection. Neuron Glia Biol. 2008, 4, 111–127. [Google Scholar] [CrossRef]
- Waehneldt, T.V. Phylogeny of myelin proteins. Ann. N. Y. Acad. Sci. 1990, 605, 15–28. [Google Scholar] [CrossRef]
- Yoshida, M.; Colman, D.R. Parallel evolution and coexpression of the proteolipid proteins and protein zero in vertebrate myelin. Neuron 1996, 16, 1115–1126. [Google Scholar] [CrossRef] [Green Version]
- Inouye, H.; Kirschner, D.A. Evolution of myelin ultrastructure and the major structural myelin proteins. Brain Res. 2016, 1641, 43–63. [Google Scholar] [CrossRef]
- Peters, A.; Palay, S.L.; Webster, H.d. The Fine Structure of the Nervous System: Neurons and Their Supporting Cells; Oxford University Press: New York, NY, USA, 1991; pp. 3–494. [Google Scholar]
- Rosenbluth, J. Multiple functions of the paranodal junction of myelinated nerve fibers. J. Neurosci. Res. 2009, 87, 3250–3258. [Google Scholar] [CrossRef]
- Djannatian, M.; Timmler, S.; Arends, M.; Luckner, M.; Weil, M.T.; Alexopoulos, I.; Snaidero, N.; Schmid, B.; Misgeld, T.; Mobius, W.; et al. Two adhesive systems cooperatively regulate axon ensheathment and myelin growth in the CNS. Nat. Commun. 2019, 10, 4794. [Google Scholar] [CrossRef] [Green Version]
- Hughes, A.N.; Appel, B. Oligodendrocytes express synaptic proteins that modulate myelin sheath formation. Nat. Commun. 2019, 10, 4125. [Google Scholar] [CrossRef] [Green Version]
- Elazar, N.; Vainshtein, A.; Rechav, K.; Tsoory, M.; Eshed-Eisenbach, Y.; Peles, E. Coordinated internodal and paranodal adhesion controls accurate myelination by oligodendrocytes. J. Cell Biol. 2019, 218, 2887–2895. [Google Scholar] [CrossRef] [Green Version]
- Czopka, T.; Lyons, D.A. Dissecting mechanisms of myelinated axon formation using zebrafish. Methods Cell Biol. 2011, 105, 25–62. [Google Scholar] [CrossRef]
- Kaya, F.; Mannioui, A.; Chesneau, A.; Sekizar, S.; Maillard, E.; Ballagny, C.; Houel-Renault, L.; Dupasquier, D.; Bronchain, O.; Holtzmann, I.; et al. Live imaging of targeted cell ablation in Xenopus: A new model to study demyelination and repair. J. Neurosci. 2012, 32, 12885–12895. [Google Scholar] [CrossRef] [Green Version]
- Mannioui, A.; Vauzanges, Q.; Fini, J.B.; Henriet, E.; Sekizar, S.; Azoyan, L.; Thomas, J.L.; Pasquier, D.D.; Giovannangeli, C.; Demeneix, B.; et al. The Xenopus tadpole: An in vivo model to screen drugs favoring remyelination. Mult. Scler. 2018, 24, 1421–1432. [Google Scholar] [CrossRef] [Green Version]
- Mannioui, A.; Zalc, B. Conditional Demyelination and Remyelination in a Transgenic Xenopus laevis. In Oligodendrocytes; Springer: Salmon Tower Building, NY, USA, 2019; pp. 239–248. [Google Scholar]
- Session, A.M.; Uno, Y.; Kwon, T.; Chapman, J.A.; Toyoda, A.; Takahashi, S.; Fukui, A.; Hikosaka, A.; Suzuki, A.; Kondo, M.; et al. Genome evolution in the allotetraploid frog Xenopus laevis. Nature 2016, 538, 336–343. [Google Scholar] [CrossRef] [Green Version]
- Van de Peer, Y.; Mizrachi, E.; Marchal, K. The evolutionary significance of polyploidy. Nat. Rev. Genet. 2017, 18, 411–424. [Google Scholar] [CrossRef]
- Ravi, V.; Venkatesh, B. Rapidly evolving fish genomes and teleost diversity. Curr. Opin. Genet. Dev. 2008, 18, 544–550. [Google Scholar] [CrossRef]
- Onimaru, K.; Motone, F.; Kiyatake, I.; Nishida, K.; Kuraku, S. A staging table for the embryonic development of the brownbanded bamboo shark (Chiloscyllium punctatum). Dev. Dyn. 2018, 247, 712–723. [Google Scholar] [CrossRef] [Green Version]
- Maxwell, E.E.; Frobisch, N.B.; Heppleston, A.C. Variability and conservation in late chondrichthyan development: Ontogeny of the winter skate (Leucoraja ocellata). Anat. Rec. 2008, 291, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Gould, R.M.; Spivack, W.D.; Gilland, E.; Pant, H.C.; Tseng, D. Localization of Myelin Proteins in the Developing Shark Spinal Cord. Biol. Bull. 1992, 183, 358–359. [Google Scholar] [CrossRef]
- Hildebrand, C.; Bowe, C.M.; Remahl, I.N. Myelination and myelin sheath remodelling in normal and pathological PNS nerve fibres. Prog. Neurobiol. 1994, 43, 85–141. [Google Scholar] [CrossRef]
- Hildebrand, C.; Remahl, S.; Persson, H.; Bjartmar, C. Myelinated nerve fibres in the CNS. Prog. Neurobiol. 1993, 40, 319–384. [Google Scholar] [CrossRef]
- Rosenbluth, J. Redundant myelin sheaths and other ultrastructural features of the toad cerebellum. J. Cell Biol. 1966, 28, 73–93. [Google Scholar] [CrossRef] [Green Version]
- Yin, X.H.; Crawford, T.O.; Griffin, J.W.; Tu, P.H.; Lee, V.M.Y.; Li, C.M.; Roder, J.; Trapp, B.D. Myelin-associated glycoprotein is a myelin signal that modulates the caliber of myelinated axons. J. Neurosci. 1998, 18, 1953–1962. [Google Scholar] [CrossRef] [Green Version]
- Edgar, J.M.; McLaughlin, M.; Werner, H.B.; McCulloch, M.C.; Barrie, J.A.; Brown, A.; Faichney, A.B.; Snaidero, N.; Nave, K.A.; Griffiths, I.R. Early ultrastructural defects of axons and axon-glia junctions in mice lacking expression of Cnp1. Glia 2009, 57, 1815–1824. [Google Scholar] [CrossRef]
- Patzig, J.; Erwig, M.S.; Tenzer, S.; Kusch, K.; Dibaj, P.; Möbius, W.; Goebbels, S.; Schaeren-Wiemers, N.; Nave, K.-A.; Werner, H.B. Septin/anillin filaments scaffold central nervous system myelin to accelerate nerve conduction. eLife 2016, 5, e17119. [Google Scholar] [CrossRef]
- Erwig, M.S.; Patzig, J.; Steyer, A.M.; Dibaj, P.; Heilmann, M.; Heilmann, I.; Jung, R.B.; Kusch, K.; Mobius, W.; Jahn, O.; et al. Anillin facilitates septin assembly to prevent pathological outfoldings of central nervous system myelin. eLife 2019, 8. [Google Scholar] [CrossRef]
- Bolis, A.; Coviello, S.; Bussini, S.; Dina, G.; Pardini, C.; Previtali, S.C.; Malaguti, M.; Morana, P.; Del Carro, U.; Feltri, M.L.; et al. Loss of Mtmr2 Phosphatase in Schwann Cells But Not in Motor Neurons Causes Charcot-Marie-Tooth Type 4B1 Neuropathy with Myelin Outfoldings. J. Neurosci. 2005, 25, 8567–8577. [Google Scholar] [CrossRef]
- Katanov, C.; Novak, N.; Vainshtein, A.; Golani, O.; Dupree, J.L.; Peles, E. N-Wasp Regulates Oligodendrocyte Myelination. J. Neurosci. 2020, 40, 6103–6111. [Google Scholar] [CrossRef]
- Raine, C.S.; Siegel, G.J.; Agranoff, B.W.; Albers, R.W.; Molinoff, P.B. Neurocellular Anatomy. In Basic Neurochemistry; Raven Press, Ltd.: New York, NY, USA, 1989; Volume 4, pp. 3–33. [Google Scholar]
- Ghabriel, M.N.; Allt, G. Incisures of Schmidt-Lanterman. Prog. Neurobiol. 1981, 17, 25–58. [Google Scholar] [CrossRef]
- Hall, S.M.; Williams, P.L. Studies on the ‘incisures’ of Schmidt and Lanterman. J. Cell Sci. 1970, 6, 767–791. [Google Scholar] [PubMed]
- Rigoard, P.; Tartarin, F.; Buffenoir, K.; Chaillou, M.; Fares, M.; D’Houtaud, S.; Wager, M.; Giot, J.P.; Quellard, N.; Fernandez, B.; et al. The Na, K-ATPase alpha3-isoform specifically localizes in the Schmidt-Lanterman incisures of human nerve. Cell Mol. Biol. 2007, 53, OL1003–OL1009. [Google Scholar] [PubMed]
- Blakemore, W.F. Schmidt-Lanterman Incisures in the central nervous system. J. Ultrastruct. Res. 1969, 29, 496–498. [Google Scholar] [CrossRef]
- Yin, X.; Kidd, G.J.; Nave, K.-A.; Trapp, B.D. P(0) Protein Is Required For and Can Induce Formation of Schmidt-Lantermann Incisures in Myelin Internodes. J. Neurosci. 2008, 28, 7068–7073. [Google Scholar] [CrossRef] [Green Version]
- de Monasterio-Schrader, P.; Jahn, O.; Tenzer, S.; Wichert, S.P.; Patzig, J.; Werner, H.B. Systematic approaches to central nervous system myelin. Cell. Mol. Life Sci. CMLS 2012. [Google Scholar] [CrossRef]
- Dhaunchak, A.S.; Huang, J.K.; De Faria Junior, O.; Roth, A.D.; Pedraza, L.; Antel, J.P.; Bar-Or, A.; Colman, D.R. A proteome map of axoglial specializations isolated and purified from human central nervous system. Glia 2010, 58, 1949–1960. [Google Scholar] [CrossRef]
- Erwig, M.S.; Hesse, D.; Jung, R.B.; Uecker, M.; Kusch, K.; Tenzer, S.; Jahn, O.; Werner, H.B. Myelin: Methods for Purification and Proteome Analysis. Methods Mol. Biol. 2019, 1936, 37–63. [Google Scholar] [CrossRef] [Green Version]
- Gopalakrishnan, G.; Awasthi, A.; Belkaid, W.; De Faria, O., Jr.; Liazoghli, D.; Colman, D.R.; Dhaunchak, A.S. Lipidome and proteome map of myelin membranes. J. Neurosci. Res. 2013, 91, 321–334. [Google Scholar] [CrossRef]
- Ishii, A.; Dutta, R.; Wark, G.M.; Hwang, S.I.; Han, D.K.; Trapp, B.D.; Pfeiffer, S.E.; Bansal, R. Human myelin proteome and comparative analysis with mouse myelin. Proc. Natl. Acad. Sci. USA 2009, 106, 14605–14610. [Google Scholar] [CrossRef] [Green Version]
- Jahn, O.; Siems, S.B.; Kusch, K.; Hesse, D.; Jung, R.B.; Liepold, T.; Uecker, M.; Sun, T.; Werner, H.B. The CNS Myelin Proteome: Deep Profile and Persistence After Post-mortem Delay. Front. Cell. Neurosci. 2020, 14, 239. [Google Scholar] [CrossRef]
- Jahn, O.; Tenzer, S.; Werner, H.B. Myelin proteomics: Molecular anatomy of an insulating sheath. Mol. Neurobiol. 2009, 40, 55–72. [Google Scholar] [CrossRef] [Green Version]
- Panfoli, I.; Bruschi, M.; Santucci, L.; Calzia, D.; Ravera, S.; Petretto, A.; Candiano, G. Myelin proteomics: The past, the unexpected and the future. Expert Rev. Proteom. 2014, 11, 345–354. [Google Scholar] [CrossRef]
- Patzig, J.; Jahn, O.; Tenzer, S.; Wichert, S.P.; de Monasterio-Schrader, P.; Rosfa, S.; Kuharev, J.; Yan, K.; Bormuth, I.; Bremer, J.; et al. Quantitative and integrative proteome analysis of peripheral nerve myelin identifies novel myelin proteins and candidate neuropathy loci. J. Neurosci. 2011, 31, 16369–16386. [Google Scholar] [CrossRef] [Green Version]
- Roth, A.D.; Ivanova, A.; Colman, D.R. New observations on the compact myelin proteome. Neuron Glia Biol. 2006, 2, 15–21. [Google Scholar] [CrossRef]
- Siems, S.B.; Jahn, O.; Eichel, M.A.; Kannaiyan, N.; Wu, L.M.N.; Sherman, D.L.; Kusch, K.; Hesse, D.; Jung, R.B.; Fledrich, R.; et al. Proteome profile of peripheral myelin in healthy mice and in a neuropathy model. eLife 2020, 9. [Google Scholar] [CrossRef]
- Thakurela, S.; Garding, A.; Jung, R.B.; Muller, C.; Goebbels, S.; White, R.; Werner, H.B.; Tiwari, V.K. The transcriptome of mouse central nervous system myelin. Sci. Rep. 2016, 6, 25828. [Google Scholar] [CrossRef]
- Weiss, T.; Taschner-Mandl, S.; Bileck, A.; Slany, A.; Kromp, F.; Rifatbegovic, F.; Frech, C.; Windhager, R.; Kitzinger, H.; Tzou, C.H.; et al. Proteomics and transcriptomics of peripheral nerve tissue and cells unravel new aspects of the human Schwann cell repair phenotype. Glia 2016, 64, 2133–2153. [Google Scholar] [CrossRef]
- Gould, R.M.; Freund, C.M.; Palmer, F.; Feinstein, D.L. Messenger RNAs located at sites of myelin assembly. J. Neurochem. 2000, 75, 1834–1844. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Palavicini, J.P.; Han, X. Lipidomics Profiling of Myelin. Methods Mol. Biol. 2018, 1791, 37–50. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, K.; Sloan, S.A.; Bennett, M.L.; Scholze, A.R.; O’Keeffe, S.; Phatnani, H.P.; Guarnieri, P.; Caneda, C.; Ruderisch, N.; et al. An RNA-Sequencing Transcriptome and Splicing Database of Glia, Neurons, and Vascular Cells of the Cerebral Cortex. J. Neurosci. 2014, 34, 11929–11947. [Google Scholar] [CrossRef]
- Marques, S.; van Bruggen, D.; Castelo-Branco, G. Single-Cell RNA Sequencing of Oligodendrocyte Lineage Cells from the Mouse Central Nervous System. Methods Mol. Biol. 2019, 1936, 1–21. [Google Scholar] [CrossRef]
- Marques, S.; van Bruggen, D.; Vanichkina, D.P.; Floriddia, E.M.; Munguba, H.; Varemo, L.; Giacomello, S.; Falcao, A.M.; Meijer, M.; Bjorklund, A.K.; et al. Transcriptional Convergence of Oligodendrocyte Lineage Progenitors during Development. Dev. Cell 2018, 46, 504–517.e7. [Google Scholar] [CrossRef] [Green Version]
- Marques, S.; Vanichkina, D.; van Bruggen, D.; Floriddia, E.; Munguba, H.; Varemo, L.; Giacomello, S.; Mendanha Falcao, A.; Meijer, M.; Samudyata, S.; et al. Single-cell transcriptomic profiling of progenitors of the oligodendrocyte lineage reveals transcriptional convergence during development. bioRxiv 2017. [Google Scholar] [CrossRef] [Green Version]
- Marques, S.; Zeisel, A.; Codeluppi, S.; van Bruggen, D.; Mendanha Falcao, A.; Xiao, L.; Li, H.; Haring, M.; Hochgerner, H.; Romanov, R.A.; et al. Oligodendrocyte heterogeneity in the mouse juvenile and adult central nervous system. Science 2016, 352, 1326–1329. [Google Scholar] [CrossRef] [Green Version]
- Marisca, R.; Hoche, T.; Agirre, E.; Hoodless, L.J.; Barkey, W.; Auer, F.; Castelo-Branco, G.; Czopka, T. Functionally distinct subgroups of oligodendrocyte precursor cells integrate neural activity and execute myelin formation. Nat. Neurosci. 2020, 23, 363–374. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Möbius, W.; Hümmert, S.; Ruhwedel, T.; Kuzirian, A.; Gould, R. New Species Can Broaden Myelin Research: Suitability of Little Skate, Leucoraja erinacea. Life 2021, 11, 136. https://doi.org/10.3390/life11020136
Möbius W, Hümmert S, Ruhwedel T, Kuzirian A, Gould R. New Species Can Broaden Myelin Research: Suitability of Little Skate, Leucoraja erinacea. Life. 2021; 11(2):136. https://doi.org/10.3390/life11020136
Chicago/Turabian StyleMöbius, Wiebke, Sophie Hümmert, Torben Ruhwedel, Alan Kuzirian, and Robert Gould. 2021. "New Species Can Broaden Myelin Research: Suitability of Little Skate, Leucoraja erinacea" Life 11, no. 2: 136. https://doi.org/10.3390/life11020136
APA StyleMöbius, W., Hümmert, S., Ruhwedel, T., Kuzirian, A., & Gould, R. (2021). New Species Can Broaden Myelin Research: Suitability of Little Skate, Leucoraja erinacea. Life, 11(2), 136. https://doi.org/10.3390/life11020136






