Abstract
The prominence of arbuscular mycorrhizal fungi (AMF) in sustainable rice production has long been recognized. However, there is little information about AMF response in aerobic rice cultivation under phosphorus (P)-deficient conditions. The aim of this experiment was to compare and determine the preeminent AMF effects on rice mycorrhizal colonization, responsiveness, P utilization, and different growth-promoting traits under P-deficient conditions. Different AMF genera viz. (Funneliformis sp., Rhizophagus sp., Glomus sp., Acaulospora sp., and Claroideoglomus sp.) in four different aerobic rice varieties developed by ICAR-NRRI, India (CR Dhan 201, CR Dhan 204, CR Dhan 205, and CR Dhan 207) were investigated using the check P-susceptible variety (IR 36) and the P-tolerant variety (Kasalath IC459373). Data analyzed through linear modeling approaches and bivariate associations found that AMF colonization was highly correlated with soil enzymes, particularly fluorescein diacetate (FDA) and plant P uptake. The microbial biomass carbon (MBC) and FDA content were significantly changed among rice varieties treated with AMF compared to uninoculated control. Out of four different rice varieties, CR Dhan 207 inoculated with AMF showed higher plant P uptake compared to other varieties. In all the rice varieties, AMF colonization had higher correlation coefficients with soil enzymes (FDA), MBC, and plant P uptake than uninoculated control. The present study indicates that AMF intervention in aerobic rice cultivation under P-deficient conditions significantly increased plant P uptake, soil enzymes activities and plant growth promotion. Thus, the information gathered from this study will help us to develop a viable AMF package for sustainable aerobic rice cultivation.
1. Introduction
Rice (Oryza sativa L.) is a major agricultural crop and staple food that feeds more than half of the world’s population, is grown in >100 countries with 90% of the total global production coming from Asia [1]. In India, rice is cultivated in an area of 45 million hectares and contributes to a great extent to national food security. Additionally, Asia alone consumes 90% of the freshwater diverted to agriculture in the entire world [2,3]. This will soon be a burden on the ecological balance in many areas, leading to water scarcity. In this case, aerobic rice cultivation is a modern practice for cultivating rice crops with durable water soil and suited, high-yielding varieties that are sown directly dry [4]. This approach saves water significantly; in China, the aerobic rice system of cultivation used 55–56% less water as compared to the traditional transplanted system of cultivation with water productivity that is 1.6–1.9 times higher [5]. To keep pace with the changing scenario, an estimated 22 varieties and 2 hybrids have been released for aerobic conditions in India [4]. According to Ghasal et al. [6], dry and aerobic soil can reduce the natural supply of phosphorus (P), making the application of P fertilizer more crucial for rice grown aerobically. P is necessary for all living organisms, and is a crucial nutrient for the expansion and development of the plants [7,8,9]. Phosphorus makes up about 0.2% of a plant’s dry biomass and is mostly present in tissue components such as phospholipids, nucleic acids, and adenosine triphosphate (ATP) [10]. P is the second most limiting nutrient after nitrogen (N) [11]. It may decrease agricultural productivity and slow down plant growth and development. P exists in three different forms in the soil: organic P, soluble inorganic P, and insoluble inorganic P [7]. The amount of total soil phosphorus varies between 30 and 65% in organic forms, which are unavailable to plants, and 35 to 70% in inorganic forms [12]. Organic P can be found in soil microorganisms and dead plants and animals. P becomes unavailable in the soil because of fixation and immobilization, and 70–90% of phosphate fertilizers become fixed in the ground [13,14]. Soil microorganisms, mainly arbuscular mycorrhizal fungi (AMF), play a key role in mobilizing phosphorus from the soil into plant-available forms [15,16,17,18]. In the root cortical cells of their host plants, AMF create highly branching fungal structures called arbuscules, with which they exchange inorganic minerals, particularly phosphorus, and carbon molecules. AMF are one of the most prevalent organisms in the mycorrhizosphere [19,20] and have interactions and colonization with more than 200,000 different species of host plants with more than 240 different AMF morphotypes [21]. The exploration of mycorrhizal symbiosis is one of the most promising methods for creating resource-efficient and resilient agricultural systems [20,22]. Several studies have reported AMF diversities in rice [23,24,25], but very limited information is available on their performance in aerobics under P-deficient conditions [26]. Additionally, some studies indicated that AMF have a host preference [27] and their performance will vary depending on different agroecosystems [28]. In aerobic rice cultivation, soil P fixation is one of the major problems which causes P deficiency in the soil resulting in yield reduction. The main idea of this study is whether the intervention of suitable AMF will resolve the issue of soil P deficiency in aerobic rice cultivation. Hence, the present study was conducted to evaluate the effect of AMF on P uptake and growth promotion in popular aerobic rice varieties under P-deficient conditions.
2. Materials and Methods
2.1. Low P Soil Sampling, Site Description, and AMF Inoculum and Propagation of AMF
Low-phosphorus (P) soil was collected from Krishi Vigyan Kendra (KVK), Santhpur, ICAR—NRRI, Cuttack, Odisha (20°27′45.08″ N; 85°52′58.76″ E) for the experiment and analysis. The initial properties of the experimental soil were analyzed (Table 1). The sterilized soil was used for a pot experiment. The soil-based single AMF inoculum viz. Funneliformis sp., Rhizophagus sp., Glomus sp., Acaulospora sp. and Claroideoglomus sp. received from Microbiology, ICAR—the National Rice Research Institute (ICAR-NRRI), India, were used in this experiment together with inoculum containing 115–120 AMF spores/g of soil, which was multiplied using finger millet (Eleusine coracana) as the host plant in sterile soil using a trap culturing method (Figure 1) [27,29].

Table 1.
Initial soil properties of the experimental soil sample.

Figure 1.
Monospecific AMF spore propagation using trap cultures and finger millet as host plant.
2.2. Experimental Site and Pot Experiment
The experiment was conducted during the 2020–2021 Rabi season (the Indian cropping season starting from the onset of winter from October-November until spring in March–April) in a controlled net house condition at Microbiology, the ICAR-National Rice Research Institute (NRRI), Cuttack, Odisha (latitude—20°25′ N, longitude—85°55′ E with an altitude of 24 m above mean sea level). The pot (5 kg) experiment was conducted with five different species of AMF and six rice varieties with three replications. The treatment details are as follows, T0: Control, T1: Funneliformis sp., T2: Rhizophagus sp., T3: Glomus sp., T4: Acaulospora sp. and T5: Claroideoglomus sp. In this experiment, four aerobic rice varieties viz. V1: CR Dhan 201, V2: CR Dhan 204, V3: CR Dhan 205, V4: CR Dhan 207 (CR Dhan 201, 204, 205, and 207 developed by ICAR-NRRI, Cuttack), and two check varieties viz. V5: IR 36 (P-susceptible) and V6: Kasalath IC459373 (P-tolerant) were used, and were collected from the Crop Improvement Division, ICAR-NRRI, Cuttack, India. After germination, three plants per pot were maintained. Soil (completely homogenized and transported to the laboratory in a cool pack) and all the plant samples from each pot were collected from all treatment after 60 days in order to estimate the AMF colonization, growth parameters (root length, shoot length, leaf area, chlorophyll, fresh and dry biomass), P uptake, soil chemical, microbial and enzymatic activities analysis [30].
2.3. Assessment of AMF Colonization and Spore Count
The method developed by Phillip and Hayman [31] was used to evaluate the rice root colonization of AMF [32]. Freshly collected root samples were gently washed to remove soil that was attached to the root surfaces, submerged in 10% potassium hydroxide (KOH) solution, and autoclaved for 15 min at 121 °C. The KOH solution was decanted, and the treated roots were rinsed with tap water three times until no brown colour appeared in the rinsed water. The treated root samples were further immersed in 2% hydrochloric acid (HCl) solution for 5 min. Without being rinsed with water, HCl was decanted, and the root samples were stained with 0.05% trypan blue (HiMedia, Maharashtra, India) in lacto-glycerol (400 mL lactic acid + 400 mL glycerol + 100 mL water) and autoclaved for 15 min at 121 °C. After autoclaving, the stained solution was decanted, and the roots were de-stained with lacto-glycerol solution to remove the excess stains and used for microscopic observations. The slide was prepared by keeping 10 segments of the stained root on a clean glass slide and observed under a compound microscope (Zeiss Stemi 508, Oberkochen, Germany). The method described by McGonigle et al. [33] was used to calculate the percentage of root colonization.
AMF root colonization was calculated using the formula:
% of colonization = no. of root segments colonized ÷ total no. of root segments × 100
2.4. Phosphorus Estimation in Plant Sample
Collected plant samples were dried in a hot air oven maintained at 60 °C for up to 5 days in order to attain a constant weight. The determination of P concentration in the plant sample was carried out using the vanadomolybdophosphoric acid method with a spectrophotometer [30,34]. A quantity of 1 gm of the dried plant sample and 10 mL of the concentrate HNO3 were added and kept overnight, following which 10 mL of tri-acid (HNO3, H2SO4, HCLO4 in a ratio of 9:4:1), was added and mixed properly. The mixture was kept in a hot plate at 100 °C for 1 h under a temperature rise up to 200 °C until the content reduced to 2–3 mL and turned colourless. The content was cooled and 10 mL of diluted HCL was added and filtered through Whatman No. 42. The filtrate volume was made up to 100 mL with distilled water. A quantity of 5 mL of the digested sample was taken and 10 mL of vanadomolybdate reagent was added (Merck, Darmstadt, Germany) and kept for 30 min. The absorption of the sample was measured at 420 nm with a spectrophotometer (Analytikjena specord-200, Jena, Germany). A standard curve was prepared with a phosphate solution (0.2195 gm of KH2PO4 in 500 mL of distilled water + 25 mL of 7N H2SO4 and made up to 1 L) and the P content of the plant sample was calculated from the standard curve.
2.5. Estimation of Soil Chemical, Enzymatic and Microbial Properties
The activity of the acid (AcP) and alkaline (AkP) phosphatase of soil samples was estimated by the method of Tabatabai and Bremner [35], using p-nitrophenyl as a substrate and expressed in l g of p-nitrophenyl phosphate (pNP) released per gram of soil per hour. Soil fluorescein diacetate activity (FDA) measurement was carried out by using Scherer and Ross [36] as modified by Adam and Duncan [37]. The concentration of fluorescein released during the assay was calculated using the calibrating graph produced from the 0–5 µg fluorescein mL−1 standard and expressed as µg fluorescein h−1g−1 soil [27]. Dehydrogenase activity (DHA) was estimated by the method of Casida et al. [38]), using triphenyltetrazolium chloride (TTC) as a substrate. Microbial biomass carbon (MBC) was determined using the chloroform fumigation extraction (CFE) method [39].
2.6. Statistical Analysis
The R version 4.2.2 [40] was used for statistical computing. For the identification of important variables related to AMF colonization in plants, a stepwise regression model was constructed using the “stepAIC” function available in the MASS package [41]. The Pearson correlation was constructed using the “ggpairs” function available in the GGally package [42].
3. Results and Discussion
Rice crops are very sensitive to water stress and reduction in water inputs with a consequent decline in yield [43]. Approximately 75% of the rice is produced by a conventional flooding method, and 3000–5000 L of water is needed to produce 1 kg of grains [4,44]. Researchers have developed several technologies to reduce water inputs in rice such as alternate wetting and drying, raised bed rice cultivation, saturated soil culture, a system of rice intensification, ground cover systems, and raised bed systems [45]. Some of the modern technologies additionally require puddling and ponded water during crop growth. In rice cultivation, the aerobic rice has been introduced to minimize the use of water, which is one of the promising water-saving technologies in rice production [46,47]. Aerobic rice reduces water use by 27–51% by limiting water loss due to seepage, percolation, and evaporation and increases water productivity by 32–88% [48]. It has been well documented that microorganisms enhance plant growth under abiotic stress [49].
3.1. Seed Germination of Rice Varieties
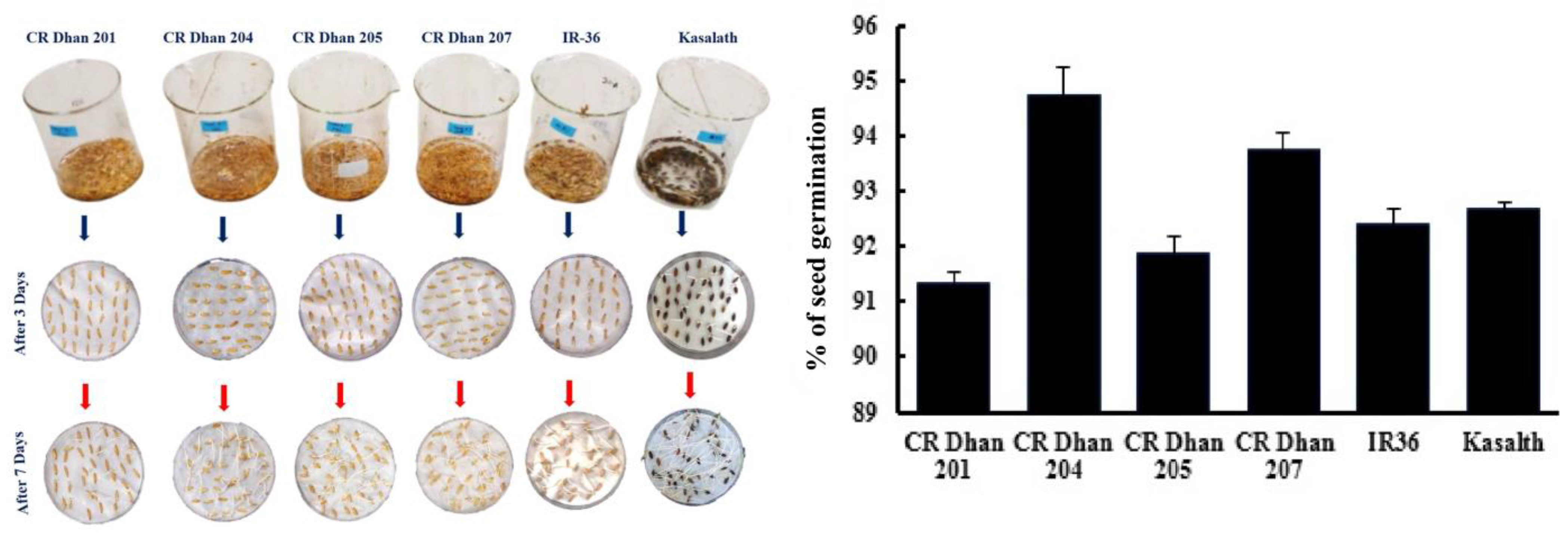
The seed germination percentages of four different aerobic rice varieties (CR Dhan 201, CR Dhan 204, CR Dhan 205, and CR Dhan 207), as well as another P-susceptible variety (IR 36) and P-tolerant variety (Kasalath IC459373) are given in Figure 2. CR Dhan 204 and 207 rice varieties showed the highest germination percentages. However, all the rice types had germination rates of >90%.
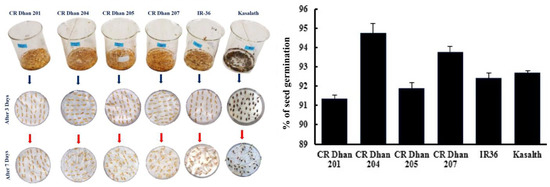
Figure 2.
Percentage of seed germination of six rice varieties.
3.2. AMF Root Colonization in Different Aerobic Rice Varieties
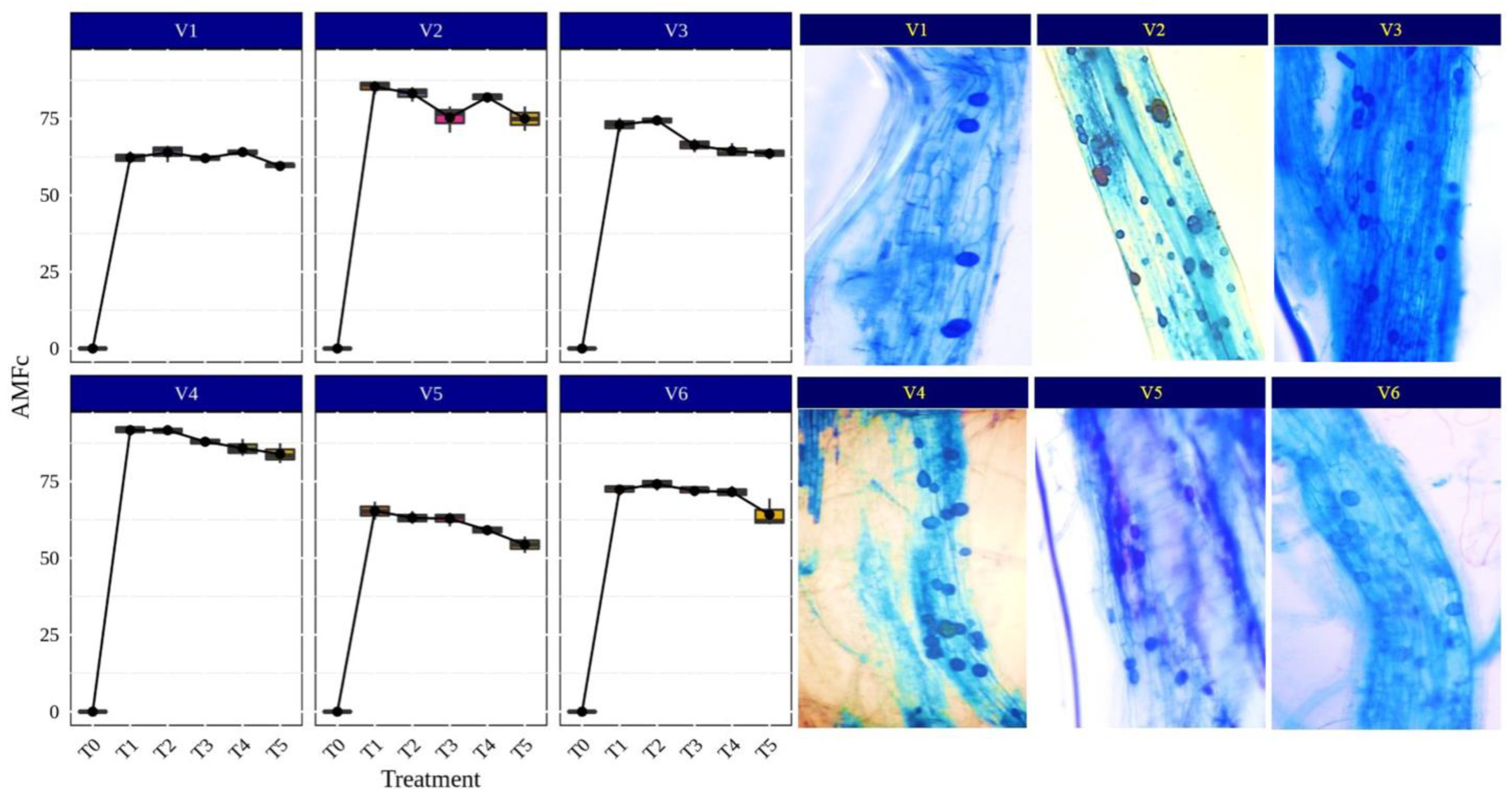
AMF symbiosis increases nutrient and water uptake in plants by external hyphae, regulation of stomatal conductance and the increased activity of antioxidant enzymes. Under aerobic conditions, rice plants readily form mycorrhizal associations as compared to submerged conditions where the anoxic environment limits the mycorrhizal infection process [50,51]. Rice can also be grown with alternate irrigation to reduce the water input and to create aerobic conditions for better AMF fungi colonization in rice roots. Therefore, an investigation was undertaken to understand the benefits of AMF association for rice plant growth and development under aerobic conditions. Narwal et al. [44] found a 20% increase in the plant biomass and 58% higher colonization of Glomus intraradices and G. mosseae (currently Funneliformis mosseae) in upland rice varieties (Pyari, Satyabhama, CR Dhan 205 and CR Dhan 202) compared to lowland rice varieties (Pusa Basmati (PB) 1509, PB 1121, Pusa Sugandha 5 and PB 1612) in pot experiments with sterile soil. The AM plants enhanced the activities of glutamine synthetase and nitrate reductase; the rice genotypes with higher nitrate reductase and glutamine synthetase (Pyari and Satyabhama) also exhibited more (20%) biomass production and plant N content by 36% [44]. In our study, the results of the different AMF-inoculated rice varieties and its root colonization, presented in Figure 3, indicated that Funneliformis sp., Rhizophagus sp. and Glomus sp. showed higher colonization in CR Dhan 207 (91.75, 91.72 and 87.97%, respectively) and CR Dhan 204 (85.43, 83.19, and 75.37%, respectively), while the other genera of AMF recorded a root colonization in the range of 54.38–74.98%.
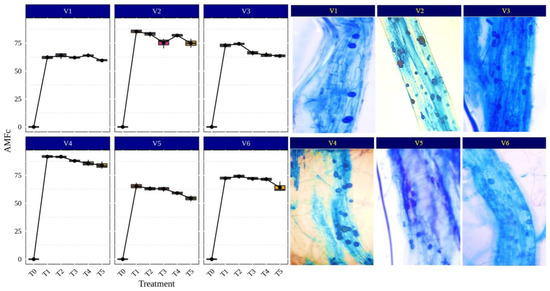
Figure 3.
Percentage of AMF colonization (AMFc) in different rice varieties. Abbreviation: percentage of AMF colonization (AMFc).
3.3. Effect of AMF Inoculation on Physiological and Agronomic Properties
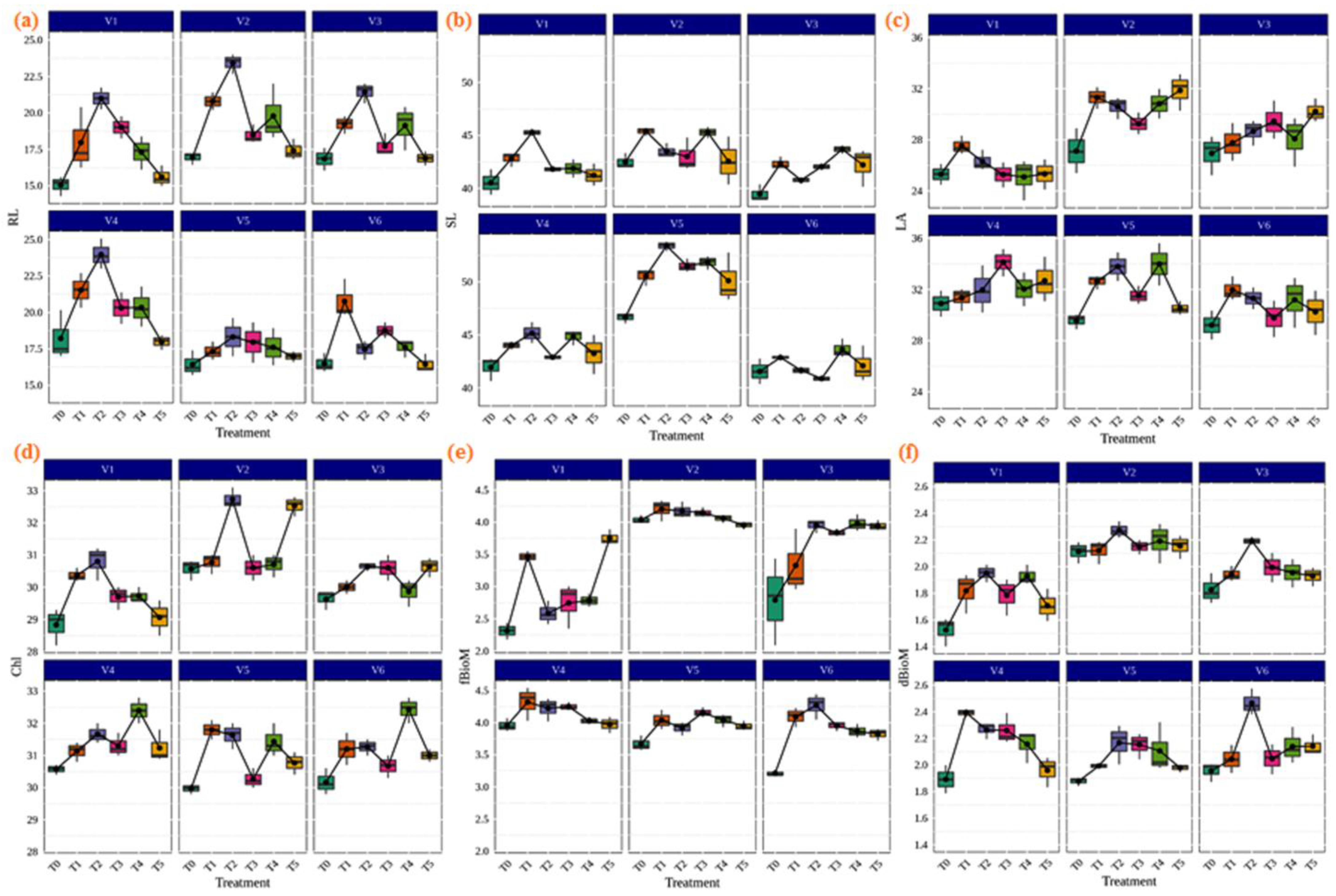
Inoculation of AMF played an important role in the improvement of the biomass chlorophyll contents and physiological and agronomic parameters of the plant. It is widely believed that the inoculation of AMF provides the highest efficiency to host plants for plant growth. As shown in Figure 4, our results demonstrated that AMF inoculation in different rice varieties significantly increased the agronomic parameters, including root length (cm), shoot length (cm), leaf area (m2), chlorophyll (SPAD), fresh biomass (gm), and dry biomass (gm) compared to the control. The highest shoot and root lengths were found in IR36 (53.40 cm) and CR Dhan 207 (23.973 cm) with the treatment of Rhizophagus sp. (Figure 4a,b). In the rice variety CR Dhan 207 (34.127 m2), treatment with Glomus sp. showed the best improvements for the leaf area (Figure 4c). Chlorophyll (SPAD) levels were highest in CR Dhan 204 (32.73) with Rhizophagus sp.; CR Dhan 204 (32.53) with Claroideoglomus sp.; Kasalath IC459373 (32.43) and CR Dhan 207 (32.40) with Acaulospora sp. treatment (Figure 4d). The Funneliformis sp. treated with CR Dhan 207 (4.32 gm) and Rhizophagus sp. treated with Kasalath IC459373 (2.466 gm) had the maximum performance in terms of plant fresh biomass and dried biomass, respectively (Figure 4e,f). However, the plant growth parameters viz. root length, leaf area, chlorophyll and plant biomass showed themselves to be significantly higher in CR Dhan 207 and CR Dhan 204 inoculated with Rhizophagus sp., Glomus sp., Funneliformis sp., and Acaulospora sp.
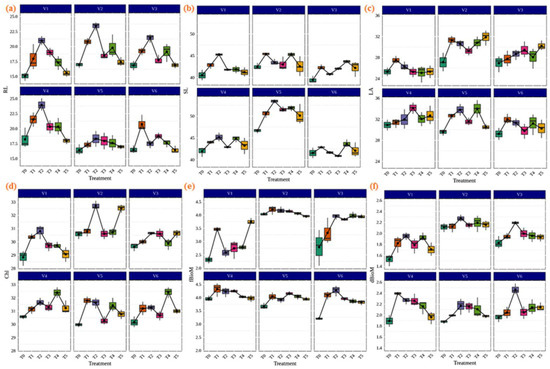
Figure 4.
Enhancement of plant growth parameters due to AMF inoculation in different aerobic rice varieties. Abbreviations: (a) root length in cm. (RL); (b) shoot length in cm. (SL); (c) leaf area m2 (LA); (d) chlorophyll SPAD (Chl); (e) fresh biomass in gm. (fBioM); (f) dry biomass in gm. (dBioM).
3.4. Effects of AMF on Uptake of Plant P
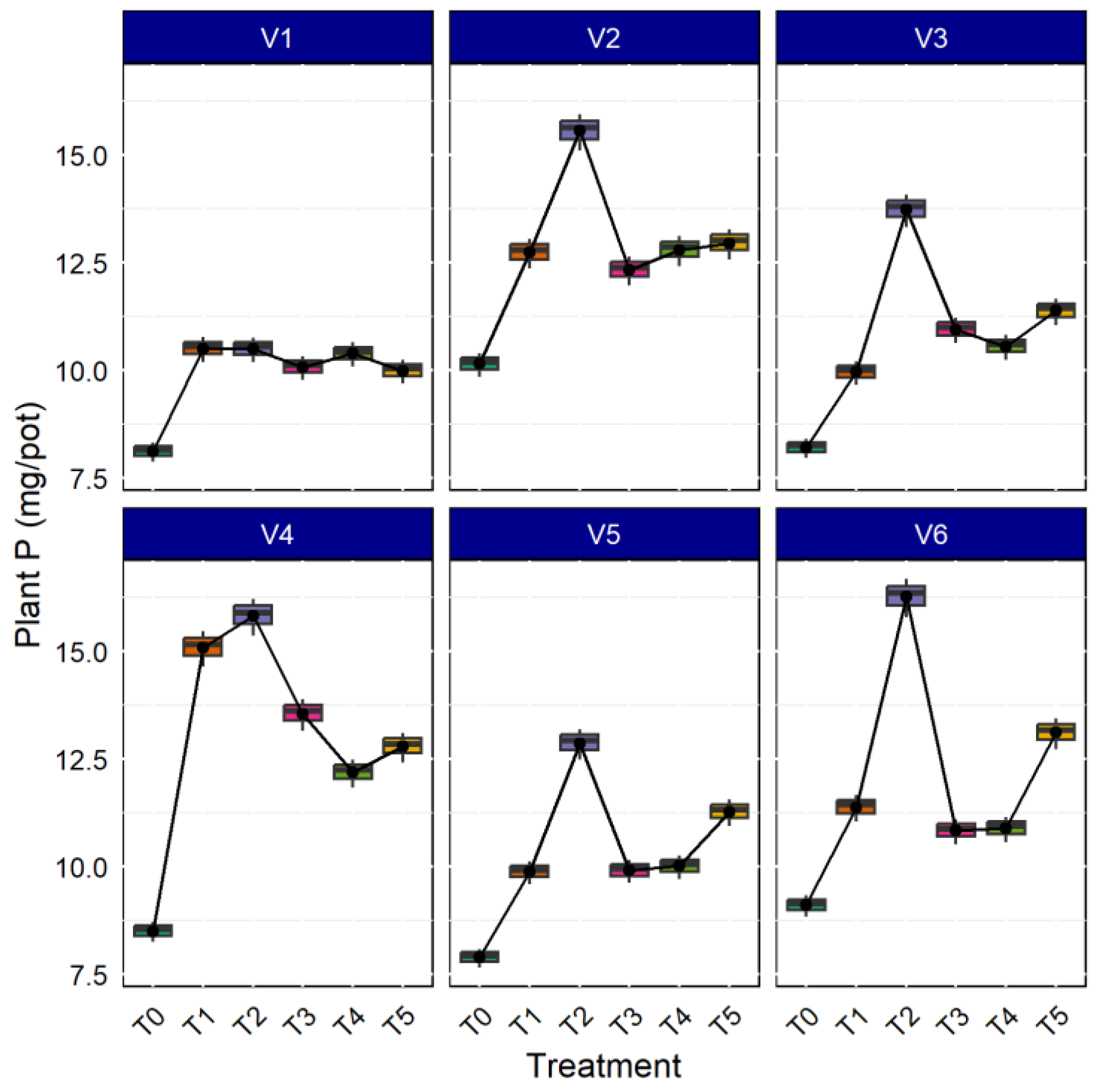
AMF both in aerobic and anaerobic rice cultivation increases nutrient concentration in the rice plant tissue; the bioavailability of nutrients increased in the soil solution due to mycorrhizae inoculation [52]. As shown in Figure 5, the P concentration in plants was higher in the rice variety CR Dhan 207 (14.796 mg. pot−1), followed by Kasalath IC459373 (14.186 mg. pot−1) and CR Dhan 204 (14.156 mg. pot−1). Additionally, all the rice varieties inoculated with Rhizophagus sp., showed maximum P uptake, followed by Funneliformis sp., and Glomus sp. inoculation. The results deciphered 16.60–28.50% higher P uptake with AMF inoculation in all the rice varieties, compared to the uninoculated control.
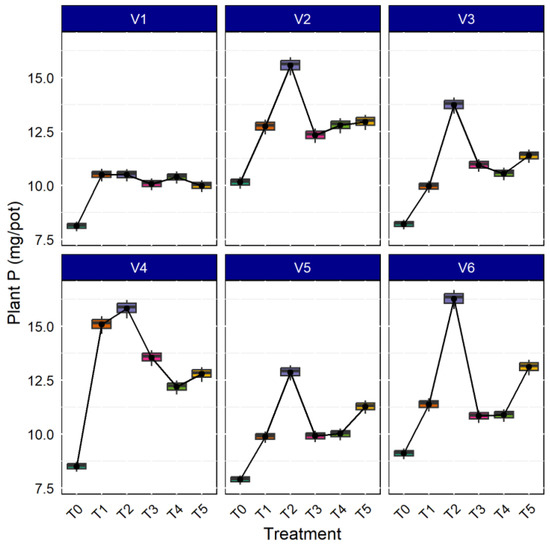
Figure 5.
AMF inoculation on uptake of plant P in different aerobic rice varieties.
3.5. Responses of AMF on Soil Enzyme and Microbial Properties
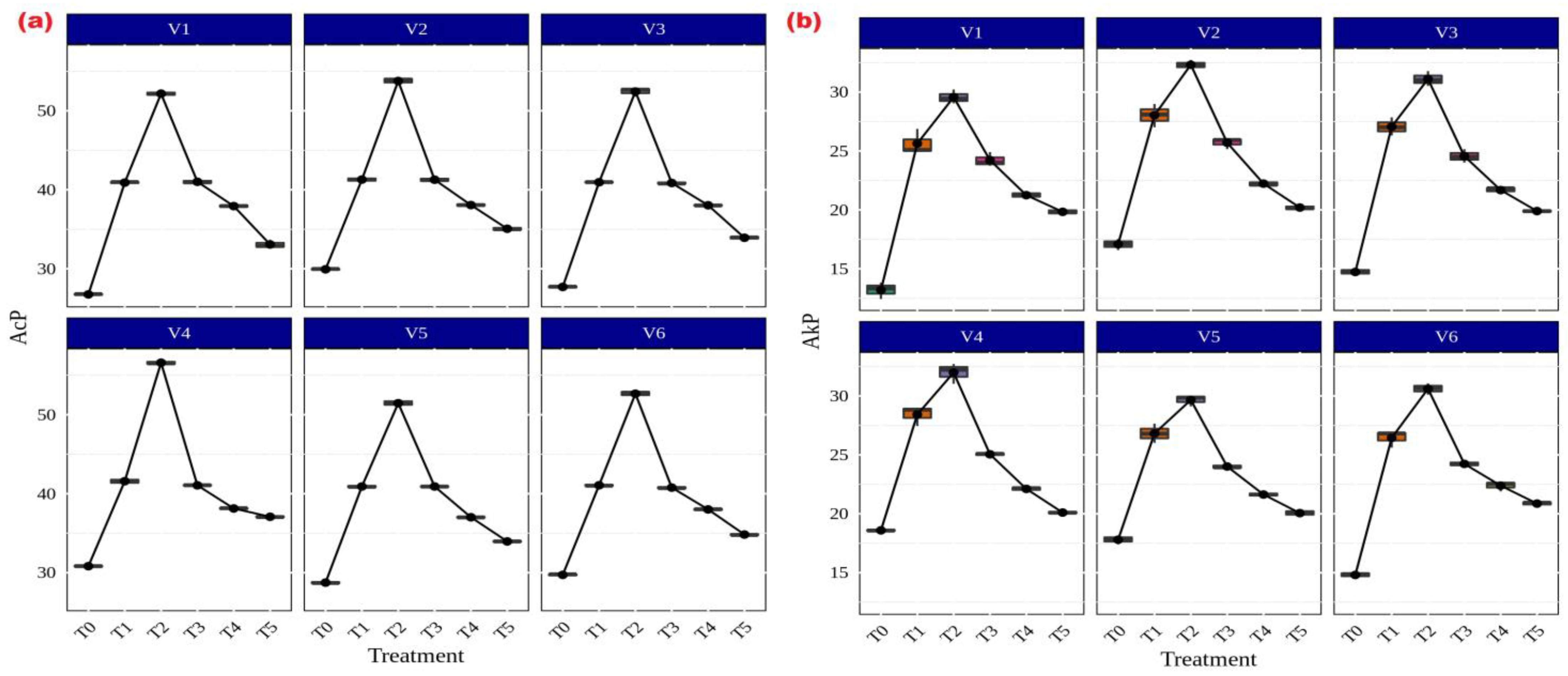
Among the several AMF treatments, Rhizophagus sp. (56.59 g p-nitrophenol released h−1 g−1 soil) and Funneliformis sp. (31.99 g p-nitrophenol released h−1 g−1 soil) showed the highest levels of both acid (Figure 6a) and alkaline (Figure 6b) phosphatase activity in CR Dhan 207. Irrespective of the treatments, all rice varieties showed significantly higher acid and alkaline phosphatase activity in AMF-inoculated treatments as compared to the uninoculated control.
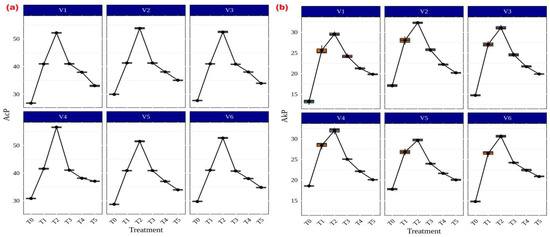
Figure 6.
Enhancement of acid and alkaline phosphatase activities in different aerobic rice varieties. Abbreviations: (a) acid phosphatase (AcP) [µg p-nitrophenol released h−1 g−1 soil]; (b) alkaline phosphatase (AkP) [µg p-nitrophenol released h−1 g−1 soil].
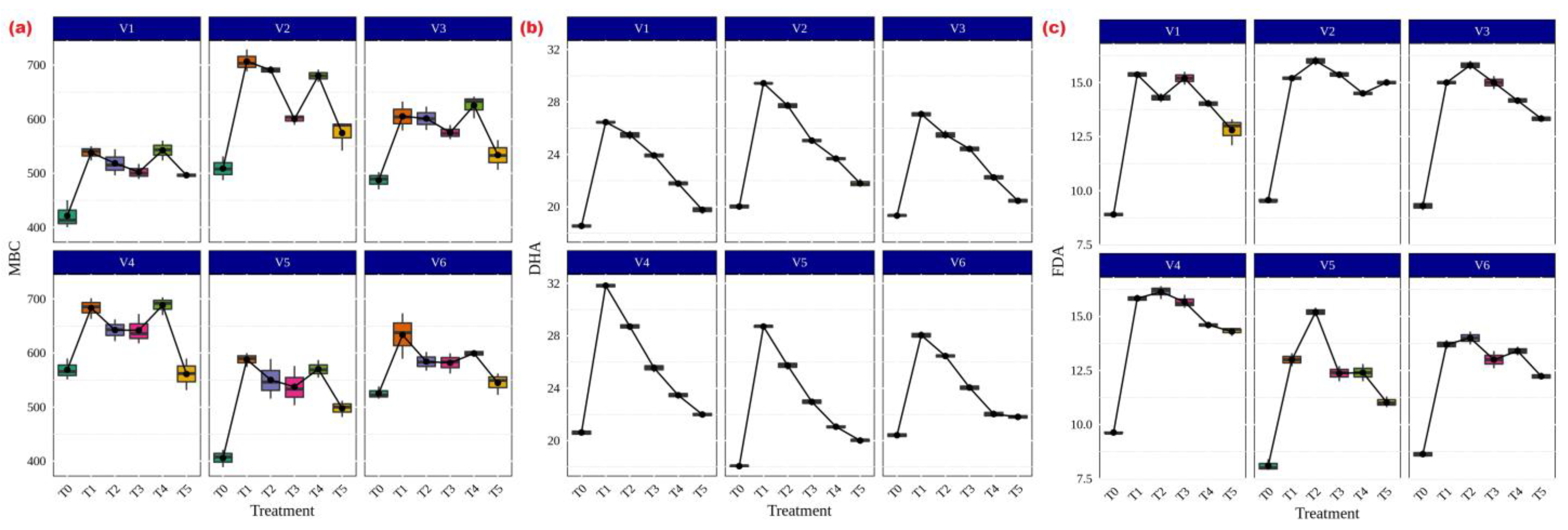
In terms of microbial properties, Funneliformis sp., Rhizophagus sp., Glomus sp., Acaulospora sp. and Claroideoglomus sp. treatments significantly increased MBC in CR Dhan 204 and CR Dhan 207 (706.8 and 688.4 µg g−1 soil) (Figure 7a). A similar trend was also noticed in DHA (29.43 and 31.82 µgTPF h−1g−1 soil) (Figure 7b) and FDA (15.37 and 16.13 µg fluorescein h−1g−1 soil) (Figure 7c).
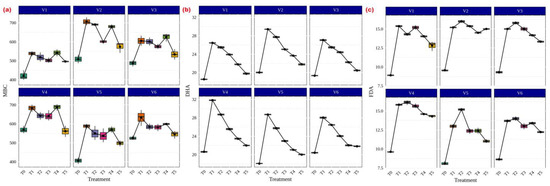
Figure 7.
AMF and its influence on enhancement of microbial properties in different aerobic rice varieties. Abbreviations: (a) microbial biomass carbon (MBC) [µg g−1 soil]; (b) dehydrogenase activity (DHA) [µgTPF h−1g−1 soil]; (c) fluorescein diacetate assay (FDA) [µg fluorescein h−1g−1 soil].
Through increasing microbial activity in the soil or by the exudation of enzymes by plants, AMF can also have an impact on soil enzyme activity as well as plant growth promotion [53,54,55]. Several studies have described how AMF intervention could stimulate soil enzyme activity through soil microorganisms [20,27,56,57]. Generally, soil enzymes are primarily produced by microorganisms; others, such as phosphatase [58], urease, and peroxidases, are also secreted by plant roots. Reports [59,60,61] have shown that the effects of AMF on various soil enzyme activities and growth-promoting compounds, which release the more biologically accessible nutrients from complex materials, were positively correlated with increasing ratios of soil-available P and plant biomass as well as strongly abiotic context-dependent factors, with beneficial implications for plant growth. All of the aforementioned data made it very evident that AMF will increase soil enzyme activity, which could improve nutrient cycling.
3.6. Assessing the Mycorrhizal Responsiveness in Different Aerobic Rice Varieties
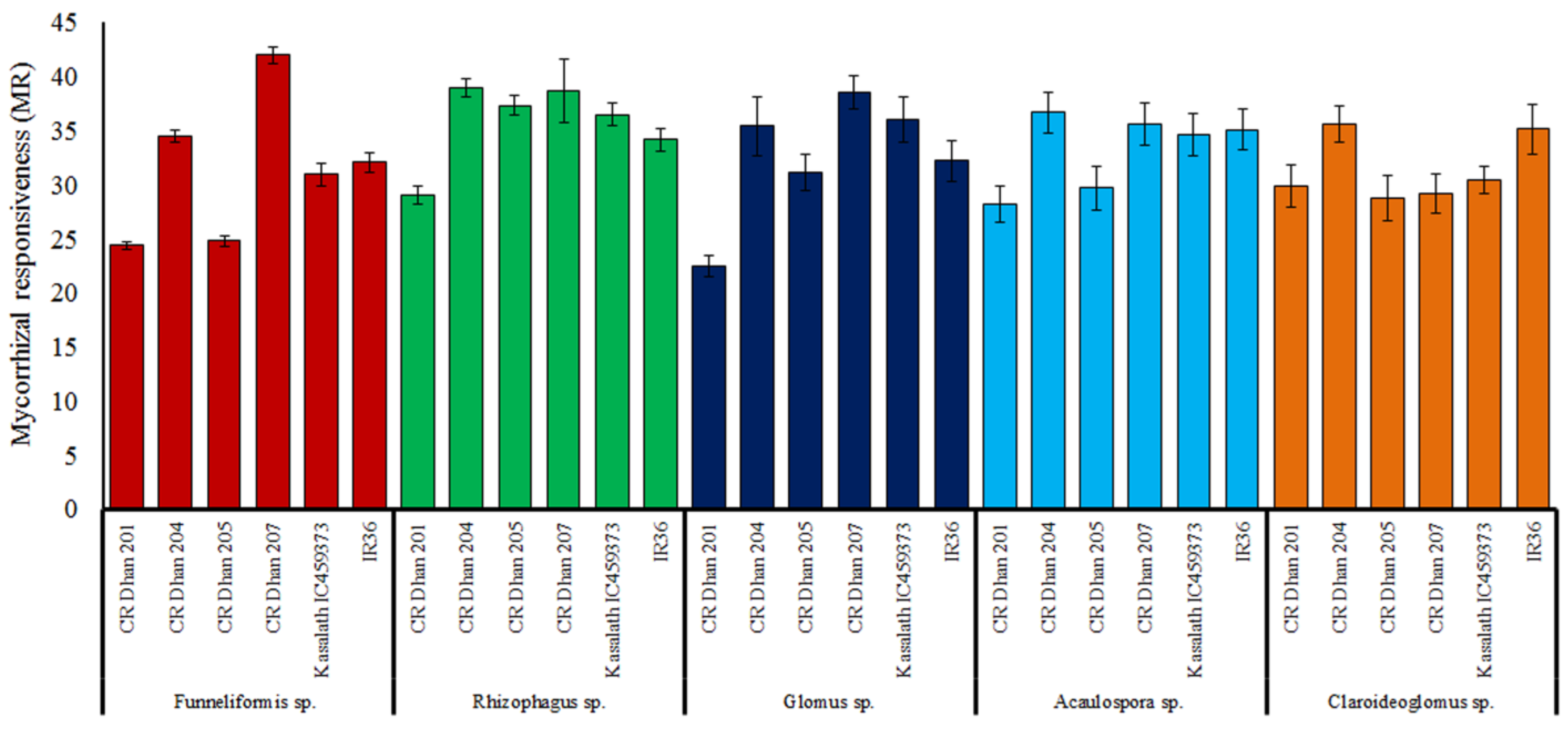
Out of the selected rice varieties, mycorrhizal responsiveness was found highest in CR Dhan 207 followed by CR Dhan 204, CR Dhan 205 and Kasalath IC459373 with the application of Funneliformis sp. and Rhizophagus sp. under P-deficient conditions (Figure 8); however, the AMF responsiveness varies with different rice varieties.
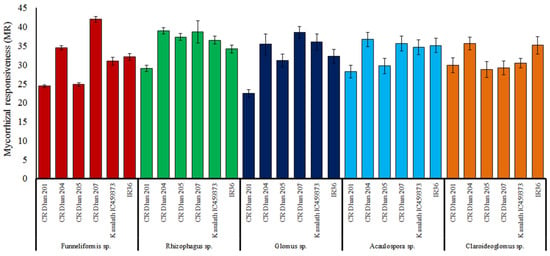
Figure 8.
Mycorrhizal responsiveness in six aerobic rice varieties with five AMF inoculum effects.
3.7. Correlation of AMF Colonization with Soil and Plant Properties Using Linear Models
The linear model was used to select the important parameters linked to AMF colonization and to calculate the correlation of the important variables (Table 2).

Table 2.
Identification of important parameters using step regression model.
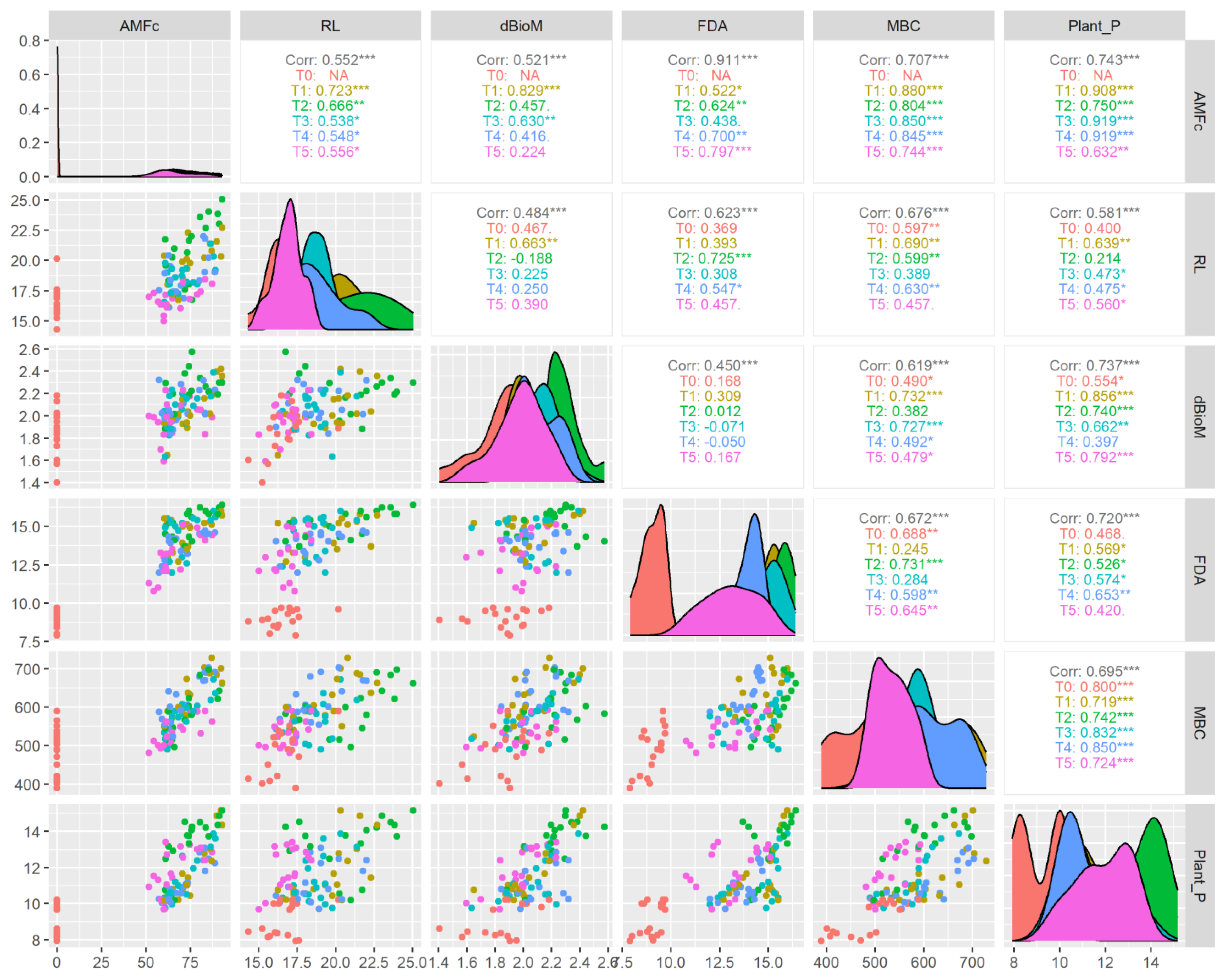
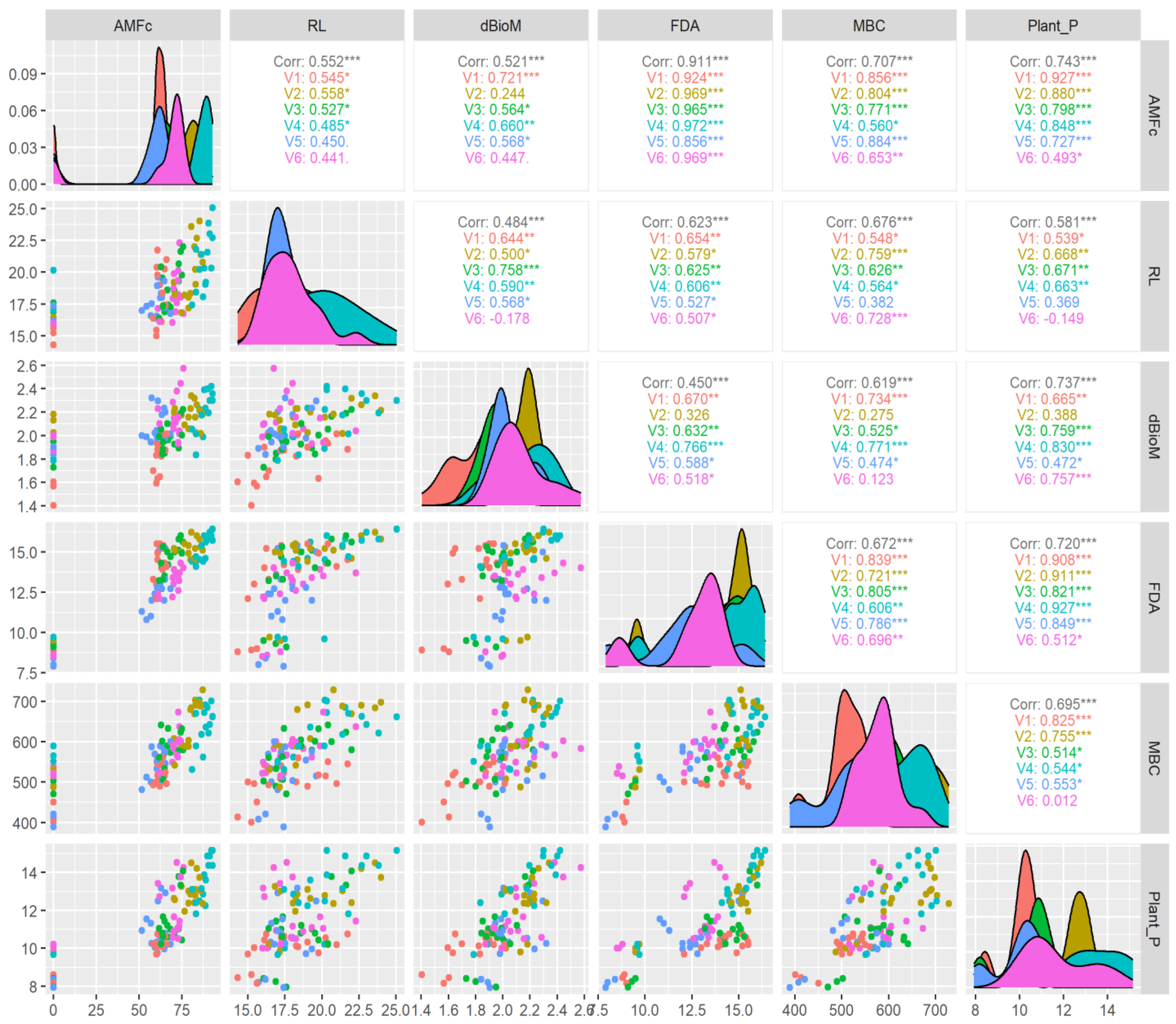
The correlation analysis (Figure 9) showed that AMF colonization had a significant (p < 0.001) positive correlation with FDA (R2 = 0.911), MBC (R2 = 0.707) and plant-available P (R2 = 0.743). The correlation between AMF colonization and FDA, the Claroideoglomus sp. (R2 = 0.797) and Acaulospora sp. (R2 = 0.700) treatments, showed a higher coefficient than other treatments. Similarly, with AMF colonization and MBC correlation, the higher coefficients were recorded in the treatment Funneliformis sp. (R2 = 0.880) followed by Glomus sp. (R2 = 0.850), Acaulospora sp. (R2 = 0.845), —Rhizophagus sp. (R2 = 0.804) and Claroideoglomus sp. (R2 = 0.744) at p < 0.011 levels of significance. The correlation coefficient between AMF colonization and plant P was significantly (p < 0.01) at par for microbial treatments Acaulospora sp. (R2 = 0.919), Glomus sp. (R2 = 0.919), Funneliformis sp. (R2 = 0.908), Rhizophagus sp. (R2 = 0.705), and Claroideoglomus sp. (R2 = 0.632). Similarly, many scientific reports have well documented that AMF plays a crucial role in soils for improving microbial activity, nutrient cycling, soil structure and plant–soil microbe interactions [62,63,64,65,66].
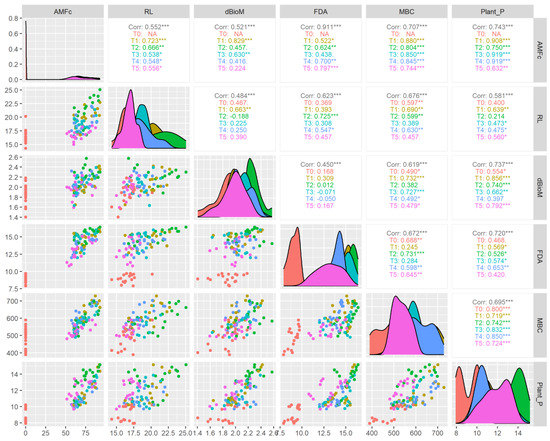
Figure 9.
Correlation of AMF treatments in different aerobic rice varieties on plant P uptake and soil microbial properties. * p < 0.05, ** p < 0.01, *** p < 0.001.
Further correlation studies among varieties given in Figure 10 show that CR Dhan 207 (R2 = 0.972), CR Dhan 204 (R2 = 0.969), and Kasalath IC459373 (R2 = 0.969) had the maximum coefficients between AMF colonization and FDA (R2 = 0.911) among the different aerobic varieties. However, the correlation between AMF colonization and MBC (R2 = 0.707) indicated that, among the varieties, IR 36 (R2 = 0.884) and CR Dhan 201 (R2 = 0.856) had the highest coefficient values, whereas CR Dhan 207 (R2 = 0.560) and Kasalath IC459373 (R2 = 0.653) registered the lowest coefficient among other varieties. Regarding the correlation between varieties and plant P uptake (R2 = 0.743), the highest coefficient was found in CR Dhan 207 (R2 = 0.927), at p < 0.001 significance. This finding clearly indicates that the response of AMF differs based on the type of variety. Thus, the selection of the right type of AMF is essential for exploring the maximum benefit from AMF symbiosis. Das et al. [67] reported that the application of Glomus spp. inoculation improved rice crop yields with better P availability in the rhizosphere under alternate wetting and drying irrigation.
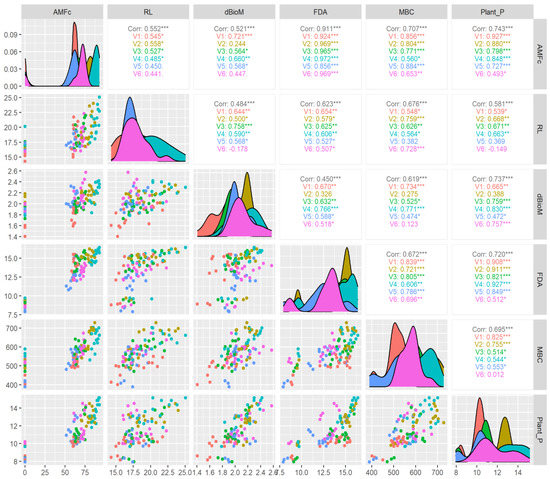
Figure 10.
Response of aerobic rice varieties in AMF colonization correlation with plant P and soil microbial properties using Pearson correlation. * p < 0.05, ** p < 0.01, *** p < 0.001.
4. Conclusions
Soil phosphorus deficiency is one of the major problems in aerobic rice cultivation. The fixation of this element in the soil makes it unavailable for plant uptake. The present study revealed that AMF intervention could significantly increase the plant growth and enhance P uptake by 16.60–28.50% compared to the control. Among the four different aerobic rice varieties, the mycorrhizal responsiveness was found to be superior in CR Dhan 207, followed by CR Dhan 204, CR Dhan 205, and CR Dhan 201. The linear modelling approach found that the AMF colonization in all the rice varieties had significant (p < 0.001) positive correlation with FDA, MBC, and P uptake, deciphering the importance of AMF association in rice for the improvement of phosphate availability to plants. The present findings require further field validation. However, results suggest that the external application of suitable AMF is essential for improving the plant growth and enhancing the uptake of P in aerobic rice in P-deficient soil.
Author Contributions
Conceptualization, P.P., P.K.D.M. and D.M.; methodology, P.P., P.K.D.M. and D.M.; software, A.S. and D.M.; validation, P.P. and P.C.; formal analysis, D.M.; investigation, P.P., P.C., A.K.N. and P.K.D.M.; data curation, D.M.; writing—original draft preparation, D.M., P.P. and A.S.; visualization, D.M. and A.S.; supervision, P.P. and P.K.D.M.; project administration, P.P. and A.K.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Acknowledgments
The authors are grateful to the Honourable Director, ICAR—the National Rice Research Institute, India; the Honourable Vice-Chancellor, Raiganj University, India; the Department of Biotechnology, Government of India (BT/PR36476/ NNT/28/1723/2020) and Project no 2.7 (ICAR-NRRI, Cuttack) for support. DM is grateful to the Government of West Bengal, India, for a Swami Vivekananda Merit Cum Means Ph.D. Scholarship (WBP191584588825). The authors wish to extend special thanks to A. Anandan, Principal Scientist (Genetics and Plant Breeding), ICAR-NRRI, Cuttack, for providing seeds and support for this experiment. The research article is a part of Debasis Mitra’s Ph.D. research programme, which was supervised by P. Panneerselvam, Principal Scientist, CPD, ICAR-NRRI, Cuttack, and Pradeep K. Das Mohapatra, Associate Professor and Head, Department of Microbiology, Raiganj University, India.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fukagawa, N.K.; Ziska, L.H. Rice: Importance for global nutrition. J. Nutr. Sci. Vitaminol. 2019, 65, S2–S3. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, V. Water and agriculture in India. In Background Paper for the South Asia Expert Panel during the Global Forum for Food and Agriculture; OAV—German Asia-Pacific Business Association: Hamburg, Germany, 2017; Volume 28, pp. 80–85. [Google Scholar]
- Tuong, T.P.; Bouman, B.A. Rice production in water-scarce environments. In Water Productivity in Agriculture: Limits and Opportunities for Improvement; Kijne, J.W., Barker, R., Molden, D., Eds.; CABI: Wallingford, CT, USA, 2003; Volume 1, pp. 13–42. [Google Scholar]
- Anandan, A.; Pradhan, S.K.; Panda, S.; Dash, S.K.; Panneerselvam, P.; Meher, J.; Patra, B.C. Aerobic dry direct seeded rice: A system of rice cultivation for water shortfall irrigated and lowland areas. In Proceedings of the NRRI Research Bulletin No. 33, ICAR-National Rice Research Institute, Cuttack-753006, Odisha, India, 23 April 2021; p. 32. [Google Scholar]
- Priyanka, S.; Jitesh, B.; Babu, S. Aerobic rice, a new approach of rice cultivation. Int. J. Res. Biol. Sci. 2012, 1, 1–6. [Google Scholar]
- Ghasal, K.; Pathak, K.; Mohan, G. Performance of Aerobic Rice under Different Sources and Levels of Phosphorus. Int. J. Curr. Microbiol. App. Sci. 2020, 11, 3699–3706. [Google Scholar]
- McDowell, R.W.; Noble, A.; Pletnyakov, P.; Haygarth, P.M. A Global Database of Soil Plant Available Phosphorus. Sci. Data 2023, 10, 125. [Google Scholar] [CrossRef]
- Mitra, D.; Nayeri, F.D.; Sansinenea, E.; Ortiz, A.; Bhatta, B.B.; Adeyemi, N.O.; Janeeshma, E.; Tawfeeq Al-Ani, L.K.; Sharma, S.B.; Boutaj, H.; et al. Unraveling arbuscular mycorrhizal fungi interaction in rice for plant growth development and enhancing phosphorus use efficiency through recent development of regulatory genes. J. Plant Nutr. 2023, 46, 1–37. [Google Scholar] [CrossRef]
- Mitra, D.; Khoshru, B.; Mohapatra, P.K.D.; Panneerselvam, P. Beneficial Interaction of Arbuscular Mycorrhizal Fungi in Plant to Improve the Uptake of Phosphorus. Indian J. Plant Soil 2020, 7, 61–63. [Google Scholar]
- Kayoumu, M.; Iqbal, A.; Muhammad, N.; Li, X.; Li, L.; Wang, X.; Gui, H.; Qi, Q.; Ruan, S.; Guo, R.; et al. Phosphorus Availability Affects the Photosynthesis and Antioxidant System of Contrasting Low-P-Tolerant Cotton Genotypes. Antioxidants 2023, 12, 466. [Google Scholar] [CrossRef]
- Prasad, R.; Chakraborty, D. Phosphorus basics: Understanding phosphorus forms and their cycling in the soil. Ala. Coop. Ext. Syst. 2019, ANR-2535. Available online: https://www.aces.edu/blog/topics/crop-production/understanding-phosphorus-forms-and-their-cycling-in-the-soil/ (accessed on 3 April 2023).
- Steffens, D.; Leppin, T.; Luschin-Ebengreuth, N.; Min Yang, Z.; Schubert, S. Organic soil phosphorus considerably contributes to plant nutrition but is neglected by routine soil-testing methods. J. Plant Nutr. Soil Sci. 2010, 173, 765–771. [Google Scholar] [CrossRef]
- Ibrahim, M.; Iqbal, M.; Tang, Y.T.; Khan, S.; Guan, D.X.; Li, G. Phosphorus Mobilization in Plant–Soil Environments and Inspired Strategies for Managing Phosphorus: A Review. Agronomy 2022, 12, 2539. [Google Scholar] [CrossRef]
- Johan, P.D.; Ahmed, O.H.; Omar, L.; Hasbullah, N.A. Phosphorus transformation in soils following co-application of charcoal and wood ash. Agronomy 2021, 11, 2010. [Google Scholar] [CrossRef]
- Panneerselvam, P.; Das Mohapatra, P.K.; Nayak, A.K.; Mitra, D.; Velmourougane, K.; Santos-Villalobos, S.D.L. Arbuscular Mycorrhizal Fungi: For Nutrient, Abiotic and Biotic Stresses Management in Rice, 1st ed.; CRC Press: Boca Raton, FL, USA, 2023; p. 232. [Google Scholar]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of arbuscular mycorrhizal fungi in plant growth regulation: Implications in abiotic stress tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef] [PubMed]
- Wilkes, T.I. Arbuscular Mycorrhizal Fungi in Agriculture. Encyclopedia 2021, 1, 1132–1154. [Google Scholar] [CrossRef]
- Panneerselvam, P.; Kumar, U.; Sugitha, T.C.K.; Parameswaran, C.; Sahoo, S.; Binodh, A.K.; Jahan, A.; Anandan, A. Arbuscular mycorrhizal fungi (AMF) for sustainable rice production. In Advances in Soil Microbiology: Recent Trends and Future Prospects: Volume 2: Soil-Microbe-Plant Interaction; Adhya, T.K., Lal, B., Mohapatra, B., Paul, D., Das, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 99–126. [Google Scholar]
- Sahoo, S.; Panneerselvam, P.; Chowdhury, T.; Kumar, A.; Kumar, U.; Jahan, A.; Senapati, A.; Anandan, A. Understanding the AM fungal association in flooded rice under elevated CO2 condition. ORYZA-Int. J. Rice 2017, 54, 290–297. [Google Scholar] [CrossRef]
- Mythili, M.; Ramalakshmi, A. Unraveling the distribution of AMF communities and their metabolites associated with soils of minor millets. Rhizosphere 2022, 21, 100473. [Google Scholar] [CrossRef]
- Mitra, D.; Guerra, B.E.; Khoshru, B.; De Los Santos Villalobos, S.; Belz, C.; Chaudhary, P.; Shahri, F.N.; Djebaili, R.; Adeyemi, N.O.; El-Ballat, E.M.; et al. Impacts of arbuscular mycorrhizal fungi on rice growth, development, and stress management with a particular emphasis on strigolactone effects on root development. Commun. Soil Sci. Plant Anal. 2021, 52, 1591–1621. [Google Scholar] [CrossRef]
- Baki, M.Z.I.; Suzuki, K.; Takahashi, K.; Chowdhury, S.A.; Asiloglu, R.; Harada, N. Molecular genetic characterization of arbuscular mycorrhizal fungi associated with upland rice in Bangladesh. Rhizosphere 2021, 18, 100357. [Google Scholar] [CrossRef]
- Sarkodee-Addo, E.; Yasuda, M.; Gyu Lee, C.; Kanasugi, M.; Fujii, Y.; Ansong Omari, R.; Oppong Abebrese, S.; Bam, R.; Asuming-Brempong, S.; Mohammad Golam Dastogeer, K.; et al. Arbuscular mycorrhizal fungi associated with rice (Oryza sativa L.) in Ghana: Effect of regional locations and soil factors on diversity and community assembly. Agronomy 2020, 10, 559. [Google Scholar] [CrossRef]
- Chareesri, A.; De Deyn, G.B.; Sergeeva, L.; Polthanee, A.; Kuyper, T.W. Increased arbuscular mycorrhizal fungal colonization reduces yield loss of rice (Oryza sativa L.) under drought. Mycorrhiza 2020, 30, 315–328. [Google Scholar] [CrossRef]
- Narwal, E.; Annapurna, K.; Choudhary, J.; Sangwan, S. Effect of arbuscular mycorrhizal fungal colonization on nutrient uptake in rice aerobic conditions. Int. J. Curr. Microbiol. App. Sci. 2018, 7, 1072–1093. [Google Scholar] [CrossRef]
- Panneerselvam, P.; Sahoo, S.; Senapati, A.; Kumar, U.; Mitra, D.; Parameswaran, C.; Anandan, A.; Kumar, A.; Jahan, A.; Nayak, A.K. Understanding interaction effect of arbuscular mycorrhizal fungi in rice under elevated carbon dioxide conditions. J. Basic Microbiol. 2019, 59, 1217–1228. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Kuyper, T.W.; Zou, C.; Zhang, F.; Hoffland, E. Mycorrhizal responsiveness of aerobic rice genotypes is negatively correlated with their zinc uptake when nonmycorrhizal. Plant Soil 2007, 290, 283–291. [Google Scholar] [CrossRef]
- Trejo-Aguilar, D.; Banuelos, J. Isolation and culture of arbuscular mycorrhizal fungi from field samples. Methods Mol. Biol. 2020, 2146, 1–18. [Google Scholar] [PubMed]
- Nayak, A.K.; Bhattacharya, P.; Shahid, M.; Tripathi, R. Modern Techniques in Soil and Plant Analysis; KALYANI Publisher: Cuttack, India, 2016; p. 272. [Google Scholar]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158-IN18. [Google Scholar] [CrossRef]
- Ganeshamurthy, A.N.; Sharma, K.; Mitra, D.; Radha, T.K.; Rupa, T.R. Isolation and characterization of Arbuscular mycorrhizal fungi and their role in plants growing under harsh environments. Mycorrhiza News-Mycorrhizae 2017, 29, 7–12. [Google Scholar]
- McGonigle, T.P.; Miller, M.H.; Evans, D.G.; Fairchild, G.L.; Swan, J.A. A new method which gives an objective measure of colonization of roots by vesicular—Arbuscular mycorrhizal fungi. New Phytol. 1990, 115, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Arrhenius, O. Phosphorus determination by the molybdenum blue method. Arch. Suikerind. 1927, 35, 903–911. [Google Scholar]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Schnrer, J.; Rosswall, T. Fluorescein diacetate hydrolysis as a measure of total microbial activity in soil and litter. Appl. Environ. Microbiol. 1982, 43, 1256–1261. [Google Scholar] [CrossRef]
- Adam, G.; Duncan, H. Development of a sensitive and rapid method for the measurement of total microbial activity using fluorescein diacetate (FDA) in a range of soils. Soil Biol. Biochem. 2001, 33, 943–951. [Google Scholar] [CrossRef]
- Casida, L.E., Jr.; Klein, D.A.; Santoro, T. Soil dehydrogenase activity. Soil Sci. 1964, 98, 371–376. [Google Scholar] [CrossRef]
- Witt, C.; Gaunt, J.L.; Galicia, C.C.; Ottow, J.C.; Neue, H.U. A rapid chloroform-fumigation extraction method for measuring soil microbial biomass carbon and nitrogen in flooded rice soils. Biol. Fertil. Soils 2000, 30, 510–519. [Google Scholar] [CrossRef]
- R Core Team. R Language Definition; R Foundation for Statistical Computing: Vienna, Austria, 2000; Volume 3. [Google Scholar]
- Ripley, B.; Venables, B.; Bates, D.M.; Hornik, K.; Gebhardt, A.; Firth, D.; Ripley, M.B. Package ‘Mass’. Cran r; 2013; Volume 538, pp. 113–120. [Google Scholar]
- Schloerke, B.; Crowley, J.; Cook, D. Package ‘GGally’. In Extension to ‘ggplot2’; 2018; p. 713. [Google Scholar]
- Panda, D.; Mishra, S.S.; Behera, P.K. Drought tolerance in rice: Focus on recent mechanisms and approaches. Rice Sci. 2021, 28, 119–132. [Google Scholar] [CrossRef]
- Narwal, E.; Annapurna, K.; Choudhary, J.; Dhakar, R.; Singh, Y. Bioprospecting aerobic rice (Oryza sativa) and mycorrhizal interaction for nutrient uptake and plant growth. Indian J. Agric. Sci. 2021, 91, 1236–1277. [Google Scholar] [CrossRef]
- Kadiyala, S.; Gillespie, S.; Thorat, S. Tackling the Agriculture-Nutrition disconnect in India: The way forward. In Tackling the Agriculture-Nutrition Disconnect in India; Kadiyala, S., Gillespie, S., Thorat, S., Eds.; Draft Book; International Food Policy Research Institute: Washington, DC, USA, 2012. [Google Scholar]
- Mahapatra, B.S.; Bhupenchandra, I.; Devi, S.H.; Kumar, A.; Chongtham, S.K.; Singh, R.; Babu, S.; Bora, S.S.; Devi, E.L.; Verma, G. Aerobic Rice and its significant perspective for sustainable crop production. Indian J. Agron. 2021, 66, 383–392. [Google Scholar]
- Jana, K.; Karmakar, R.; Banerjee, S.; Sana, M.; Goswami, S.; Puste, A.M. Aerobic rice cultivation system: Eco-friendly and water saving technology under changed climate. Agric. Res. Technol. 2018, 13, 40–44. [Google Scholar]
- Bouman, B.A.; Humphreys, E.; Tuong, T.P.; Barker, R. Rice and water. Adv. Agron. 2007, 92, 187–237. [Google Scholar]
- Ruiz-Sanchez, M.C.; Domingo, R.; Castel, J.R. Deficit irrigation in fruit trees and vines in Spain. Span. J. Agric. Res. 2010, 8, 5–20. [Google Scholar] [CrossRef]
- Watanarojanaporn, N.; Boonkerd, N.; Tittabutr, P.; Longtonglang, A.; Young, J.P.W.; Teaumroong, N. Effect of rice cultivation systems on indigenous arbuscular mycorrhizal fungal community structure. Microbes Environ. 2013, 28, 316–324. [Google Scholar] [CrossRef]
- Xavier Martins, W.F.; Rodrigues, B.F. Identification of dominant arbuscular mycorrhizal fungi in different rice ecosystems. Agric. Res. 2020, 9, 46–55. [Google Scholar] [CrossRef]
- Iqbal, M.T.; Ahmed, I.A.; Isik, M.; Sultana, F.; Ortaş, I. Role of mycorrhizae inoculations on nutrient uptake in rice grown under aerobic and anaerobic water management. J. Plant Nutr. 2021, 44, 550–568. [Google Scholar] [CrossRef]
- Xu, H.; Shao, H.; Lu, Y. Arbuscular mycorrhiza fungi and related soil microbial activity drive carbon mineralization in the maize rhizosphere. Ecotoxicol. Environ. Saf. 2019, 182, 109476. [Google Scholar] [CrossRef] [PubMed]
- Etesami, H.; Jeong, B.R.; Glick, B.R. Contribution of arbuscular mycorrhizal fungi, phosphate–solubilizing bacteria, and silicon to P uptake by plant. Front. Plant Sci. 2021, 12, 699618. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, C.; Kilburn, M.R.; Clode, P.L.; Fuchslueger, L.; Koranda, M.; Cliff, J.B.; Solaiman, Z.M.; Murphy, D.V. Exploring the transfer of recent plant photosynthates to soil microbes: Mycorrhizal pathway vs. direct root exudation. New Phytol. 2015, 205, 1537–1551. [Google Scholar] [CrossRef] [PubMed]
- Nottingham, A.T.; Turner, B.L.; Winter, K.; Chamberlain, P.M.; Stott, A.; Tanner, E.V. Root and arbuscular mycor-rhizal mycelial interactions with soil microorganisms in lowland tropical forest. FEMS Microbiol. Ecol. 2013, 85, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Panneerselvam, P.; Kumar, U.; Senapati, A.; Parameswaran, C.; Anandan, A.; Kumar, A.; Jahan, A.; Padhy, S.R.; Nayak, A.K. Influence of elevated CO2 on arbuscular mycorrhizal fungal community elucidated using Illumina MiSeq platform in sub-humid tropical paddy soil. Appl. Soil Ecol. 2020, 145, 103344. [Google Scholar] [CrossRef]
- Tisserant, B.; Gianinazzi-Pearson, V.; Gianinazzi, S.; Gollotte, A. In planta histochemical staining of fungal alkaline phosphatase activity for analysis of efficient arbuscular mycorrhizal infections. Mycol. Res. 1993, 97, 245–250. [Google Scholar] [CrossRef]
- Qin, M.; Zhang, Q.; Pan, J.; Jiang, S.; Liu, Y.; Bahadur, A.; Peng, Z.; Yang, Y.; Feng, H. Effect of arbuscular mycorrhizal fungi on soil enzyme activity is coupled with increased plant biomass. Eur. J. Soil Sci. 2020, 71, 84–92. [Google Scholar] [CrossRef]
- Li, S.; Bi, Y.; Kong, W.; Yu, H.; Lang, Q.; Miao, Y. Effects of arbuscular mycorrhizal fungi on ecological restoration in coal mining areas. Russ. J. Ecol. 2015, 46, 431–437. [Google Scholar] [CrossRef]
- Mitra, D.; Dam, P.; Mondal, R.; Mahakur, B.; Al-Tawaha, A.R.M.; Sangeetha, J. Application of arbuscular mycorrhiza fungi in agricultural and horticultural crops. In Mycorrhizal Technology: Managing Plant Stress and Mitigating Climate Change Using Mycorrhaizae; Apple Academic Press, Inc.: Cambridge, MA, USA, 2023; pp. 51–64. [Google Scholar]
- Luo, Y.Q.; Zhou, X.H. Soil Respiration and the Environment; Academic Press: San Diego, CA, USA, 2010. [Google Scholar]
- Gougoulias, C.; Clark, J.M.; Shaw, L.J. The role of soil microbes in the global carbon cycle: Tracking the below-ground microbial processing of plant-derived carbon for manipulating carbon dynamics in agricultural systems. J. Sci. Food Agric. 2014, 94, 2362–2371. [Google Scholar] [CrossRef]
- Leifheit, E.F.; Veresoglou, S.D.; Lehmann, A.; Morris, E.K.; Rillig, M.C. Multiple factors influence the role of arbuscular mycorrhizal fungi in soil aggregation—A meta-analysis. Plant Soil 2014, 374, 523–537. [Google Scholar] [CrossRef]
- Wang, Z.G.; Bi, Y.L.; Jiang, B.; Zhakypbek, Y.; Peng, S.P.; Liu, W.W.; Liu, H. Arbuscular mycorrhizal fungi enhance soil carbon sequestration in the coalfields, northwest China. Sci. Rep. 2016, 6, 34336. [Google Scholar] [CrossRef] [PubMed]
- Thirkell, T.J.; Pastok, D.; Field, K.J. Carbon for nutrient exchange between arbuscular mycorrhizal fungi and wheat varies according to cultivar and changes in atmospheric carbon dioxide concentration. Glob. Chang. Biol. 2020, 26, 1725–1738. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Ullah, H.; Himanshu, S.K.; Tisarum, R.; Cha-Um, S.; Datta, A. Arbuscular mycorrhizal fungi inoculation and phosphorus application improve growth, physiological traits, and grain yield of rice under alternate wetting and drying irrigation. J. Plant Physiol. 2022, 278, 153829. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).