Improvement in the Sequential Extraction of Phycobiliproteins from Arthrospira platensis Using Green Technologies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microalgal Production
2.2. Chlorophyll and Carotenoid Pigments Using Supercritical Fluid Extraction
2.3. Sequential Phycobiliprotein Extraction
2.4. C-Phycocyanin Isolation
2.5. Carotenoid and Chlorophyll Determination
2.6. Phycobiliprotein Determination
2.7. Extract Purity
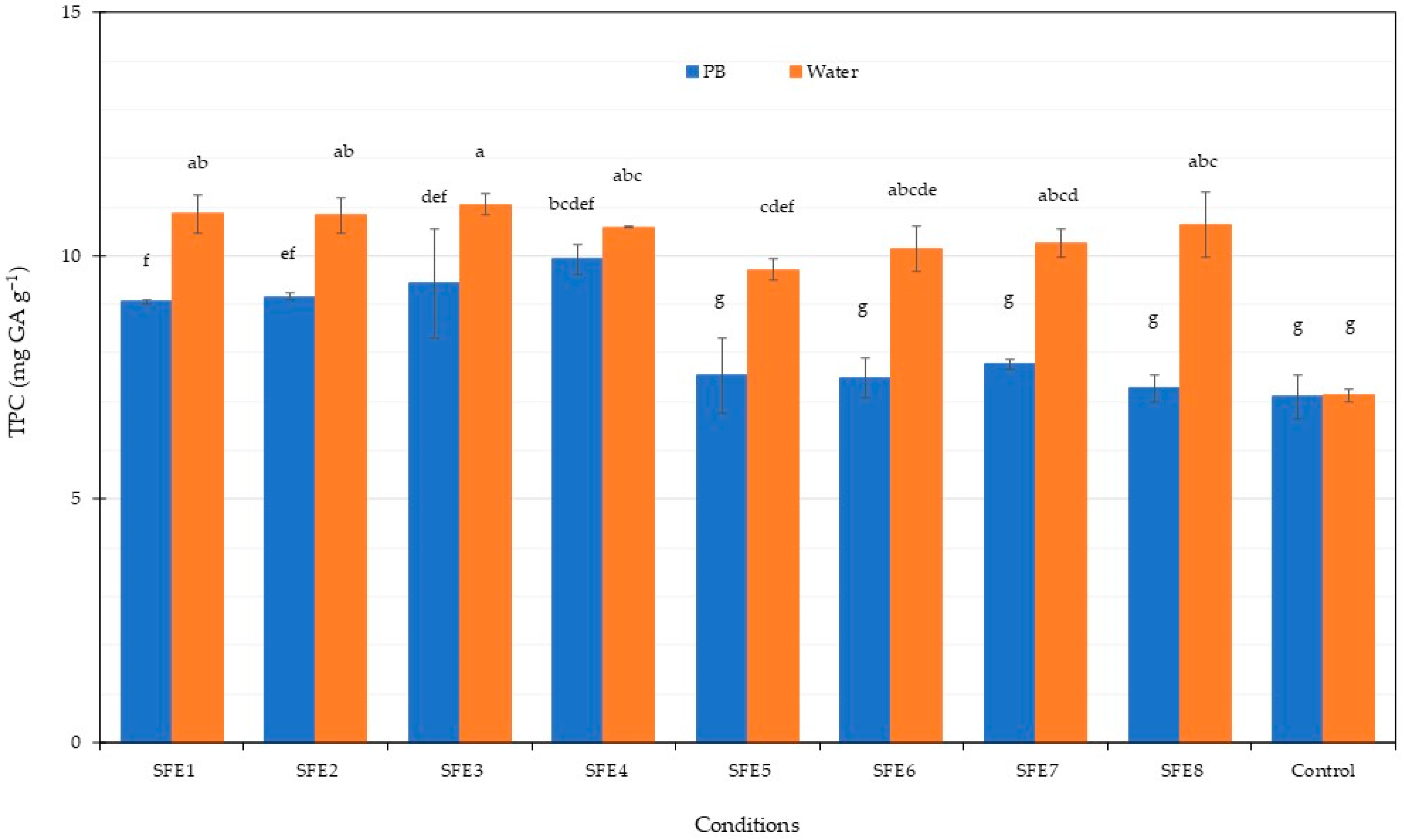
2.8. Total Phenolic Content
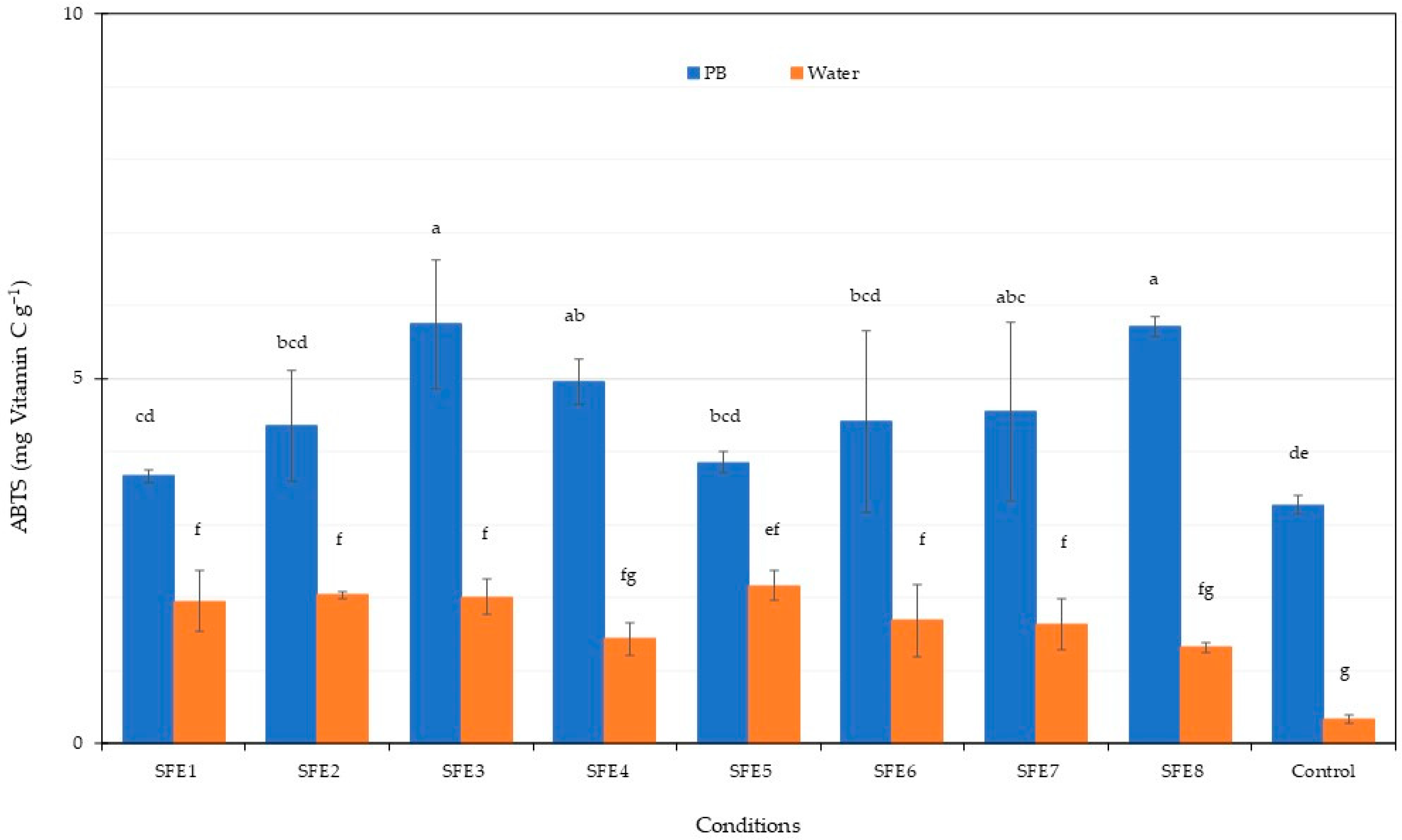
2.9. ABTS Assay
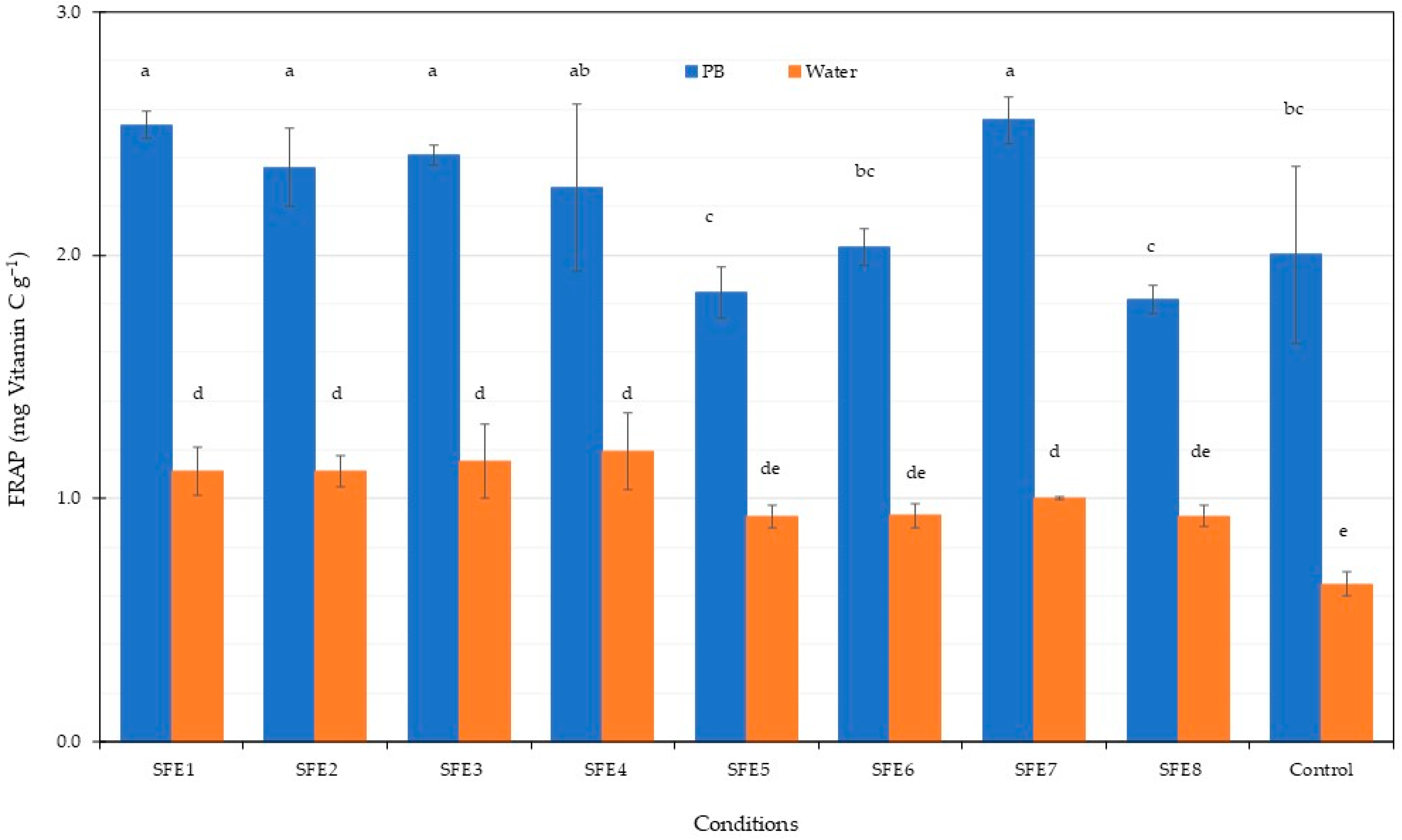
2.10. FRAP Assay
2.11. Statistical Analysis
3. Results and Discussion
3.1. Supercritical Fluid Extraction (SFE)
3.2. Sequential Phycobiliprotein Extraction
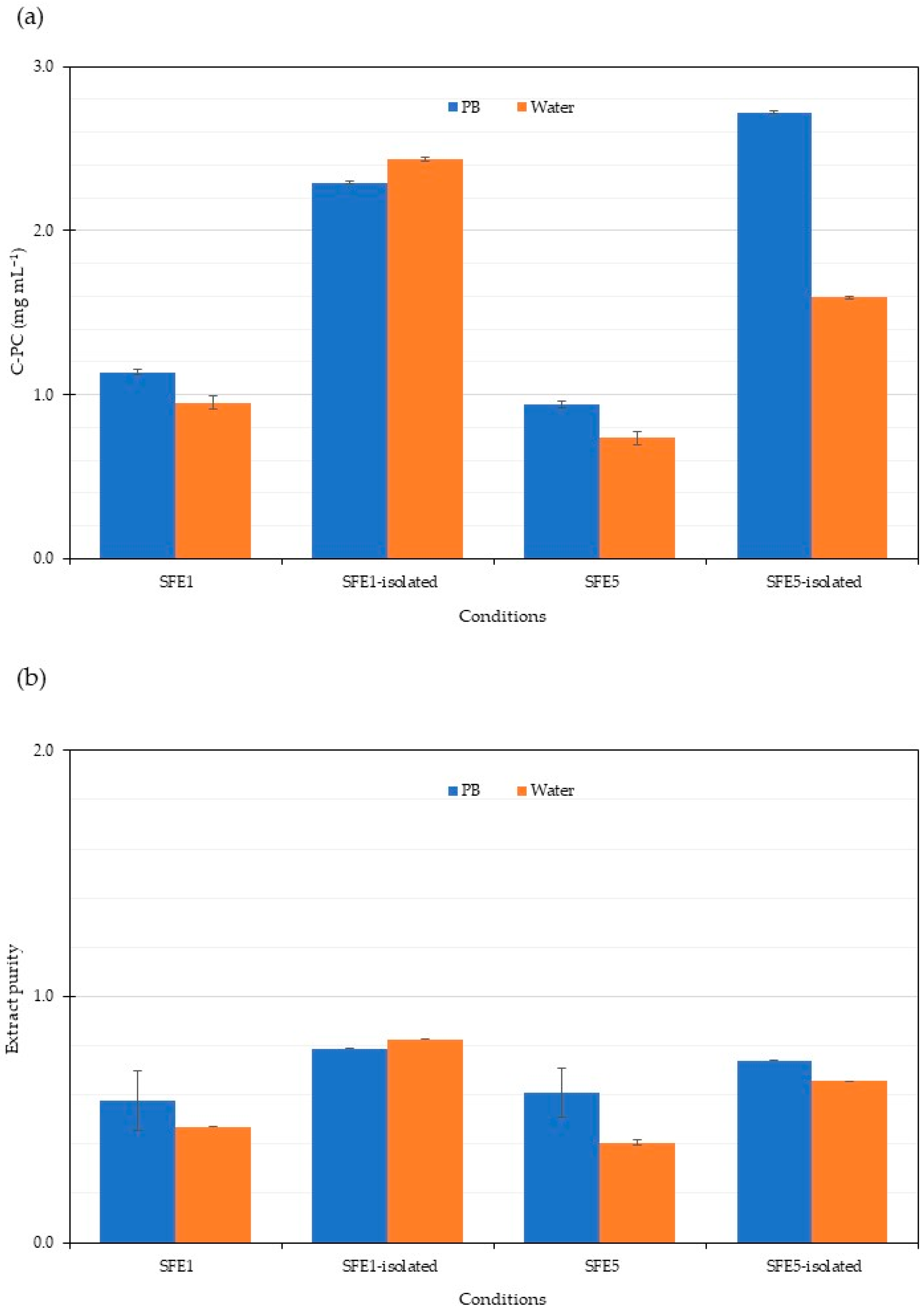
3.3. C-Phycocyanin Isolation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Masten Rutar, J.; Hudobivnik, M.J.; Nečemer, M.; Vogel Mikuš, K.; Arčon, I.; Ogrinc, N. Nutritional Quality and Safety of the Spirulina Dietary Supplements Sold on the Slovenian Market. Foods 2022, 11, 849. [Google Scholar] [CrossRef] [PubMed]
- AlFadhly, N.K.Z.; Alhelfi, N.; Altemimi, A.B.; Verma, D.K.; Cacciola, F.; Narayanankutty, A. Trends and Technological Advancements in the Possible Food Applications of Spirulina and Their Health Benefits: A Review. Molecules 2022, 27, 5584. [Google Scholar] [CrossRef] [PubMed]
- Wollina, U.; Voicu, C.; Gianfaldoni, S.; Lotti, T.; França, K.; Tchernev, G. Arthrospira platensis—Potential in Dermatology and Beyond. Open Access Maced. J. Med. Sci. 2018, 6, 176–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, C.H.G.; Braune, S.; Waldeck, P.; Küpper, J.-H.; Petrick, I.; Jung, F. Morphology and Growth of Arthrospira platensis during Cultivation in a Flat-Type Bioreactor. Life 2021, 11, 536. [Google Scholar] [CrossRef] [PubMed]
- Altmann, B.A.; Rosenau, S. Spirulina as Animal Feed: Opportunities and Challenges. Foods 2022, 11, 965. [Google Scholar] [CrossRef]
- Ebhodaghe, S.O.; Imanah, O.E.; Ndibe, H. Biofuels from microalgae biomass: A review of conversion processes and procedures. Arab. J. Chem. 2021, 15, 103591. [Google Scholar] [CrossRef]
- Iglina, T.; Iglin, P.; Pashchenko, D. Industrial CO2 Capture by Algae: A Review and Recent Advances. Sustainability 2022, 14, 3801. [Google Scholar] [CrossRef]
- Jaeschke, D.P.; Mercali, G.D.; Marczak, L.D.F.; Müller, G.; Frey, W.; Gusbeth, C. Extraction of valuable compounds from Arthrospira platensis using pulsed electric field treatment. Bioresour. Technol. 2019, 283, 207–212. [Google Scholar] [CrossRef]
- Finamore, A.; Palmery, M.; Bensehaila, S.; Peluso, I. Antioxidant, Immunomodulating, and Microbial-Modulating Activities of the Sustainable and Ecofriendly Spirulina. Oxidat. Med. Cell. Longev. 2017, 2017, 3247528. [Google Scholar] [CrossRef] [Green Version]
- Salla, A.C.V.; Margarites, A.C.; Seibel, F.I.; Holz, L.C.; Brião, V.B.; Bertolin, T.E.; Colla, L.M.; Costa, J.A.V. Increase in the carbohydrate content of the microalgae Spirulina in culture by nutrient starvation and the addition of residues of whey protein concentrate. Bioresour. Technol. 2016, 209, 133–141. [Google Scholar] [CrossRef]
- Soni, R.A.; Sudhakar, K.; Rana, R.S. Spirulina—From growth to nutritional product: A review. Trends Food Sci. Technol. 2017, 69, 157–171. [Google Scholar] [CrossRef] [Green Version]
- Soni, R.A.; Sudhakar, K.; Rana, R.S. Comparative study on the growth performance of Spirulina platensis on modifying culture media. Energy Rep. 2019, 5, 327–336. [Google Scholar] [CrossRef]
- Koli, D.K.; Rudra, S.G.; Bhowmik, A.; Pabbi, S. Nutritional, Functional, Textural and Sensory Evaluation of Spirulina Enriched Green Pasta: A Potential Dietary and Health Supplement. Foods 2022, 11, 979. [Google Scholar] [CrossRef] [PubMed]
- Park, W.S.; Kim, H.-J.; Li, M.; Lim, D.H.; Kim, J.; Kwak, S.-S.; Kang, C.-M.; Ferruzzi, M.G.; Ahn, M.-J. Two Classes of Pigments, Carotenoids and C-Phycocyanin, in Spirulina Powder and Their Antioxidant Activities. Molecules 2018, 23, 2065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chethana, S.; Nayak, C.A.; Madhusudhan, M.C.; Raghavarao, K.S.M.S. Single step aqueous two-phase extraction for downstream processing of C-phycocyanin from Spirulina platensis. J. Food Sci. Technol. 2014, 52, 2415–2421. [Google Scholar] [CrossRef] [Green Version]
- Martins, M.; Albuquerque, C.M.; Pereira, C.F.; Coutinho, J.A.P.; Neves, M.G.P.M.S.; Pinto, D.C.G.A.; Faustino, M.A.F.; Ventura, S.P.M. Recovery of Chlorophyll a Derivative from Spirulina maxima: Its Purification and Photosensitizing Potential. ACS Sustain. Chem. Eng. 2021, 9, 1772–1780. [Google Scholar] [CrossRef]
- Fischer, W.W.; Hemp, J.; Johnson, J.E. Evolution of oxygenic photosynthesis. Annu. Rev. Earth Planet. Sci. 2016, 44, 647–683. [Google Scholar] [CrossRef]
- Falkowski, P.G.; Raven, J.A. Aquatic Photosynthesis; Princeton University Press: Princeton, NJ, USA, 2013. [Google Scholar]
- Assunção, L.S.; Bezerra, P.Q.M.; Poletto, V.S.H.; Rios, A.D.O.; Ramos, I.G.; Ribeiro, C.D.F.; Machado, B.A.S.; Druzian, J.I.; Costa, J.A.V.; Nunes, I.L. Combination of carotenoids from Spirulina and PLA/PLGA or PHB: New options to obtain bioactive nanoparticles. Food Chem. 2020, 346, 128742. [Google Scholar] [CrossRef]
- Liang, R.; Shoemaker, C.F.; Yang, X.; Zhong, F.; Huang, Q. Stability and Bioaccessibility of β-Carotene in Nanoemulsions Stabilized by Modified Starches. J. Agric. Food Chem. 2013, 61, 1249–1257. [Google Scholar] [CrossRef]
- Christaki, E.; Bonos, E.; Giannenas, I.; Florou-Paneri, P. Functional properties of carotenoids originating from algae. J. Sci. Food Agric. 2013, 93, 5–11. [Google Scholar] [CrossRef]
- Kannaujiya, V.K.; Sundaram, S.; Sinha, R.P. Phycobiliproteins: Recent Developments and Future Applications; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Bermejo, R.; Ruiz, E.; Acien, F. Recovery of B-phycoerythrin using expanded bed adsorption chromatography: Scale-up of the process. Enzym. Microb. Technol. 2007, 40, 927–933. [Google Scholar] [CrossRef]
- Christaki, E.; Bonos, E.; Florou-Paneri, P. Chapter 14—Innovative Microalgae Pigments as Functional Ingredients in Nutrition. In Handbook of Marine Microalgae; Kim, S.-K., Ed.; Academic Press: Boston, MA, USA, 2015; pp. 233–243. [Google Scholar]
- Tavanandi, H.A.; Mittal, R.; Chandrasekhar, J.; Raghavarao, K. Simple and efficient method for extraction of C-Phycocyanin from dry biomass of Arthospira platensis. Algal Res. 2018, 31, 239–251. [Google Scholar] [CrossRef]
- De la Jara, A.; Ruano-Rodriguez, C.; Polifrone, M.; Assunçao, P.; Brito-Casillas, Y.; Wägner, A.M.; Serra-Majem, L. Impact of dietary Arthrospira (Spirulina) biomass consumption on human health: Main health targets and systematic review. J. Appl. Phycol. 2018, 30, 2403–2423. [Google Scholar] [CrossRef]
- Ali, S.K.; Saleh, A.M. Spirulina—An overview. Int. J. Pharm. Pharm. Sci. 2012, 4, 9–15. [Google Scholar]
- Kaur, S.; Khattar, J.I.S.; Singh, Y.; Singh, D.P.; Ahluwalia, A.S. Extraction, purification and characterisation of Phycocyanin from Anabaena fertilissima PUPCCC 410.5: As a natural and food grade stable pigment. J. Appl. Phycol. 2019, 31, 1685–1696. [Google Scholar] [CrossRef]
- Vinothkanna, A.; Sekar, S. Diagnostic Applications of Phycobiliproteins. In Pigments from Microalgae Handbook; Springer: Berlin/Heidelberg, Germany, 2020; pp. 585–610. [Google Scholar]
- Li, W.; Su, H.-N.; Pu, Y.; Chen, J.; Liu, L.-N.; Liu, Q.; Qin, S. Phycobiliproteins: Molecular structure, production, applications, and prospects. Biotechnol. Adv. 2019, 37, 340–353. [Google Scholar] [CrossRef]
- Chiong, T.; Acquah, C.; Lau, S.Y.; Khor, E.H.; Danquah, M.K. Chapter 12—Microalgal-Based Protein By-Products: Extraction, Purification, and Applications. In Protein Byproducts; Singh Dhillon, G., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 213–234. [Google Scholar]
- Sekar, S.; Chandramohan, M. Phycobiliproteins as a commodity: Trends in applied research, patents and commercialization. J. Appl. Phycol. 2007, 20, 113–136. [Google Scholar] [CrossRef]
- Lauceri, R.; Chini Zittelli, G.; Torzillo, G. A simple method for rapid purification of phycobiliproteins from Arthrospira platensis and Porphyridium cruentum biomass. Algal Res. 2019, 44, 101685. [Google Scholar] [CrossRef]
- Dagnino-Leone, J.; Figueroa, C.P.; Castañeda, M.L.; Youlton, A.D.; Vallejos-Almirall, A.; Agurto-Muñoz, A.; Pavón Pérez, J.; Agurto-Muñoz, C. Phycobiliproteins: Structural aspects, functional characteristics, and biotechnological perspectives. Comput. Struct. Biotechnol. J. 2022, 20, 1506–1527. [Google Scholar] [CrossRef]
- Sala, L.; Moraes, C.C.; Kalil, S.J. Cell pretreatment with ethylenediaminetetraacetic acid for selective extraction of C-phycocyanin with food grade purity. Biotechnol. Prog. 2018, 34, 1261–1268. [Google Scholar] [CrossRef]
- Hosikian, A.; Lim, S.; Halim, R.; Danquah, M.K. Chlorophyll Extraction from Microalgae: A Review on the Process Engineering Aspects. Int. J. Chem. Eng. 2010, 2010, 391632. [Google Scholar] [CrossRef]
- Montero, L.; Sedghi, M.; García, Y.; Almeida, C.; Safi, C.; Engelen-Smit, N.; Cifuentes, A.; Mendiola, J.A.; Ibáñez, E. Pressurized Liquid Extraction of Pigments from Chlamydomonas sp. and Chemical Characterization by HPLC–MS/MS. J. Anal. Test. 2018, 2, 149–157. [Google Scholar] [CrossRef]
- Nemer, G.; Louka, N.; Vorobiev, E.; Salameh, D.; Nicaud, J.-M.; Maroun, R.G.; Koubaa, M. Mechanical Cell Disruption Technologies for the Extraction of Dyes and Pigments from Microorganisms: A Review. Fermentation 2021, 7, 36. [Google Scholar] [CrossRef]
- Pagels, F.; Pereira, R.N.; Vicente, A.A.; Guedes, A.C. Extraction of Pigments from Microalgae and Cyanobacteria—A Review on Current Methodologies. Appl. Sci. 2021, 11, 5187. [Google Scholar] [CrossRef]
- Banayan, S.; Jahadi, M.; Khosravi-Darani, K. Pigment Productions by Spirulina platensis as a Renewable Resource. J. Appl. Biotechnol. Rep. 2022, 9, 614–621. [Google Scholar] [CrossRef]
- Martí-Quijal, F.J.; Ramon-Mascarell, F.; Pallarés, N.; Ferrer, E.; Berrada, H.; Phimolsiripol, Y.; Barba, F.J. Extraction of Antioxidant Compounds and Pigments from Spirulina (Arthrospira platensis) Assisted by Pulsed Electric Fields and the Binary Mixture of Organic Solvents and Water. Appl. Sci. 2021, 11, 7629. [Google Scholar] [CrossRef]
- Basa’Ar, O.; Fatema, S.; Alrabie, A.; Mohsin, M.; Farooqui, M. Supercritical carbon dioxide extraction of Triognella foenum graecum Linn seeds: Determination of bioactive compounds and pharmacological analysis. Asian Pac. J. Trop. Biomed. 2017, 7, 1085–1091. [Google Scholar] [CrossRef]
- Sahena, F.; Zaidul, I.; Jinap, S.; Karim, A.; Abbas, K.; Norulaini, N.; Omar, A. Application of supercritical CO2 in lipid extraction—A review. J. Food Eng. 2009, 95, 240–253. [Google Scholar] [CrossRef]
- Ruiz-Domínguez, M.C.; Medina, E.; Salinas, F.; Bugueño, W.; Fuentes, J.-L.; Vílchez, C.; Garbayo, I.; Cerezal-Mezquita, P. Methodological Optimization of Supercritical Fluid Extraction of Valuable Bioactive Compounds from the Acidophilic Microalga Coccomyxa onubensis. Antioxidants 2022, 11, 1248. [Google Scholar] [CrossRef]
- Díaz-Reinoso, B.; Moure, A.; Domínguez, H.; Parajó, J.C. Supercritical CO2 Extraction and Purification of Compounds with Antioxidant Activity. J. Agric. Food Chem. 2006, 54, 2441–2469. [Google Scholar] [CrossRef]
- Wei, M.-C.; Wang, C.-S.; Wei, D.-H.; Yang, Y.-C. Insights into the Supercritical CO2 Extraction of Perilla Oil and Its Theoretical Solubility. Processes 2021, 9, 239. [Google Scholar] [CrossRef]
- Bahadar, A.; Khan, M.B.; Asim, M.A.; Jalwana, K. Chapter 21—Supercritical Fluid Extraction of Microalgae (Chlorella vulagaris) Biomass. In Handbook of Marine Microalgae; Kim, S.-K., Ed.; Academic Press: Boston, MA, USA, 2015; pp. 317–330. [Google Scholar]
- Vafaei, N.; Rempel, C.B.; Scanlon, M.G.; Jones, P.J.H.; Eskin, M.N.A. Application of Supercritical Fluid Extraction (SFE) of Tocopherols and Carotenoids (Hydrophobic Antioxidants) Compared to Non-SFE Methods. AppliedChem 2022, 2, 68–92. [Google Scholar] [CrossRef]
- Molino, A.; Mehariya, S.; Di Sanzo, G.; LaRocca, V.; Martino, M.; Leone, G.P.; Marino, T.; Chianese, S.; Balducchi, R.; Musmarra, D. Recent developments in supercritical fluid extraction of bioactive compounds from microalgae: Role of key parameters, technological achievements and challenges. J. CO2 Util. 2020, 36, 196–209. [Google Scholar] [CrossRef]
- Uwineza, P.A.; Waśkiewicz, A. Recent Advances in Supercritical Fluid Extraction of Natural Bioactive Compounds from Natural Plant Materials. Molecules 2020, 25, 3847. [Google Scholar] [CrossRef]
- Patias, L.D.; Fernandes, A.S.; Petry, F.C.; Mercadante, A.Z.; Jacob-Lopes, E.; Zepka, L.Q. Carotenoid profile of three microalgae/cyanobacteria species with peroxyl radical scavenger capacity. Food Res. Int. 2017, 100, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Poojary, M.M.; Barba, F.J.; Aliakbarian, B.; Donsì, F.; Pataro, G.; Dias, D.A.; Juliano, P. Innovative Alternative Technologies to Extract Carotenoids from Microalgae and Seaweeds. Mar. Drugs 2016, 14, 214. [Google Scholar] [CrossRef] [Green Version]
- Pyne, S.; Paria, K. Optimization of extraction process parameters of caffeic acid from microalgae by supercritical carbon dioxide green technology. BMC Chem. 2022, 16, 1–11. [Google Scholar] [CrossRef]
- Kankala, R.K.; Zhang, Y.S.; Wang, S.-B.; Lee, C.-H.; Chen, A.-Z. Supercritical Fluid Technology: An Emphasis on Drug Delivery and Related Biomedical Applications. Adv. Healthc. Mater. 2017, 6, 1700433. [Google Scholar] [CrossRef] [Green Version]
- Chakravarty, P.; Famili, A.; Nagapudi, K.; Al-Sayah, M.A. Using Supercritical Fluid Technology as a Green Alternative During the Preparation of Drug Delivery Systems. Pharmaceutics 2019, 11, 629. [Google Scholar] [CrossRef] [Green Version]
- Zarrouk, C. Contribution à l’étude d’une Cyanophycée. Influence de Divers Facteurs Physiques et Chimiques sur la Croissanceet la Photosynthèse de Spirulina maxima. Ph.D. Thesis, University of Paris, Paris, France, 1996. [Google Scholar]
- Pan-Utai, W.; Poopat, N.; Parakulsuksatid, P. Photoautotrophic Cultivation of Arthrospira maxima for Protein Accumulation under Minimum Nutrient Availability. Appl. Food Biotechnol. 2020, 7, 225–234. [Google Scholar] [CrossRef]
- Deniz, I.; Ozen, M.O.; Yesil-Celiktas, O. Supercritical fluid extraction of phycocyanin and investigation of cytotoxicity on human lung cancer cells. J. Supercrit. Fluids 2016, 108, 13–18. [Google Scholar] [CrossRef]
- Esquivel-Hernández, D.A.; López, V.H.; Rodríguez-Rodríguez, J.; Alemán-Nava, G.S.; Cuéllar-Bermúdez, S.P.; Rostro-Alanis, M.; Parra-Saldívar, R. Supercritical Carbon Dioxide and Microwave-Assisted Extraction of Functional Lipophilic Compounds from Arthrospira platensis. Int. J. Mol. Sci. 2016, 17, 658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan-Utai, W.; Iamtham, S. Extraction, purification and antioxidant activity of phycobiliprotein from Arthrospira platensis. Process Biochem. 2019, 82, 189–198. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Analysis 1983, 11, 591–592. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Chen, X.-L.; Zhang, X.-Y.; Zhang, Y.-Z.; Zhou, B.-C. One-step chromatography method for efficient separation and purification of R-phycoerythrin from Polysiphonia urceolata. J. Biotechnol. 2005, 116, 91–100. [Google Scholar] [CrossRef]
- De Amarante, M.C.A.; Braga, A.R.C.; Sala, L.; Kalil, S.J. Colour stability and antioxidant activity of C-phycocyanin-added ice creams after in vitro digestion. Food Res. Int. 2020, 137, 109602. [Google Scholar] [CrossRef]
- Renugadevi, K.; Nachiyar, C.V.; Sowmiya, P.; Sunkar, S. Antioxidant activity of phycocyanin pigment extracted from marine filamentous cyanobacteria Geitlerinema sp. TRV57. Biocatal. Agric. Biotechnol. 2018, 16, 237–242. [Google Scholar] [CrossRef]
- Crampon, C.; Boutin, O.; Badens, E. Supercritical Carbon Dioxide Extraction of Molecules of Interest from Microalgae and Seaweeds. Ind. Eng. Chem. Res. 2011, 50, 8941–8953. [Google Scholar] [CrossRef]
- Michalak, I.; Dmytryk, A.; Wieczorek, P.P.; Rój, E.; Łęska, B.; Górka, B.; Messyasz, B.; Lipok, J.; Mikulewicz, M.; Wilk, R.; et al. Supercritical Algal Extracts: A Source of Biologically Active Compounds from Nature. J. Chem. 2015, 2015, 597140. [Google Scholar] [CrossRef] [Green Version]
- Priyadarsani, S.; Patel, A.S.; Kar, A.; Dash, S. Process optimization for the supercritical carbondioxide extraction of lycopene from ripe grapefruit (Citrus paradisi) endocarp. Sci. Rep. 2021, 11, 10273. [Google Scholar] [CrossRef]
- Macías-Sánchez, M.; Mantell, C.; Rodríguez, M.; de la Ossa, E.M.; Lubián, L.; Montero, O. Supercritical fluid extraction of carotenoids and chlorophyll a from Synechococcus sp. J. Supercrit. Fluids 2007, 39, 323–329. [Google Scholar] [CrossRef]
- Ariff, M.A.M.; Yusri, A.M.; Razak, N.A.A.; Jaapar, J. Effect of CO2 flow rate, co-solvent and pressure behavior to yield by supercritical CO2 extraction of Mariposa Christia Vespertilionis leaves. AIP Conf. Proc. 2018, 2045, 020072. [Google Scholar] [CrossRef]
- Radzali, S.A.; Markom, M.; Saleh, N.M. Co-Solvent Selection for Supercritical Fluid Extraction (SFE) of Phenolic Compounds from Labisia pumila. Molecules 2020, 25, 5859. [Google Scholar] [CrossRef] [PubMed]
- Macías-Sánchez, M.D.; Serrano, C.M.; Rodríguez, M.R.; de la Ossa, E.M.; Lubián, L.M.; Montero, O. Extraction of carotenoids and chlorophyll from microalgae with supercritical carbon dioxide and ethanol as cosolvent. J. Sep. Sci. 2008, 31, 1352–1362. [Google Scholar] [CrossRef]
- Santana, A.; Jesus, S.; Larrayoz, M.; Filho, R. Supercritical Carbon Dioxide Extraction of Algal Lipids for the Biodiesel Production. Procedia Eng. 2012, 42, 1755–1761. [Google Scholar] [CrossRef] [Green Version]
- Macías-Sánchez, M.D.; Mantell, C.; Rodriguez, M.; de la Ossa, E.M.; Lubian, L.; Montero, O. Comparison of supercritical fluid and ultrasound-assisted extraction of carotenoids and chlorophyll a from Dunaliella salina. Talanta 2009, 77, 948–952. [Google Scholar] [CrossRef]
- Palavra, A.; Coelho, J.; Barroso, J.; Rauter, A.; Fareleira, J.; Mainar, A.; Urieta, J.; Nobre, B.; Gouveia, L.; Mendes, R.; et al. Supercritical carbon dioxide extraction of bioactive compounds from microalgae and volatile oils from aromatic plants. J. Supercrit. Fluids 2011, 60, 21–27. [Google Scholar] [CrossRef]
- Zhang, Y.; Rehmmann, L. Chapter 19—Extraction of high-value compounds from marine biomass via ionic liquid-based techniques. In Innovative and Emerging Technologies in the Bio-Marine Food Sector; Garcia-Vaquero, M., Rajauria, G., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 417–439. [Google Scholar]
- Castro-Puyana, M.; Marina, M.L.; Plaza, M. Water as green extraction solvent: Principles and reasons for its use. Curr. Opin. Green Sustain. Chem. 2017, 5, 31–36. [Google Scholar] [CrossRef]
- Choi, W.Y.; Lee, H.Y. Effect of Ultrasonic Extraction on Production and Structural Changes of C-Phycocyanin from Marine Spirulina maxima. Int. J. Mol. Sci. 2018, 19, 220. [Google Scholar] [CrossRef] [Green Version]
- Aftari, R.V.; Rezaei, K.; Mortazavi, A.; Bandani, A.R. The Optimized Concentration and Purity of Spirulina platensis C-Phycocyanin: A Comparative Study on Microwave-Assisted and Ultrasound-Assisted Extraction Methods. J. Food Process. Preserv. 2015, 39, 3080–3091. [Google Scholar] [CrossRef]
- López, A.; Rico, M.; Rivero, A.; de Tangil, M.S. The effects of solvents on the phenolic contents and antioxidant activity of Stypocaulon scoparium algae extracts. Food Chem. 2011, 125, 1104–1109. [Google Scholar] [CrossRef]
- Vieira, M.V.; Turkiewicz, I.P.; Tkacz, K.; Fuentes-Grünewald, C.; Pastrana, L.M.; Fuciños, P.; Wojdyło, A.; Nowicka, P. Microalgae as a Potential Functional Ingredient: Evaluation of the Phytochemical Profile, Antioxidant Activity and In-Vitro Enzymatic Inhibitory Effect of Different Species. Molecules 2021, 26, 7593. [Google Scholar] [CrossRef] [PubMed]
- Aoki, J.; Sasaki, D.; Asayama, M. Development of a method for phycocyanin recovery from filamentous cyanobacteria and evaluation of its stability and antioxidant capacity. BMC Biotechnol. 2021, 21, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-L.; Wang, G.-H.; Xiang, W.-Z.; Li, T.; He, H. Stability and Antioxidant Activity of Food-Grade Phycocyanin Isolated from Spirulina platensis. Int. J. Food Prop. 2016, 19, 2349–2362. [Google Scholar] [CrossRef] [Green Version]
- Sukhinov, D.V.; Gorin, K.V.; Romanov, A.O.; Gotovtsev, P.M.; Sergeeva, Y.E. Increased C-phycocyanin extract purity by flocculation of Arthrospira platensis with chitosan. Algal Res. 2021, 58, 102393. [Google Scholar] [CrossRef]
- Kissoudi, M.; Sarakatsianos, I.; Samanidou, V. Isolation and purification of food-grade C-phycocyanin from Arthrospira platensis and its determination in confectionery by HPLC with diode array detection. J. Sep. Sci. 2017, 41, 975–981. [Google Scholar] [CrossRef]
- De Amarante, M.C.A.; Braga, A.R.C.; Sala, L.; Moraes, C.C.; Kalil, S.J. Design strategies for C-phycocyanin purification: Process influence on purity grade. Sep. Purif. Technol. 2020, 252, 117453. [Google Scholar] [CrossRef]
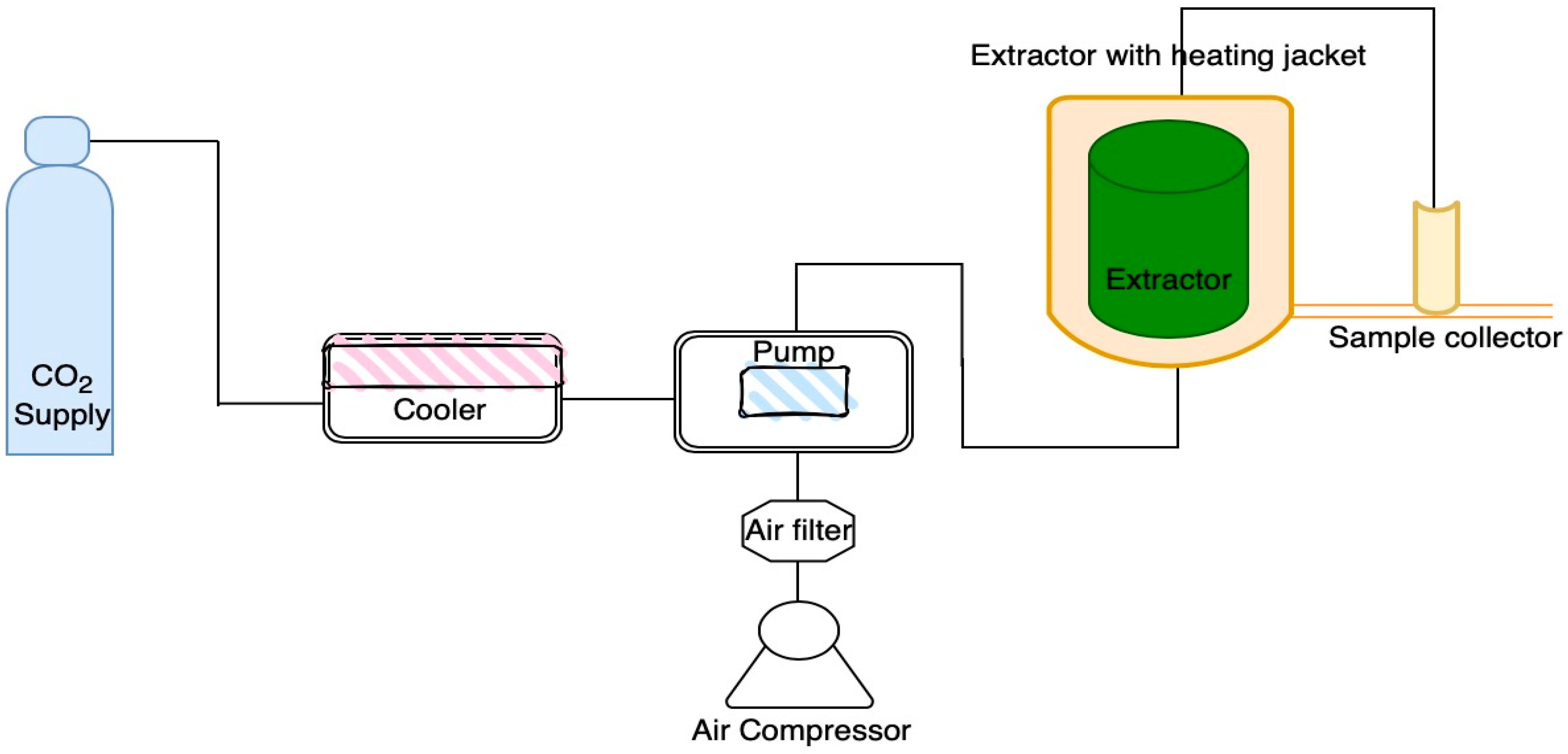
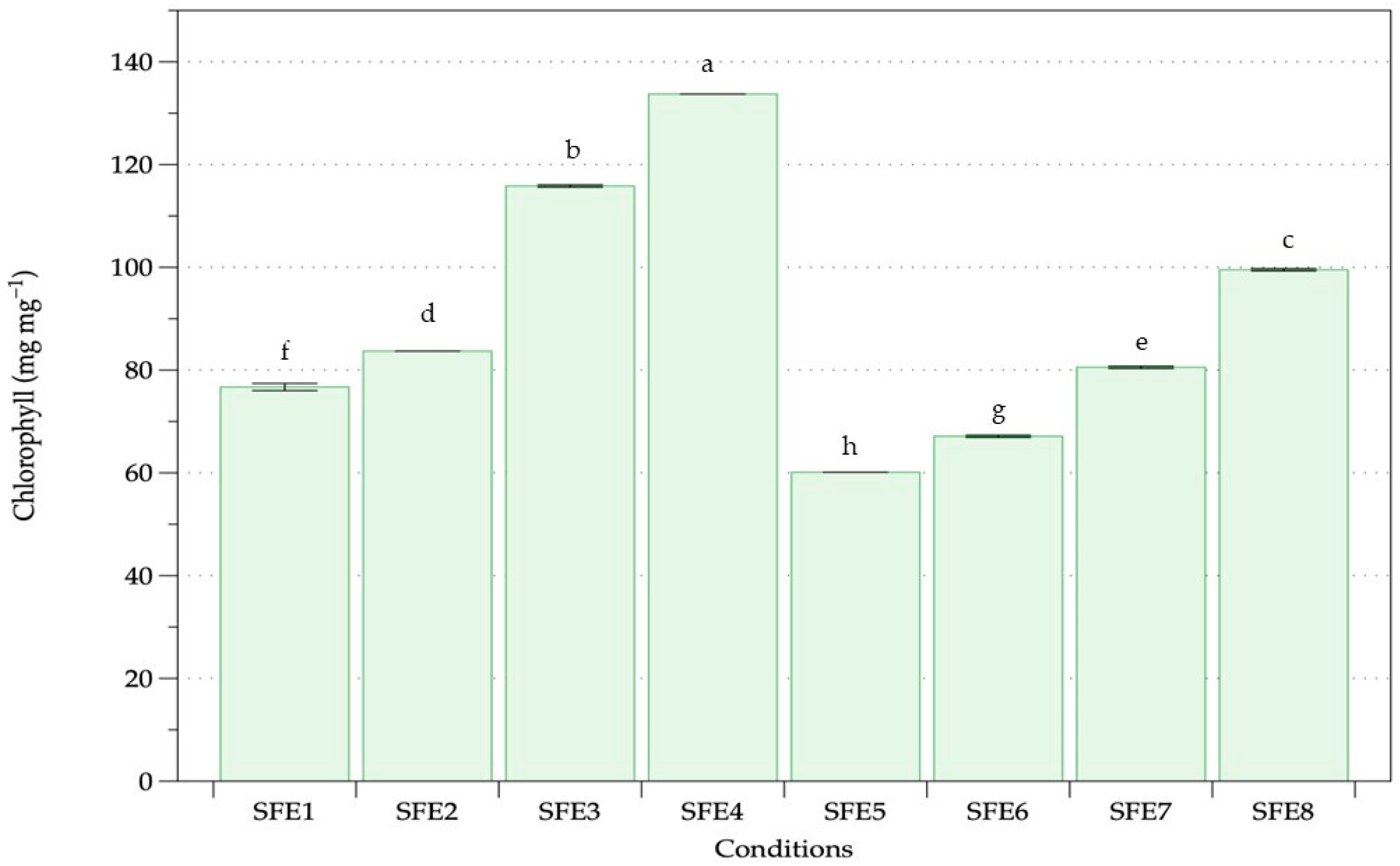
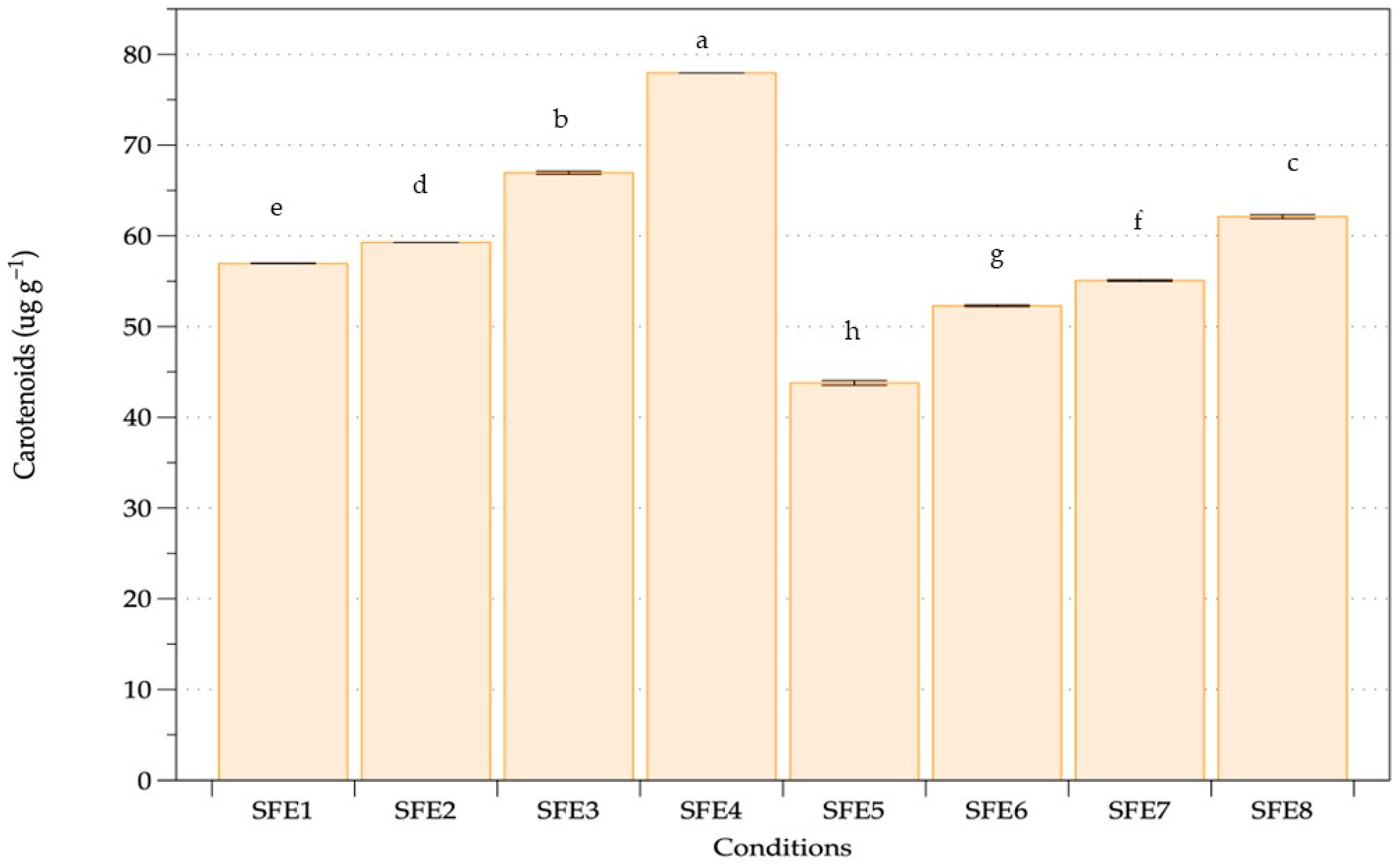
| Experiment | Pressure | Temperature | Cosolvent |
|---|---|---|---|
| (bar) | (°C) | (% w/w) | |
| SFE1 | 250 | 40 | None |
| SFE2 | 250 | 50 | None |
| SFE3 | 350 | 40 | None |
| SFE4 | 350 | 50 | None |
| SFE5 | 250 | 40 | 10% ethanol |
| SFE6 | 250 | 50 | 10% ethanol |
| SFE7 | 350 | 40 | 10% ethanol |
| SFE8 | 350 | 50 | 10% ethanol |
| Experiment | Phycobiliprotein Concentration (mg·mL−1) | |||
|---|---|---|---|---|
| C-PC | APC | PE | PBP | |
| Phosphate buffer extraction | ||||
| SFE1 | 1.139 a ± 0.02 | 0.307 a ± 0.01 | 0.094 a ± 0.00 | 1.540 a ± 0.03 |
| SFE2 | 1.140 a ± 0.03 | 0.298 ab ± 0.01 | 0.093 ab ± 0.01 | 1.530 a ± 0.01 |
| SFE3 | 1.156 a ± 0.02 | 0.275 b ± 0.01 | 0.082 c ± 0.00 | 1.513 a ± 0.02 |
| SFE4 | 1.174 a ± 0.03 | 0.291 ab ± 0.01 | 0.085 bc ± 0.01 | 1.550 a ± 0.04 |
| SFE5 | 0.945 b ± 0.02 | 0.200 c ± 0.01 | 0.062 d ± 0.00 | 1.207 b ± 0.03 |
| SFE6 | 0.886 bcd ± 0.01 | 0.184 cd ± 0.01 | 0.059 de ± 0.00 | 1.129 bcd ± 0.03 |
| SFE7 | 0.959 b ± 0.07 | 0.207 c ± 0.02 | 0.065 d ± 0.01 | 1.231 b ± 0.10 |
| SFE8 | 0.860 bcde ± 0.09 | 0.189 cd ± 0.02 | 0.060 d ± 0.01 | 1.109 bcd ± 0.12 |
| Control | 0.766 ef ± 0.09 | 0.142 f ± 0.01 | 0.045 f ± 0.00 | 0.953 ef ± 0.11 |
| Water extraction | ||||
| SFE1 | 0.953 b ± 0.04 | 0.193 c ± 0.01 | 0.059 de ± 0.00 | 1.205 b ± 0.06 |
| SFE2 | 0.960 b ± 0.02 | 0.200 c ± 0.01 | 0.063 d ± 0.00 | 1.223 b ± 0.02 |
| SFE3 | 0.911 bc ± 0.07 | 0.196 c ± 0.01 | 0.064 d ± 0.00 | 1.171 bc ± 0.07 |
| SFE4 | 0.957 b ± 0.03 | 0.196 c ± 0.01 | 0.060 d ± 0.00 | 1.214 b ± 0.03 |
| SFE5 | 0.737 f ± 0.04 | 0.135 f ± 0.01 | 0.043 f ± 0.00 | 0.915 f ± 0.05 |
| SFE6 | 0.788 def ± 0.00 | 0.148 ef ± 0.00 | 0.046 f ± 0.00 | 0.982 ef ± 0.01 |
| SFE7 | 0.839 cde ± 0.00 | 0.167 de ± 0.00 | 0.051 ef ± 0.00 | 1.056 cde ± 0.01 |
| SFE8 | 0.806 def ± 0.01 | 0.155 ef ± 0.00 | 0.049 f ± 0.00 | 1.011 def ± 0.01 |
| Control | 0.568 g ± 0.02 | 0.079 g ± 0.01 | 0.025 g ± 0.00 | 0.672 g ± 0.03 |
| Experiments | Phycobiliprotein Extraction Yield (mg·g−1) | |||
|---|---|---|---|---|
| C-PC | APC | PE | PBP | |
| Phosphate buffer extraction | ||||
| SFE1 | 54.072 a ± 0.93 | 14.550 a ± 0.01 | 4.460 a ± 0.05 | 73.082 a ± 0.98 |
| SFE2 | 54.486 a ± 2.27 | 14.216 ab ± 0.15 | 4.417 a ± 0.40 | 73.119 a ± 1.72 |
| SFE3 | 56.091 a ± 0.73 | 13.325 b ± 0.66 | 3.988 b ± 0.27 | 73.404 a ± 1.66 |
| SFE4 | 55.021 a ± 0.27 | 13.636 ab ± 0.05 | 3.985 b ± 0.04 | 72.643 a ± 0.17 |
| SFE5 | 46.864 bc ± 1.20 | 9.926 c ± 0.45 | 3.053 c ± 0.15 | 59.843 bc ± 1.80 |
| SFE6 | 43.038 cde ± 0.84 | 8.930 cd ± 0.13 | 2.873 cd ± 0.04 | 54.841 cde ± 0.76 |
| SFE7 | 46.428 bc ± 2.84 | 10.015 c ± 1.02 | 3.160 c ± 0.45 | 59.603 bc ± 4.31 |
| SFE8 | 41.206 de ± 2.90 | 9.034 cd ± 0.92 | 2.874 cd ± 0.18 | 53.114 def ± 3.99 |
| Control | 38.965 ef ± 4.65 | 7.227 f ± 0.67 | 2.300 e ± 0.02 | 48.492 fg ± 5.35 |
| Water extraction | ||||
| SFE1 | 46.945 bc ± 2.25 | 9.525 c ± 0.57 | 2.897 cd ± 0.09 | 59.367 bc ± 2.91 |
| SFE2 | 47.703 b ± 0.94 | 9.924 c ± 0.35 | 3.139 c ± 0.07 | 60.765 b ± 1.36 |
| SFE3 | 45.344 bcd ± 3.01 | 9.770 c ± 0.36 | 3.203 c ± 0.01 | 58.316 bcd ± 3.37 |
| SFE4 | 47.462 bc ± 1.46 | 9.706 c ± 0.28 | 2.998 c ± 0.03 | 60.167 bc ± 1.71 |
| SFE5 | 36.610 f ± 2.00 | 6.710 f ± 0.44 | 2.145 e ± 0.08 | 45.465 g ± 2.52 |
| SFE6 | 39.238 ef ± 0.19 | 7.349 ef ± 0.03 | 2.302 e ± 0.10 | 48.889 fg ± 0.33 |
| SFE7 | 41.770 de ± 0.12 | 8.303 de ± 0.11 | 2.521 de ± 0.07 | 52.594 ef ± 0.30 |
| SFE8 | 40.135 ef ± 0.16 | 7.734 ef ± 0.02 | 2.463 e ± 0.12 | 50.333 efg ± 0.30 |
| Control | 29.180 g ± 1.14 | 4.052 g ± 0.33 | 1.306 f ± 0.14 | 34.539 h ± 1.61 |
| Experiments | Extract Purity | ||
|---|---|---|---|
| C-PC | APC | PE | |
| Phosphate buffer extraction | |||
| SFE1 | 0.578 ab ± 0.12 | 0.240 a ± 0.05 | 0.299 ab ± 0.06 |
| SFE2 | 0.472 cd ± 0.02 | 0.193 bcde ± 0.01 | 0.243 cde ± 0.02 |
| SFE3 | 0.498 bcd ± 0.01 | 0.195 bcd ± 0.01 | 0.250 bcd ± 0.01 |
| SFE4 | 0.536 abc ± 0.06 | 0.214 abc ± 0.03 | 0.270 abc ± 0.03 |
| SFE5 | 0.611 a ± 0.10 | 0.228 ab ± 0.04 | 0.303 a ± 0.05 |
| SFE6 | 0.412 d ± 0.00 | 0.153 defg ± 0.00 | 0.205 de ± 0.00 |
| SFE7 | 0.485 bcd ± 0.01 | 0.183 cdef ± 0.00 | 0.242 cde ± 0.00 |
| SFE8 | 0.398 d ± 0.06 | 0.151 efg ± 0.03 | 0.200 de ± 0.03 |
| Control | 0.418 d ± 0.00 | 0.148 fg ± 0.00 | 0.204 de ± 0.01 |
| Water extraction | |||
| SFE1 | 0.470 cd ± 0.00 | 0.173 cdef ± 0.00 | 0.231 cde ± 0.00 |
| SFE2 | 0.473 cd ± 0.01 | 0.176 cdef ± 0.01 | 0.235 cde ± 0.01 |
| SFE3 | 0.448 cd ± 0.03 | 0.169 defg ± 0.01 | 0.226 cde ± 0.01 |
| SFE4 | 0.497 bcd ± 0.01 | 0.183 cdef ± 0.00 | 0.245 cd ± 0.01 |
| SFE5 | 0.407 d ± 0.01 | 0.144 fg ± 0.01 | 0.199 de ± 0.01 |
| SFE6 | 0.414 d ± 0.01 | 0.148 fg ± 0.00 | 0.202 de ± 0.00 |
| SFE7 | 0.419 d ± 0.02 | 0.153 defg ± 0.01 | 0.205 de ± 0.01 |
| SFE8 | 0.434 cd ± 0.01 | 0.156 defg ± 0.00 | 0.213 de ± 0.00 |
| Control | 0.405 d ± 0.01 | 0.130 g ± 0.00 | 0.190 e ± 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan-utai, W.; Iamtham, S.; Boonbumrung, S.; Mookdasanit, J. Improvement in the Sequential Extraction of Phycobiliproteins from Arthrospira platensis Using Green Technologies. Life 2022, 12, 1896. https://doi.org/10.3390/life12111896
Pan-utai W, Iamtham S, Boonbumrung S, Mookdasanit J. Improvement in the Sequential Extraction of Phycobiliproteins from Arthrospira platensis Using Green Technologies. Life. 2022; 12(11):1896. https://doi.org/10.3390/life12111896
Chicago/Turabian StylePan-utai, Wanida, Siriluck Iamtham, Sumitra Boonbumrung, and Juta Mookdasanit. 2022. "Improvement in the Sequential Extraction of Phycobiliproteins from Arthrospira platensis Using Green Technologies" Life 12, no. 11: 1896. https://doi.org/10.3390/life12111896
APA StylePan-utai, W., Iamtham, S., Boonbumrung, S., & Mookdasanit, J. (2022). Improvement in the Sequential Extraction of Phycobiliproteins from Arthrospira platensis Using Green Technologies. Life, 12(11), 1896. https://doi.org/10.3390/life12111896







