Abstract
Human colostrum (HC) is essential for oral health as it is rich in probiotics that could affect the growth of the cariogenic S. mutans and its biofilm formation; hindering dental caries in advance. In this study, HC was collected from 36 healthy mothers 1–3 days postpartum. The effect of HC on oral health was carried out by assessing the impact of HC and its derived probiotics’ cell-free supernatants (CFS) on the growth of S. mutans (using modified well diffusion) and its biofilm formation (using microtiter plate assay). Moreover, the effect of whole HC on L. rhamnosus, a probiotic oral bacterium, was examined. Probiotics were isolated and identified phenotypically by API 50 CH carbohydrate fermentation and genotypically by 16S rRNA amplification. The in vitro study revealed that HC has cariogenic activity and is associated with biofilm formation. Biofilm strength was inversely proportional to HC dilution (p-value < 0.0001). Nevertheless, HC and colostrum-derived probiotics improve oral health by inhibiting the growth of caries-inducing S. mutans with lower inhibition to L. rhamnosus probiotics. The CFS of isolated probiotics reduced the biofilm formation via the cariogenic S. mutans. These results are not only promising for caries eradication, but they also highlight the importance of breastfeeding infants from their first hours to shape healthy oral microbiota, protecting them from various diseases including dental caries.
1. Introduction
Despite being multifactorial, involving injudicious eating habits and poor oral health, dental caries remains a first-rate microbiological disease. Bacterial biofilm is the initial caries precursor and Streptococcus mutans (S. mutans) is the major contributor [1,2]. Inhibition of S. mutans and the associated biofilm, therefore, could hinder the caries process. S. mutans is both acidogenic and aciduric. Moreover, S. mutans synthesizes glucan and fructan from sucrose by the action of glucosyltransferase and fructosyltransferase, respectively [3]. These exopolysaccharides increase bacterial retention on tooth surfaces with subsequent demineralization. Various strategies have been adopted for inhibiting S. mutans; the use of probiotics is one of the most recent ones [4,5].
Probiotics are microorganisms that can hinder other pathogens, enhancing the quality of the microbiota and preserving a stable eubiosis; thus, they can prevent or even cure diseases [6,7]. Probiotics are suggested to exert their action via competition with the pathogen for nutrition and binding sites, and/or enhancing arginine deiminase system activity. The latter forms an alkaline ecology unfavorable for aciduric cariogenic microorganisms [8]. Probiotics could be obtained from several natural sources such as yogurt, kefir, pickles, and some types of cheese, and they are present in the mouth and colostrum of Mammalia [9].
Colostrum is the first milk type produced by mammals immediately postpartum [10]. It includes an abundance of immune proteins such as immunoglobulins, lactoferrin, and numerous cytokines that resist digestion, prolonging their biological activity. Colostrum also contains oligosaccharides that may serve as a selective food source (prebiotic) and favor the growth of various beneficial bacteria [11]. Additionally, it was proven that human colostrum has useful bacteria that can serve as probiotics as well [12].
It is well known that human milk has cariogenic activity [13]. As S. mutans were also detected in the saliva of some predentate children [11], studies are needed to evaluate the impact of human colostrum (HC) on dental health. Therefore, this study was carried out to evaluate the effect of HC and their derived probiotics’ cell-free supernatants (CFSs) on oral health by assessing their effect on the growth of S. mutans and their biofilm. Moreover, the effect of whole HC on L. rhamnosus, a probiotic oral bacterium, is carried out. The colostrum and their derived probiotics, if proven effective, could be used in dental health prophylaxis [5].
2. Materials and Methods
2.1. Ethical Approval
The study protocol was accepted by the Committee of Research Ethics of the Faculty of Dental Medicine for Girls, Al-Azhar University, Cairo, Egypt (P-PD-22-13). This study coincides with the Declaration of Helsinki guidelines [14].
2.2. Sample Size Calculation
The calculation of the sample size was guided by the Kim et al. study [15]. According to this study, the minimally accepted sample size was 29. When the response within each subject group was normally distributed with a standard deviation of 1.3, the estimated mean difference was 1 when the power was 80% and the type I error probability was 0.05. The sample size was increased to 36 to compensate for the 20% dropout. The sample size was calculated using P.S.power 3.1.6.
2.3. Collection of HC Samples
Following written consent, about 30 mL of HC was collected from each of the 36 healthy mothers 1–3 days postpartum. It was reported that all contributors had received antibiotics after delivery. The samples were cooled till transferred to the lab within 4 h, centrifuged (3000 rpm for 10 min at 4 °C), filter-sterilized (0.45 µm) (Millipore, Bedford, MA, USA), and kept at −20 °C until used [16].
2.4. Bacterial Strains and Growth Conditions
S. mutans strain ATTC 25175 was isolated from dentin caries, cultured at 37 °C on brain-heart infusion (BHI) medium (Oxoid Ltd., Hampshire, UK), and then incubated for 48 h in 5% CO2 at 37 °C. DeMan, Rogosa, Sharpe (MRS) agar and broth (Oxoid Ltd.), however, were used for the isolation of probiotic bacteria from HC. Cultures were incubated for 24 h in 5% CO2 at 37 °C [3,17].
2.5. Effect of the Whole HC on the Oral Bacteria S. mutans, and L. rhamnosus
The effect of the whole HC was examined using the modified well diffusion method [18] to learn the effect on the growth of the oral bacteria S. mutans (ATCC 25175), and L. rhamnosus (ATCC 7469) (probiotic bacteria that inhabit the mouth and protect against tooth decay via S. mutans [19]). A total of 20 mL of S. mutans or L. rhamnosus suspension (1.5 × 108 CFU/mL) was added to 200 mL of melted MRS agar and kept at 45 °C. The mix was poured into plates and left to solidify. A total of 150 µL of HC was added to the wells, while other wells contained 2% chlorhexidine acetate, the standard antibacterial, as a positive control (Madam Health Pharmaceutical Co., Ltd., Shanghai, China). After incubation (24 h at 37 °C), the growth inhibition diameters were determined [4].
2.6. Effect of the Whole HC on S. mutans Biofilm Formation
The microtiter plate assay (MTP) was performed as dictated by Allison et al. (2015) [16]. Various dilutions of HC (1:3, 1:9, 1:27, 1:81, 1:243, 1:729, and 1:2187) were investigated. HC samples were mixed with BHI (190 µL total volume) and 10 µL of an overnight culture of S. mutans (106 CFU/mL) in seven rows (one row/each dilution) out of eight of the 96-well-flat bottom sterile polystyrene microtiter plates for 24 h. BHI with and without S. mutans were used as positive and negative controls, respectively, in the remaining row of wells. After incubation, each well was triple-washed (300 µL of phosphate buffer saline), heat-fixed (60 °C for 1 h), crystal violet-stained (150 µL for 15 min), and the stain was extracted by absolute ethanol (150 µL) (Merck, Darmstadt, Germany). Finally, stained adherent biofilms were graded, as shown in Table 1. According to Pui et al. (2017) and Salem et al. (2022), depending on their optical densities, an OD of 630 nm was recorded by a Stat Fax® 2100 microtiter reader (Awareness Technologies Inc., Westport, CT, USA). The test was repeated three times and the results were averaged [20,21].

Table 1.
Grading of biofilm formation.
2.7. HC Probiotics Isolation and Identification
Of each HC sample, 10 µL was cultured in sterile 5 mL MRS broth (72 h, 5% CO2 at 37 °C), then sub-cultured twice on MRS agar plates to obtain pure colonies in case of the presence of mixed colonies. Samples were first cultured on broth to maximize the yield as initial culturing of HC on MRSA agar plates did not show any bacterial growth. The bacterial growth was indicated by showing bacterial colonies. The resultant colonies were initially identified by their morphology on MRS agar plates, Gram staining, and catalase reaction. Catalase-negative isolates were species identified using API 50 CH carbohydrate fermentation strips [22] (bioMerieux, Craponne, France) and 16S ribosomal ribonucleic acid 16S rRNA [23]. Following the isolates’ genomic DNA extraction using NucliSENS easyMAG (bioMerieux), 16S rRNA genes were PCR amplified. The amplicons were sequenced in Cosmo Gentech, Seoul, Republic of Korea, using an Applied Biosystems 3730x1 DNA Analyzer (Thermo Fisher, Seoul, Republic of Korea). Isolates with the highest Basic Local Alignment Search Tool (BLAST) score were identified against the GenBank DNA database (www.ncbi.nlm.nih.gov/Genbank, accessed on 10 November 2022) [24,25].
2.8. Preparation of Cell-Free Supernatant (CFS) of Isolated Probiotics
For CFS preparation, each probiotic isolate was inoculated into 30 mL of MRS broth at (108 CFU/mL) concentration, followed by incubation (24 h in 5% CO2 at 37 °C), centrifugation (4500 rpm for 10 min at 4 °C), and filtration (0.45 µm syringe filter) (Millipore) [26].
2.9. Anti-S. mutans Effect of CFS of Isolated Probiotics
Using the modified well diffusion method [18], 20 mL of S. mutans suspension (1.5 × 108 CFU/mL) was added to 200 mL of melted MRS agar and kept at 45 °C. The mix was poured into plates and left to solidify. The CFS (150 µL) of isolated probiotics were added to the wells, while other wells contained 2% chlorhexidine acetate, the standard antibacterial, as a positive control (Madam Health Pharmaceutical Co., Ltd., Shanghai, China). After incubation (24 h at 37 °C), the S. mutans growth inhibition diameters were determined [4].
2.10. Anti-S. mutans Biofilm Effect of CFS of Isolated Probiotics (MTP Assay)
The previously mentioned MTP assay was used for assessing the effect of the CFS of the isolated probiotics on S. mutans biofilm formation. The CFS of each isolated bacteria were used in a dilution that did not inhibit the growth of S. mutans [27]. The ODs of stained retained biofilms were triple assessed [26] using a microtiter plate reader, at an OD of 570 nm. The percentages of biofilm inhibition were calculated according to the following equation [28]:
% inhibition = (1 − OD sample/OD positive control) × 100.
2.11. Statistical Analysis
Statistical analysis was performed with SPSS 20®, Graph Pad Prism®, and Microsoft Excel 2016. All quantitative data were explored for normality using a Shapiro–Wilk normality test and presented as means and standard deviation (SD) values. Qualitative data, however, were presented as frequencies and percentages.
3. Results
3.1. The Cariogenic Activity of HC
Effect of the Whole HC on S. mutans Biofilm Formation
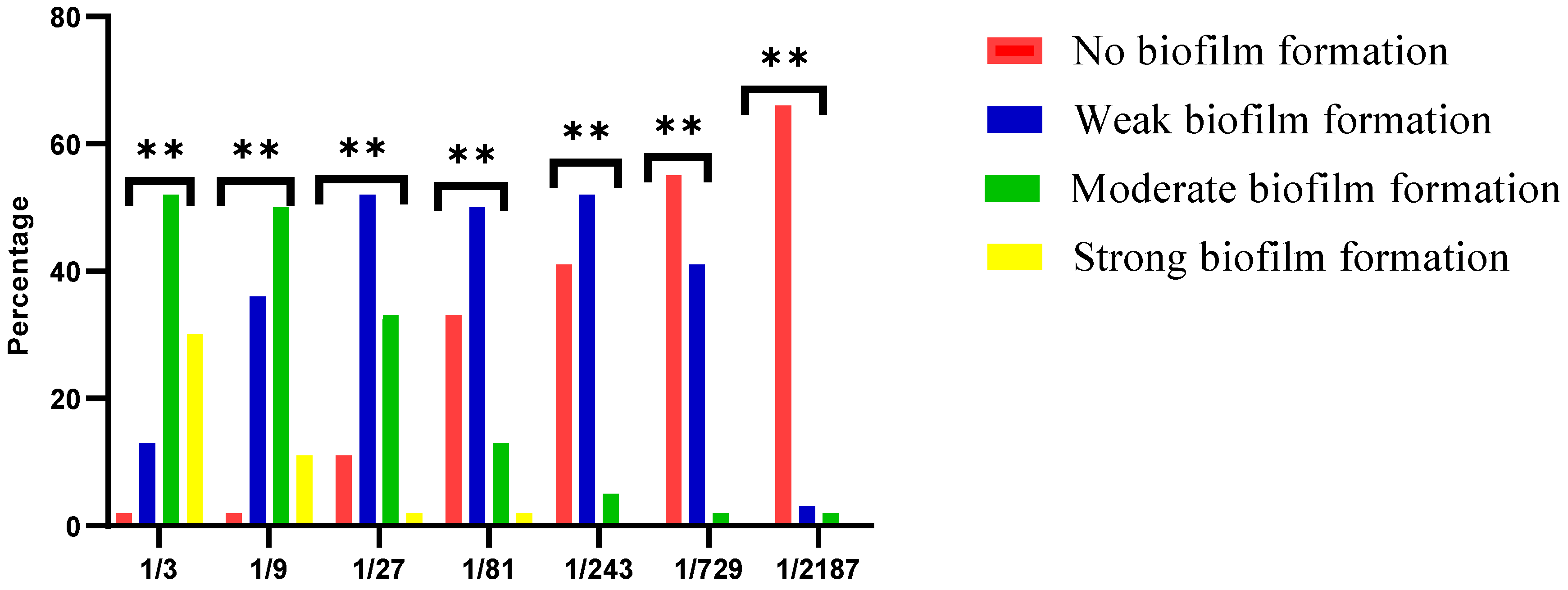
Whole HC samples were associated with biofilm formation; the biofilm strength was inversely proportional to HC dilution. The frequencies and percentages of S. mutans biofilm formation of different HC dilutions are shown in Figure 1. A Chi-square test revealed a significant difference among different HC dilutions (p ≤ 0.05).
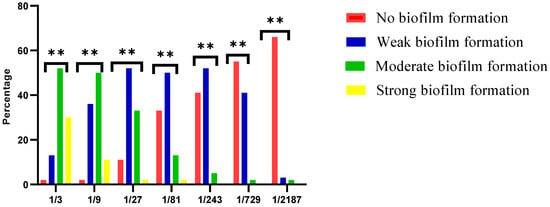
Figure 1.
Effect of different dilutions of the whole HC on S. mutans biofilm formation. **: Significance.
3.2. The Anti-Caries Potential of HC
3.2.1. Effect of the Whole HC on the Oral Bacteria S. mutans and L. rhamnosus
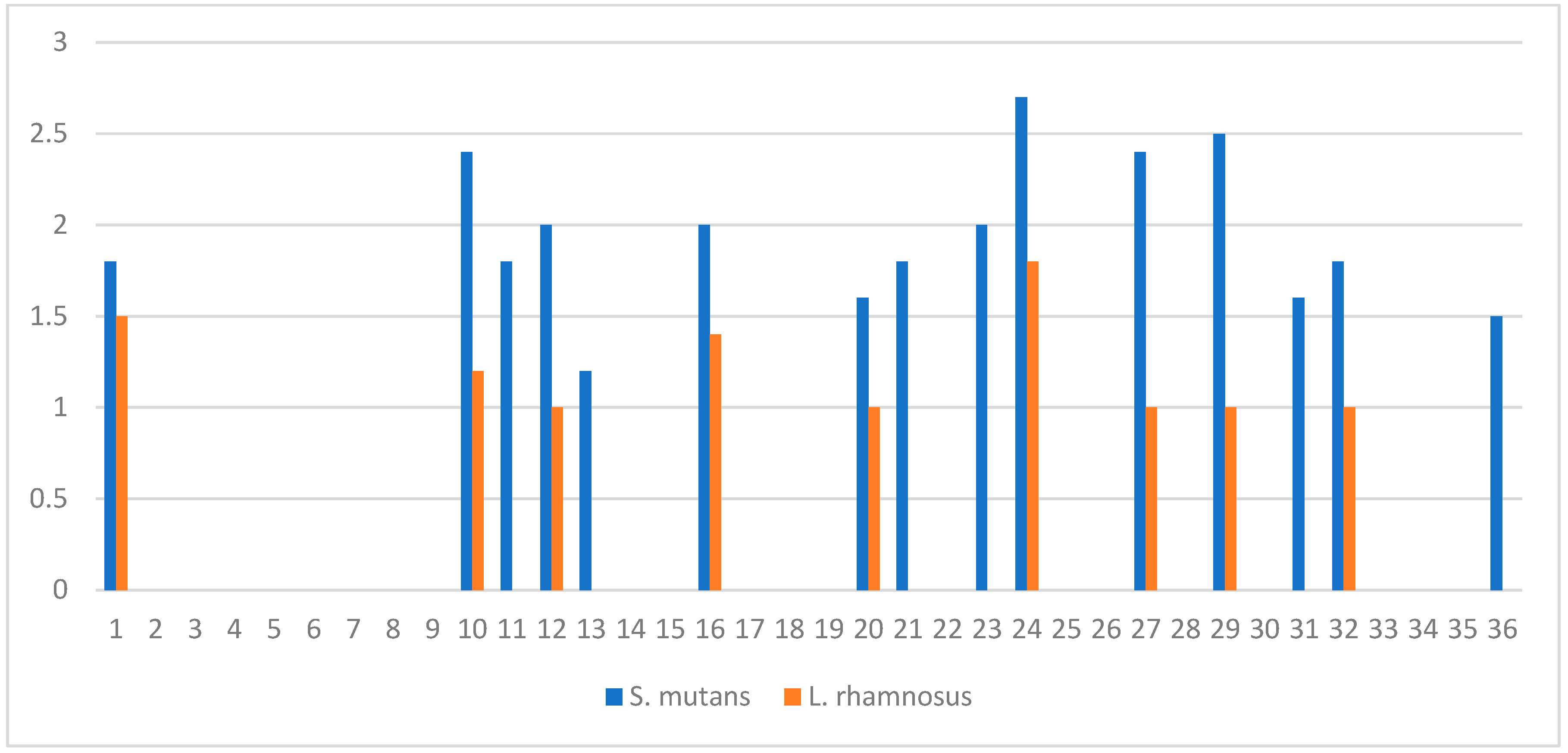
The well-diffusion test showed that HC inhibits the growth of the cariogenic bacteria, S. mutans, with inhibition zone diameters ranging from 12 to 27 mm, while the inhibition zone diameters for the probiotic bacteria, L. rhamnosus, ranged from 10 to 18 mm (Figure 2).

Figure 2.
Inhibition zone diameters (cm) of whole colostrum against S. mutans (blue pars), and L. rhamnosus (orange bars) for all collected colostrum (n = 36). Well diameter = 0.8 cm.
3.2.2. Identification of Probiotic Bacteria Isolated in MRS from HC
From nine colostrum samples, ten bacteria were isolated in MRS agar and phenotypically identified: Lactobacillus rhamnosus (n = 1), Pediococcus acidilactici (n = 2), Pediococcus pentosaceus (n = 1), Leuconostoc lactis (n = 1), Leuconostoc mesenteroides (n = 1), Lactococcus lactis ssp lactis 1 (n = 1), Staphylococcus lugdunensis (n = 1), Pseudomonas helleri (n = 1), and Staphylococcus epidermidis (n = 1). Genotypically identified species were recorded in the GenBank DNA database, along with their accession numbers, and are listed in Table 2.

Table 2.
Accession numbers of genotypically identified isolated probiotics.
3.2.3. Anti-S. mutans Effect of CFS of Isolated Probiotics
Most isolated (7/10) probiotics have successfully inhibited the growth of S. mutans with variable degrees. The difference among different isolated probiotics regarding S. mutans inhibition was statistically insignificant (p = 0.51) according to one-way ANOVA (p > 0.05). The effect of CFS of isolated probiotics on the growth of S. mutans, was presented as mean and standard deviation values of inhibition zone diameters (in mm) as shown in Table 3.

Table 3.
Effect of CFS of isolated probiotics on the growth of S. mutans.
3.2.4. Anti-S. mutans Biofilm Effect of CFS of Isolated Probiotics
The CFS of isolated probiotics showed anti-S. mutans biofilm efficacy that varied among different probiotic species. The difference among them, however, was statistically insignificant (p = 0.14), as evidenced by one-way ANOVA (p > 0.05). The mean and standard deviation values of % inhibition of S. mutans biofilm formation by different isolated bacteria CFS are presented in Table 4 [15,28].

Table 4.
Effect of CFS of isolated bacteria on % inhibition of biofilm formation by S. mutans.
4. Discussion
Dental caries is still a widespread public health disease, even with progress in treatment strategies. Nowadays, most trends are directed toward prevention [29]. Being the outcome of host/microbiome imbalance [30], caries could be prevented as early as possible via modifying oral microbiomes and controlling caries-implicated strains such as S. mutans [31]. There is a growing interest in using probiotics, especially naturally driven species, for this purpose [30].
Breast milk was evidenced to include a diverse bacterial community that could formulate the infant’s microbiome [23]. The inclusion of more than 200 bacterial species makes breast milk a good source of natural probiotics [28]. HC was selected as a source of probiotics as the first administered nutrient capable of altering the oral ecology [32]. This study investigated the effect of different dilutions of HC on the formation of biofilm by cariogenic S. mutans. Results showed that almost all HC samples increased S. mutans biofilm formation. Biofilm strength was inversely proportional to the degree of HC dilution. This could be attributed to the fact that human milk, as a whole, contains both cariogenic (e.g., casein) and anti-cariogenic constituents (e.g., IgA and probiotics), as evidenced in the Allison et al. study [16]. Such results are further supported by the occurrence of nursing caries, a disease in which dental caries cause severe damage to the anterior teeth of the suckling baby as a result of prolonged breastfeeding, esp. during sleeping at night, in the absence of the mother’s awareness of oral hygiene [33].
The anticaries potential of HC was also demonstrated in this study by revealing that whole colostrum inhibited the growth of cariogenic S. mutans with inhibition zone diameters ranging from 12 to 27 mm. This inhibitory activity could be attributed to its constituents of probiotic bacteria and secretory immunoglobulin (IgA) [11,12]. Surprisingly, this bacterial inhibitory activity was lower for the beneficial oral probiotic L. rhamnosus as their zone of inhibition ranged from 10 to 18 mm. Some HC specimens that inhibited S. mutans did not affect the growth of L. rhamnosus, which is an added advantage indicating that HC had preserved this probiotic bacterium, an effect that has a positive impact on oral health and oral microbiome.
It is noted that only 25% (9/36) of HC showed antibacterial activity. Only ten beneficial bacteria including Lactobacillus rhamnosus (n = 1), Pediococcus acidilactici (n = 2), Pediococcus pentosaceus (n = 1), Leuconostoc lactis (n = 1), Leuconostoc mesenteroides (n = 1), Lactococcus lactis ssp lactis 1 (n = 1), Staphylococcus lugdunensis (n = 1), Pseudomonas helleri (n = 1), and Staphylococcus epidermidis (n = 1) were isolated from the HC of Egyptian mothers. Colostrum is less diverse than mature milk [28]. This could explain obtaining only 10 isolates belonging to six genera from HC samples: Pediococcus (30%, 3/10), Leuconostoc (20%, 2/10), Lactococcus (10%, 1/10), Lactobacillus (10%, 1/10), Staphylococcus (20%, 2/10), and Pseudomonas (10%, 1/10). This complies with the results of other studies [28,34]. Although Lactobacillus and Bifidobacterium are usual probiotics of breast milk, they formed only 0.4% and 1.7% of isolated probiotics. This was also shown by Chen et al. (2020), who have even recorded their absence in some milk samples [6]. Breast milk microbiome could be individually varied according to genetics, diet, lifestyle, lactation period, and way of delivery [35]. This reported low count of isolated bacteria is in agreement with another study that mentioned that due to low microbial DNA amounts or sterility, 35% of breast milk from healthy postpartum women could not be isolated or even sequenced. In addition, systemic antibiotic administration to almost all Egyptian mothers after delivery might have reduced microbial count and diversity in HC samples [23]. The sterility of most HC isolated from Egyptian mothers is attributed to inappropriate use of antibiotics after delivery, as the misuse of antibiotics leads to the destruction of microbiota in addition to the emergence of antibiotic resistance [36,37]. These results showed the importance of antibiotic restriction, especially postpartum, so that the newborn benefits from varieties of the mother’s microbiomes that would positively affect their dental as well as general health. Several studies reported the effect of antibiotics on the microbiome [38]. Specific studies are needed to determine the effect of antibiotics administration on the composition of human colostrum and human breastmilk and their relation to oral health and the emergence of resistant genes.
In the current study, diluted CFS of most isolated probiotics has inhibited S. mutans and its biofilm formation by different grades. L. rhamnosus, for example, caused 34.58 ± 1.91% biofilm inhibition following the Kim et al. study, where CFS of L. rhamnosus and L. brevis had inhibited the biofilm formation by 41.45% and 35.28%, respectively [39]. Most Lactobacillus strains are known to exhibit anti-S. mutans activity by their bioactive agents [40]. However, in agreement with other studies, the current study revealed that in addition to Lactobacillus, S. mutans biofilm was inhibited by other probiotic species such as Pediococcus pentosaceus, Pediococcus acidilactici, Leuconostoc mesenteroides, Leuconostoc lactis, and Lactococcus lactis ssp lactis 1 [34,39,41].
On the other hand, isolated Staphylococcus epidermidis, Staphylococcus lugdunensis, and Pseudomonas helleri exhibited limited efficacy. Nevertheless, they are still capable of fighting S. mutans, antagonizing it, and preventing colonization [42,43,44]. Moreover, they could be used for the isolation of new antibiotics with proven bactericidal effects [45,46]. The abovementioned effects support our findings in which S. mutans biofilm formation was inhibited by the CFS of Staphylococcus epidermidis, Staphylococcus lugdunensis, and Pseudomonas helleri by 16.67, 22.92, and 22.92% respectively, confirming that HC and human milk are potential sources for obtaining solution for different health problems. Although the fact that human milk and human colostrum have some cariogenic activity due to their content of sucrose [47], they are rich sources of probiotic bacteria that maintain oral ecology and oral microbiome. In a previous study that examined the effect of probiotic bacteria on S. mutans and C. albicans using a thorough multispecies biofilm model that simulated high caries risk clinical situations, they found that probiotics inhibited biofilm formation and growth of S. mutans and C. albicans and suppressed the components of exopolysaccharides. EPS production, carbohydrate metabolism, glycan biosynthesis, and metabolism-related genes in S. mutans and C. albicans were dramatically downregulated. More importantly, there was also a considerable downregulation of genes associated with C. albicans resistance to antifungal drugs (ERG4), fungal cell wall chitin remodeling (CHT2), and resistance to oxidative stress (CAT1). The antimicrobial peptide plantaricin is produced by the Lactobacillus genes plnD, plnG, and plnN, which were considerably elevated [48].
The results of the present study revealed the in vitro cariogenic activity of HC by enhancing biofilm formation of S. mutans. At the same time, whole HC provides anticaries potential and is a source of probiotic and beneficial bacteria that fight S. mutans and its biofilm formation. An interesting finding is that the whole HC did not inhibit S. mutans biofilm formation, while isolated probiotics showed different degrees of cariogenic S. mutans inhibition. HC contains both cariogenic components (e.g., casein) and anti-cariogenic ones (such as probiotics and immunoglobulins). The cariogenic potential of milk and its substitutes directly depends on the way they are used. Caries is a multi-factorial disease requiring the presence of fermentable carbohydrates, cariogenic bacteria, and caries-susceptible surface (i.e., tooth surface). In the case of newborns, there are no teeth; accordingly, they can benefit from the probiotics in the colostrum in formulating healthy oral microbiota with no caries susceptibility till teeth eruption. The already-shaped healthy oral microbiota would later decrease caries risk, even after teeth eruption. It is noteworthy that in our in vitro study, the strength of the biofilm formed in the whole HC test was inversely proportional to HC dilution, probably due to the dilution of both cariogenic and anti-cariogenic components. It is therefore logical that, when the anti-cariogenic probiotics were isolated, the inhibition of S. mutans was more pronounced [1,2,49].
Probiotic supplements and a prebiotic diet are encouraged for children who are deprived of natural breastfeeding to restore their oral microbiome and ensure good oral and dental health [50,51]. To reverse the undesired changes in the microbiota’s composition and function brought on by antibiotic treatments or caesarean delivery, a probiotic mixture in addition to at least partial breastfeeding is highly recommended [52]. More studies are needed to report the effect of antibiotic misuse in Egypt on several aspects of health, including oral health. This study shed a light on the benefits of the probiotics on oral health in addition to the previously known effects on intestinal health or prevention of toxicity [53,54].
The limitations of this study involved the inability to use the metagenomic analysis that might reveal several microorganisms other than bacteria that help in restoring oral ecology. More studies targeting the details of HC’s whole components and in vivo studies are needed to confirm the impact of HC on dental health. Moreover, retrospective studies are encouraged to evaluate the oral health of individuals who had received human milk in their early life. Analysis of human colostrum and cell-free supernatants of isolated probiotics will add a significant discovery to understanding the power of human milk.
5. Conclusions
The present study’s findings demonstrate the in vitro cariogenic activity of HC by encouraging S. mutans to produce biofilms. On the other hand, CFS of isolated HC probiotics was proven to have good anti-S. mutans and antibiofilm effects; opening the way for early oral microbiome control and pursuing caries uprooting while emphasizing the importance of breastfeeding from the first hours of an infant’s life. Other in vivo research with greater sample sizes assessing S. mutans in the saliva of newborns before and after colostrum administration, microbiome alteration after the transition to mature breast milk, and comparing colostrum to mature breast milk after a normal delivery is highly advocated.
Author Contributions
Conceptualization, S.N.H. and O.K.M.R.; methodology, S.R.E.-S., O.K.M.R. and M.S.E.M.B.; software, S.N.H. and S.R.E.-S.; validation, S.A.Z., S.N.H., O.K.M.R. and M.S.E.M.B.; formal analysis, S.A.Z., S.N.H., O.K.M.R. and M.S.E.M.B.; investigation, S.A.Z., S.N.H., O.K.M.R. and M.S.E.M.B.; resources, S.A.Z., S.N.H., O.K.M.R., S.R.E.-S., H.M.R.M.S., S.I.B. and M.S.E.M.B.; data curation, S.A.Z., S.N.H., O.K.M.R., S.R.E.-S., H.M.R.M.S., S.I.B. and M.S.E.M.B.; writing—original draft preparation, S.A.Z., O.K.M.R. and M.S.E.M.B.; writing—review and editing, S.A.Z., S.N.H., O.K.M.R., S.R.E.-S., H.M.R.M.S. and S.I.B.; visualization, O.K.M.R., S.A.Z. and S.N.H.; supervision, S.N.H., S.A.Z. and O.K.M.R.; project administration, O.K.M.R., S.N.H. and S.A.Z.; funding acquisition, H.M.R.M.S. and S.I.B. All authors have read and agreed to the published version of the manuscript.
Funding
Funding was received from Researcher Supporting Project number (RSP2023R497) at King Saud University, Riyadh, Saudi Arabia, to publish this study.
Institutional Review Board Statement
The Committee of Research Ethics at the Faculty of Dental Medicine for Girls at Al-Azhar University in Cairo, Egypt, approved the study protocol (P-PD-22-13). This research is timed to the Helsinki Declaration.
Informed Consent Statement
The patients have given their written informed consent for this study’s participation and publication.
Data Availability Statement
All data generated during this study are included in the published article.
Acknowledgments
The authors would like to extend their sincere appreciation to Researcher Supporting Project number (RSP2023R497) at King Saud University, Riyadh, Saudi Arabia. Finally, the authors acknowledge mothers who donated their colostrum for this study.
Conflicts of Interest
The authors have no conflict of interest relevant to this article.
References
- Poorni, S.; Nivedhitha, M.; Srinivasan, M.R.; Arthi, B. Association between oral probiotic streptococcus supplements and salivary Streptococcus mutans count in human study: A systematic review. J. Clin. Diagn. Res. 2022, 16, 1–5. [Google Scholar] [CrossRef]
- Butera, A.; Maiorani, C.; Morandini, A.; Simonini, M.; Morittu, S.; Trombini, J.; Scribante, A. Evaluation of Children Caries Risk Factors: A Narrative Review of Nutritional Aspects, Oral Hygiene Habits, and Bacterial Alterations. Children 2022, 9, 262. [Google Scholar] [CrossRef] [PubMed]
- Wasfi, R.; Abd El-Rahman, O.A.; Zafer, M.M.; Ashour, H.M. Probiotic lactobacillus sp. Inhibit growth, biofilm formation and gene expression of caries- inducing Streptococcus mutans. J. Cell. Mol. Med. 2018, 22, 1972–1983. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Lu, M.; Liu, R.; Tian, Y.; Vu, V.H.; Li, Y.; Liu, B.; Kushmaro, A.; Li, Y.; Sun, Q. Inhibition of Streptococcus mutans biofilm formation and virulence by Lactobacillus plantarum K41 isolated from traditional Sichuan pickles. Front. Microbiol. 2020, 11, 1–12. [Google Scholar] [CrossRef]
- Damayanti, L.; Zulkifli, A.; Amiruddin, R.; Hadju, V.; Palutturi, S.; Syafar, M. The influent of colostrum adding in toothpaste and S IgA factor to prevent school age caries in Ujung Tanah sub district Makassar. Int. J. Adv. Sci. Tech. 2020, 29, 678–683. [Google Scholar]
- Chen, Z.; Schlafer, S.; Gostemeyer, G.; Schwendicke, F. Probiotic effects on multispecies biofilm composition, architecture and caries activity in vitro. Microorganisms 2020, 8, 1272. [Google Scholar] [CrossRef]
- Hashem, N.M.; Gonzalez-Bulnes, A. The Use of Probiotics for Management and Improvement of Reproductive Eubiosis and Function. Nutrients 2022, 14, 902. [Google Scholar] [CrossRef]
- Bijle, M.N.A.; Yiu, C.K.Y.; Ekambaram, M. Can oral ADS activity or arginine levels be a caries risk indicator? A systematic review and meta-analysis. Clin. Oral. Investig. 2018, 22, 583–596. [Google Scholar] [CrossRef]
- Dahia, D.; Nigam, P.S. The gut microbiota influenced by the intake of probiotics and functional foods with prebiotics can sustain wellness and alleviate certain ailments like gut inflammation and colon cancer. Microorganisms 2022, 10, 665. [Google Scholar] [CrossRef]
- Sanctuary, M.R.; Kain, J.N.; Chen, S.Y.; Kalanetra, K.; Lemay, D.G.; Rose, D.R.; Yang, H.T.; Tancredi, D.J.; German, J.B.; Slupsky, C.M.; et al. Pilot study of probiotic colostrum supplementation on gut function in children with autism and gastrointestinal symptoms. PLoS ONE 2019, 14, e0210064. [Google Scholar] [CrossRef]
- Bertoldo, B.B.; Silva, C.B.; Rodrigues, D.B.R.; Martins, V.R.G.; Ferriani, V.P.L.; Nogueira, R.D. Comparison of IgA response in saliva and colostrum against oral streptococci species. Braz. Oral Res. 2017, 31, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Novak, F.R.; Almeida, J.A.; Silva, G.O.; Borba, L.M. Human colostrum: A natural source of probiotics. J. Pediatr. 2001, 77, 265–270. [Google Scholar] [CrossRef]
- Ricomini Filho, A.P.; de Assis, A.C.M.; Costa Oliveira, B.E.; Cury, J.A. Cariogenic Potential of Human and Bovine Milk on Enamel Demineralization. Caries Res. 2021, 55, 260–267. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsiniki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jang, H.J.; Lee, N.K.; Paik, H.D. Antibacterial and Antibiofilm Effect of Cell-Free Supernatant of Lactobacillus brevis KCCM 202399 Isolated from Korean Fermented Food against Streptococcus mutans KCTC 5458. J. Microbiol. Biotechnol. 2022, 32, 56–63. [Google Scholar] [CrossRef]
- Allison, L.M.; Walke, L.A.; Sanders, B.J.; Yang, Z.; Eckert, G.; Gregory, R.L. Effect of human milk and its components on Streptococcus mutans biofilm formation. J. Clin. Pediatr. Dent. 2015, 39, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, D.L.; Craft, K.M.; Doster, R.S.; Weitkamp, J.H.; Aronoff, D.M.; Gaddy, J.A.; Townsend, S.D. Antimicrobial and antibiofilm activity of human milk oligosaccharides against Streptococcus agalactiae, Staphylococcus aureus and Acinetobacter baumannii. ACS Infect. Dis. 2018, 4, 315–324. [Google Scholar] [CrossRef]
- Lin, T.H.; Pan, T.M. Inhibitory effect of Lactobacillus paracasei subsp. paracasei NTU 101 on rat dental caries. J. Funct. Foods 2014, 10, 223–231. [Google Scholar] [CrossRef]
- Teanpaisan, R.; Piwat, S.; Dahlén, G. Inhibitory effect of oral Lactobacillus against oral pathogens. Lett. Appl. Microbiol. 2011, 53, 452–459. [Google Scholar] [CrossRef]
- Pui, C.F.; Apun, K.; Jalan, J.; Bilung, L.M.; Su‘ut, L.; Hashim, H.F. Microtiter plate assay for the quantification of biofilm formation by pathogenic Leptospira. Res. J. Microbiol. 2017, 12, 146–153. [Google Scholar]
- Salem, S.S.; Badawy, M.S.E.; Al-Askar, A.A.; Arishi, A.A.; Elkady, F.M.; Hashem, A.H. Green biosynthesis of selenium nanoparticles using orange peel waste: Characterization, antibacterial and antibiofilm activities against multidrug-resistant bacteria. Life 2022, 12, 893. [Google Scholar] [CrossRef] [PubMed]
- Brolazo, E.M.; Leite, D.S.; Tiba, M.R.; Villarroel, M.; Marconi, C.; Simoes, J.A. Correlation between API 50 CH and multiplex polymerase chain reaction for the identification of vaginal lactobacilli in isolates. Braz. J. Microbiol. 2011, 42, 225–232. [Google Scholar] [CrossRef]
- Damaceno, Q.S.; Gallotti, B.; Reis, I.M.M.; Totte, Y.C.P.; Assis, G.B.; Figueiredo, H.C.; Silva, T.F.; Azevedo, V.; Nicoli, J.R.; Martins, F.S. Isolation and identification of potential probiotic bacteria from human milk. Probiotics Antimicrob. Proteins 2021, 15, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.; Kim, D.H.; Kang, I.B.; Kim, H.; Song, K.Y.; Kim., H.S.; Seo, K.H. Characterization and antibacterial activity of a novel exopolysaccharide produced by Lactobacillus kefiranofaciens DN1 isolated from kefir. Food Control 2017, 78, 436–442. [Google Scholar] [CrossRef]
- Jeong, D.; Kim, D.H.; Song, K.Y.; Seo, K.H. Antimicrobial and anti-biofilm activities of Lactobacillus kefiranofaciens DD2 against oral pathogens. J. Oral Microbiol. 2018, 10, 1472985. [Google Scholar] [CrossRef] [PubMed]
- Apiwatsiri, P.; Pupa, P.; Yindee, J.; Niyomtham, W.; Sirichokchatchawan, W.; Lugsomya, K.; Shah, A.A.; Prapasarakul, N. Anticonjugation and antibiofilm evaluation of probiotic strains Lactobacillus plantarum 22F, 25F, and Pediococcus acidilactici 72N against Escherichia coli harboring mcr-1 gene. Front. Vet. Sci. 2021, 8, 614439. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Lai, C.C.; Huang, H.L.; Huang, W.Y.; Toh, H.S.; Weng, T.C.; Chuang, Y.C.; Lu, Y.C.; Tang, H.J. Antimicrobial Activity of Lactobacillus Species against Carbapenem-Resistant Enterobacteriaceae. Front. Microbiol. 2019, 10, 789. [Google Scholar] [CrossRef]
- Liu, W.; Chen, M.; Duo, L.; Wang, J.; Guo, S.; Sun, H.; Menghe, B.; Zhang, H. Characterization of potentially probiotic lactic acid bacteria and bifidobacteria isolated from human colostrum. J. Dairy Sci. 2020, 103, 4013–4025. [Google Scholar] [CrossRef] [PubMed]
- Deglovic, J.; Majtanova, N.; Majtan, J. Antibacterial and antibiofilm effect of honey in the prevention of dental caries: A recent perspective. Foods 2022, 11, 2670. [Google Scholar] [CrossRef]
- Amargianitakis, M.; Antaniadou, M.; Rahiotis, C.; Varzakas, T. Probiotics, prebiotics, synbiotics and dental caries. New perspectives, suggestions and patient coaching approach for a cavity-free mouth. Appl. Sci. 2021, 11, 5472. [Google Scholar] [CrossRef]
- Zayed, S.M.; Aboulwafa, M.M.; Hashem, A.M.; Saleh, S.E. Biofilm formation by Streptococcus mutans and its inhibition by green tea extracts. AMB Express 2021, 11, 73. [Google Scholar] [CrossRef] [PubMed]
- Sohn, K.; Kalanetra, K.M.; Mills, D.A.; Underwood, M.A. Buccal administration of human colostrum: Impact on the oral microbiota of premature infants. J. Perinat. 2016, 36, 106–111. [Google Scholar] [CrossRef] [PubMed]
- van Meijeren-van Lunteren, A.W.; Voortman, T.; Elfrink, M.E.C.; Wolvius, E.B.; Kragt, L. Breastfeeding and childhood dental caries: Results from a socially diverse birth cohort study. Caries Res. 2021, 55, 153–161. [Google Scholar] [CrossRef]
- Kang, M.S.; Kang, I.C.; Kim, S.M.; Lee, H.C.; Oh, J.S. Effect of Leuconostoc spp. on the formation of Streptococcus mutans biofilm. J. Microbiol. 2007, 45, 291–296. [Google Scholar]
- Kumar, H.; du Toit, E.; Kulkarni, A.; Aakko, J.; Linderborg, K.M.; Zhang, Y.; Nicol, M.P.; Isolauri, E.; Yang, B.; Collado, M.C.; et al. Distinct patterns in human milk microbiota and fatty acid profiles across specific geographic locations. Front. Microbiol. 2016, 7, 1619. [Google Scholar] [CrossRef]
- World Health Organization. WHO Recommendation on Prophylactic Antibiotics for Women Undergoing Caesarean Section; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Li, L.; Bao, J.; Chang, Y.; Wang, M.; Chen, B.; Yan, F. Gut Microbiota May Mediate the Influence of Periodontitis on Prediabetes. J. Dent. Res. 2021, 100, 1387–1396. [Google Scholar] [CrossRef] [PubMed]
- Patangia, D.V.; Anthony Ryan, C.; Dempsey, E.; Paul Ross, R.; Stanton, C. Impact of antibiotics on the human microbiome and consequences for host health. Microbiologyopen 2022, 11, e1260. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, S.H. Inhibitory effect of Lactococcus lactis HY 449 on cariogenic biofilm. J. Microbiol. Biotechnol. 2016, 26, 1829–1835. [Google Scholar] [CrossRef]
- Rossoni, R.D.; Velloso, M.D.S.; de Barros, P.P.; de Alvarenga, J.A.; Santos, J.D.D.; Santos Prado, A.C.C.D.; Ribeiro, F.C.; Anbinder, A.L.; Junqueira, J.C. Inhibitory effect of probiotic Lactobacillus supernatants from the oral cavity on Streptococcus mutans biofilms. Microb. Pathog. 2018, 123, 361–367. [Google Scholar] [CrossRef]
- Luan, C.; Jiang, N.; Zhou, X.; Zhang, C.; Zhao, Y.; Li, Z.; Li, C. Antibacterial and antibiofilm activities of probiotic Lactobacillus curvatus BSF206 and Pediococcus pentosaceus AC1-2 against Streptococcus mutans. Microb. Pathog. 2022, 164, 105446. [Google Scholar] [CrossRef]
- Brugger, S.D.; Bomar, L.; Lemon, K.P. Commensal-pathogen interactions along the human nasal passages. PLoS Pathog. 2016, 12, e1005633. [Google Scholar] [CrossRef]
- Sakr, A.; Brégeon, F.; Mège, J.L.; Rolain, J.M.; Blin, O. Staphylococcus aureus nasal colonization: An update on mechanisms, epidemiology, risk factors and subsequent infections. Front. Microbiol. 2018, 9, 2419. [Google Scholar] [CrossRef] [PubMed]
- Lemon, K.P.; Klepac-Ceraj, V.; Schiffer, H.K.; Brodie, E.L.; Lynch, S.V.; Kolter, R. Comparative analyses of the bacterial microbiota of the human nostril and oropharynx. mBio 2010, 1, e0129-10. [Google Scholar] [CrossRef]
- Zipperer, A.; Konnerth, M.C.; Laux, C.; Berscheid, A.; Janek, D.; Weidenmaier, C.; Burian, M.; Schilling, N.A.; Slavetinsky, C.; Marschal, M.; et al. Human commensals producing a novel antibiotic impair pathogen colonization. Nature 2016, 535, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuji, T.; Chen, T.H.; Narala, S.; Chun, K.A.; Two, A.M.; Yun, T.; Shafiq, F.; Kotol, P.F.; Bouslimani, A.; Melnik, A.V.; et al. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci. Transl. Med. 2017, 9, eaah4680. [Google Scholar] [CrossRef]
- Signori, C.; Hartwig, A.D.; Silva-Júnior, I.F.D.; Correa, M.B.; Azevedo, M.S.; Cenci, M.S. The role of human milk and sucrose on cariogenicity of microcosm biofilms. Braz. Oral. Res. 2018, 32, e109. [Google Scholar] [CrossRef]
- Zeng, Y.; Fadaak, A.; Alomeir, N.; Wu, T.T.; Rustchenko, E.; Qing, S.; Bao, J.; Gilbert, C.; Xiao, J. Lactobacillus plantarum Disrupts S. mutans-C. albicans Cross-Kingdom Biofilms. Front. Cell Infect. Microbiol. 2022, 12, 872012. [Google Scholar] [CrossRef]
- Moi, G.P.; Silva, C.B.; Garcia-Leite, E.S.; Bertaia, C.A.; Santos, R.C.; da Costa-Nobre, P.X.; Silva, A.M. Cariogenic Potential of Human Milk, Bovine Milk and Milk Substitutes in Early Childhood. Acad. J. Ped. Neonatol. 2017, 5, 555711. [Google Scholar]
- Korpela, K.; Salonen, A.; Vepsäläinen, O.; Suomalainen, M.; Kolmeder, C.; Varjosalo, M.; Miettinen, S.; Kukkonen, K.; Savilahti, E.; Kuitunen, M.; et al. Probiotic supplementation restores normal microbiota composition and function in antibiotic-treated and in caesarean-born infants. Microbiome 2018, 6, 182. [Google Scholar] [CrossRef] [PubMed]
- Widyarman, A.S.; Hartono, V.; Marjani, L.I.; Irawan, D.; Luthfi, L.; Bachtiar, B.M. Lactobacillus reuteri containing probiotic lozenges consumption reduces Streptococcus mutans, Streptococcus sobrinus, Porphyromonas gingivalis, and Aggregatibacter actinomycetemcomitans in orthodontic patients. J. Int. Dent. Med. Res. 2018, 11, 628–633. [Google Scholar]
- Twetman, S.; Jørgensen, M.R. Probiotic interventions for oral health. In Probiotic Research in Therapeutics; Pawar, S.V., Rishi, P., Eds.; Springer Nature Singapore Pte Ltd.: Singapore, 2021; pp. 253–270. ISBN 9813362367/9789813362369. [Google Scholar]
- Salem-Bekhit, M.M.; Riad, O.K.M.; Selim, H.M.R.M.; Tohamy, S.T.K.; Taha, E.I.; Al-Suwayeh, S.A.; Shazly, G.A. Box–Behnken Design for Assessing the Efficiency of Aflatoxin M1 Detoxification in Milk Using Lactobacillus rhamnosus and Saccharomyces cerevisiae. Life 2023, 13, 1667. [Google Scholar] [CrossRef] [PubMed]
- Abu-Elfotuh, K.; Selim, H.M.R.M.; Riad, O.K.M.; Hamdan, A.M.E.; Hassanin, S.O.; Sharif, A.F.; Moustafa, N.M.; Gowifel, A.M.H.; Mohamed, M.Y.A.; Atwa, A.M.; et al. The protective effects of sesamol and/or the probiotic, Lactobacillus rhamnosus, against aluminum chloride-induced neurotoxicity and hepatotoxicity in rats: Modulation of Wnt/β-catenin/GSK-3β, JAK-2/STAT-3, PPAR-γ, inflammatory, and apoptotic pathways. Front Pharmacol. 2023, 14, 1208252. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).