Association between Muscle Mass Index and Neurological Outcomes of Patients with Out-of-Hospital Cardiac Arrest
Abstract
1. Introduction
2. Materials and Methods
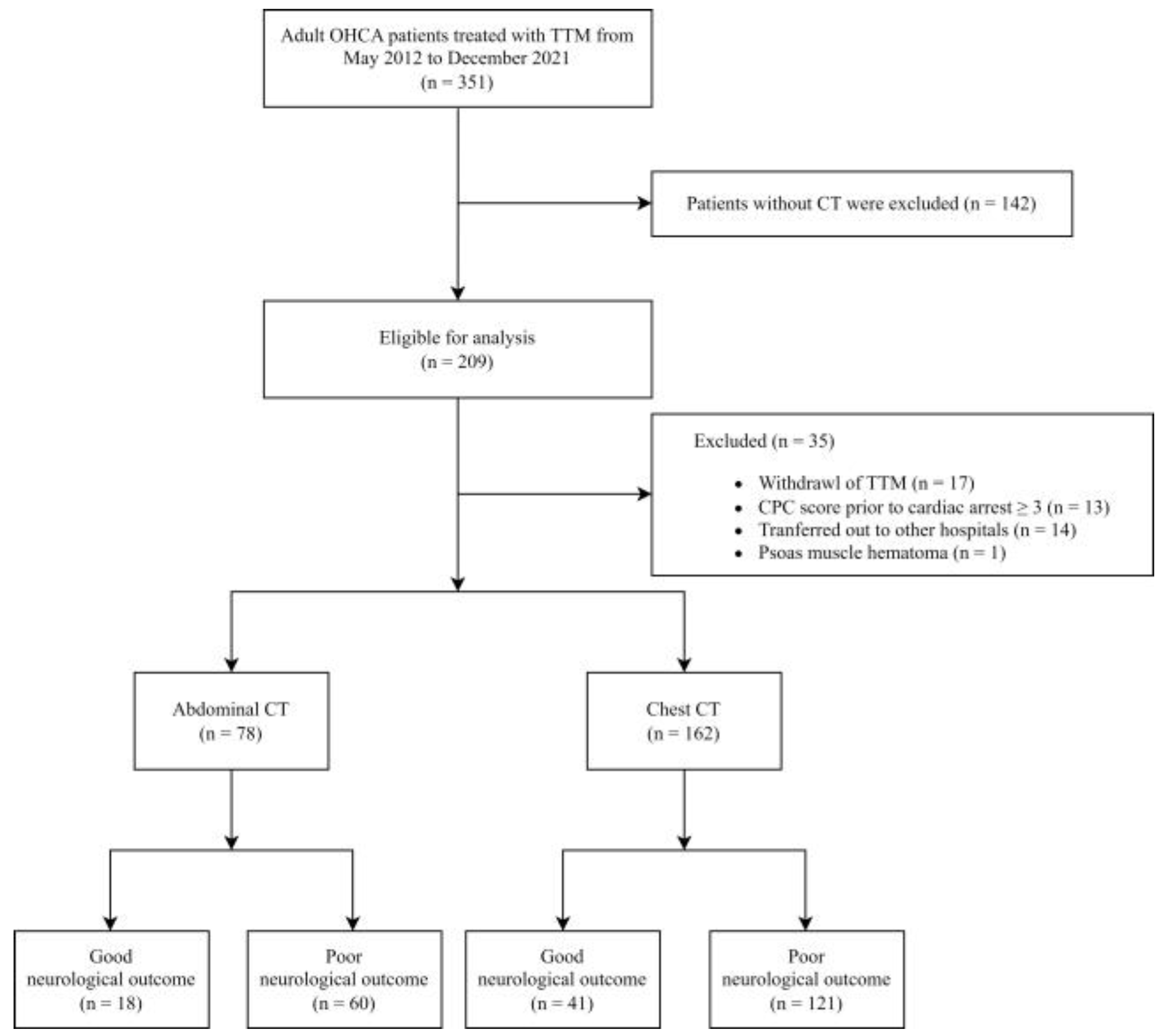
2.1. Settings and Study Population
2.2. Post-Cardiac Arrest Care and TTM
2.3. Data Collection
2.4. Muscle Mass Measurement
2.5. Statistical Analyses
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Total (n = 174) | Good Neurological Outcome (n = 101) | Poor Neurological Outcome (n = 73) | p Value | |
|---|---|---|---|---|
| Age | 63.0 [48.0–72.0] | 59.0 [45.0–70.0] | 65.0 [55.0–74.0] | 0.019 |
| Men | 123 (70.7) | 77 (76.2) | 46 (63.0) | 0.085 |
| BMI (kg/m2) | 23.4 [21.3–25.9] | 23.7 [21.4–26.3] | 22.9 [21.0–25.3] | 0.517 |
| Smoking | 0.222 | |||
| Current | 47 (27.0) | 32 (31.7) | 15 (20.5) | |
| Ex-smoker | 27 (15.5) | 16 (15.8) | 11 (15.1) | |
| Non-smoker | 100 (57.5) | 53 (52.5) | 47 (64.4) | |
| CCI | 3.0 [1.0–5.0] | 3.0 [0.0–5.0] | 4.0 [2.0–7.0] | 0.004 |
| Witnessed arrest | 115 (66.1) | 38 (52.1) | 77 (76.2) | 0.002 |
| Location | 0.153 | |||
| Residence | 76 (43.7) | 39 (38.6) | 37 (50.7) | |
| Other | 98 (56.3) | 62 (61.4) | 36 (49.3) | |
| Bystander CPR | 115 (66.1) | 74 (73.3) | 41 (56.2) | 0.029 |
| Shockable rhythm | 54 (31.0) | 45 (44.6) | 9 (12.3) | <0.001 |
| Cardiogenic cause | 84 (48.3) | 59 (58.4) | 25 (34.2) | 0.003 |
| Arrest time a (mins) | 28.5 [17.0–43.0] | 26.0 [15.0–40.0] | 37.0 [20.0–45.0] | 0.014 |
| PCI | 28 (16.1) | 21 (20.8) | 7 (9.6) | 0.076 |
| ECMO | 0.475 | |||
| ECPR | 2 (1.1) | 2 (2.0) | 0 (0.0) | |
| after ROSC | 10 (5.7) | 6 (5.9) | 4 (5.5) | |
| No ECMO | 162 (93.1) | 93 (92.1) | 69 (94.5) |
References
- Holzer, M.; Bernard, S.A.; Hachimi-Idrissi, S.; Roine, R.O.; Sterz, F.; Müllner, M.; Collaborative Group on Induced Hypothermia for Neuroprotection After Cardiac Arrest. Hypothermia for neuroprotection after cardiac arrest: Systematic review and individual patient data meta-analysis. Crit. Care Med. 2005, 33, 414–418. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Ahn, S.; Sohn, C.H.; Seo, D.-W.; Lee, Y.-S.; Lee, J.H.; Oh, B.J.; Lim, K.S.; Kim, W.Y. Long-term neurological outcomes in patients after out-of-hospital cardiac arrest. Resuscitation 2016, 101, 1–5. [Google Scholar] [CrossRef]
- Lee, H.; Shin, H.; Oh, J.; Lim, T.-H.; Kang, B.-S.; Kang, H.; Choi, H.-J.; Kim, C.; Park, J.-H. Association between Body Mass Index and Outcomes in Patients with Return of Spontaneous Circulation after Out-of-Hospital Cardiac Arrest: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 8389. [Google Scholar] [CrossRef]
- Lee, J.; Lee, H.; Oh, J.; Lim, T.H.; Kang, H.; Ko, B.S.; Cho, Y.; Korean Cardiac Arrest Research Consortium (KoCARC) Investigators. Association between Initial Serum Cholesterol Levels and Outcomes of Patients Hospitalized after Out-of-Hospital Cardiac Arrest: A Retrospective Multicenter Registry Study. J. Pers. Med. 2022, 12, 233. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Lee, B.M.; Cho, Y.; Kim, J.W.; Jeung, H.C.; Lee, I.J. Prognostic Significance of Sarcopenia in Advanced Biliary Tract Cancer Patients. Front. Oncol. 2020, 10, 1581. [Google Scholar] [CrossRef]
- Cawthon, P.M.; Peters, K.W.; Shardell, M.D.; McLean, R.R.; Dam, T.-T.L.; Kenny, A.M.; Fragala, M.S.; Harris, T.B.; Kiel, D.P.; Guralnik, J.M.; et al. Cutpoints for Low Appendicular Lean Mass That Identify Older Adults with Clinically Significant Weakness. J. Gerontol. Ser. A 2014, 69, 567–575. [Google Scholar] [CrossRef]
- Hong, S.-I.; Kim, K.W.; Ko, Y.; Kim, Y.-J.; Huh, J.W.; Hong, S.-B.; Kim, W.Y. Long-Term Outcomes After In-Hospital Cardiac Arrest: Does Pre-arrest Skeletal Muscle Depletion Matter? Front. Physiol. 2021, 12, 692757. [Google Scholar] [CrossRef]
- Bak, S.H.; Kwon, S.O.; Han, S.-S.; Kim, W.J. Computed tomography-derived area and density of pectoralis muscle associated disease severity and longitudinal changes in chronic obstructive pulmonary disease: A case control study. Respir. Res. 2019, 20, 226. [Google Scholar] [CrossRef]
- Prado, C.M.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef]
- Go, S.-I.; Park, M.J.; Song, H.-N.; Kim, H.-G.; Kang, M.H.; Lee, H.R.; Kim, Y.; Kim, R.B.; Lee, S.I.; Lee, G.-W. Prognostic impact of sarcopenia in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J. Cachexia Sarcopenia Muscle 2016, 7, 567–576. [Google Scholar] [CrossRef]
- Cosquéric, G.; Sebag, A.; Ducolombier, C.; Thomas, C.; Piette, F.; Weill-Engerer, S. Sarcopenia is predictive of nosocomial infection in care of the elderly. Br. J. Nutr. 2006, 96, 895–901. [Google Scholar] [CrossRef]
- Perkins, G.D.; Jacobs, I.G.; Nadkarni, V.M.; Berg, R.A.; Bhanji, F.; Biarent, D.; Bossaert, L.L.; Brett, S.J.; Chamberlain, D.; Caen, A.R.d.; et al. Cardiac Arrest and Cardiopulmonary Resuscitation Outcome Reports: Update of the Utstein Resuscitation Registry Templates for Out-of-Hospital Cardiac Arrest. Circulation 2015, 132, 1286–1300. [Google Scholar] [CrossRef]
- Hirlekar, G.; Jonsson, M.; Karlsson, T.; Hollenberg, J.; Albertsson, P.; Herlitz, J. Comorbidity and survival in out-of-hospital cardiac arrest. Resuscitation 2018, 133, 118–123. [Google Scholar] [CrossRef]
- Jennett, B.; Bond, M. Assessment of Outcome After Severe Brain Damage: A Practical Scale. Lancet 1975, 305, 480–484. [Google Scholar] [CrossRef]
- Langhelle, A.; Nolan, J.; Herlitz, J.; Castren, M.; Wenzel, V.; Soreide, E.; Engdahl, J.; Steen, P.A. Recommended guidelines for reviewing, reporting, and conducting research on post-resuscitation care: The Utstein style. Resuscitation 2005, 66, 271–283. [Google Scholar] [CrossRef]
- Derstine, B.A.; Holcombe, S.A.; Goulson, R.L.; Ross, B.E.; Wang, N.C.; Sullivan, J.A.; Su, G.L.; Wang, S.C. Quantifying Sarcopenia Reference Values Using Lumbar and Thoracic Muscle Areas in a Healthy Population. J. Nutr. Health Aging 2018, 22, 180–185. [Google Scholar] [CrossRef]
- Daniel, M.Z.; Caleb Everett, C.; Mark John, M. Radiographic assessment of sarcopenia in the trauma setting: A systematic review. Trauma Surg. Acute Care Open 2020, 5, e000414. [Google Scholar] [CrossRef]
- Englesbe, M.J.; Patel, S.P.; He, K.; Lynch, R.J.; Schaubel, D.E.; Harbaugh, C.; Holcombe, S.A.; Wang, S.C.; Segev, D.L.; Sonnenday, C.J. Sarcopenia and Mortality after Liver Transplantation. J. Am. Coll. Surg. 2010, 211, 271–278. [Google Scholar] [CrossRef]
- Moon, S.W.; Lee, S.H.; Woo, A.; Leem, A.Y.; Lee, S.H.; Chung, K.S.; Kim, E.Y.; Jung, J.Y.; Kang, Y.A.; Park, M.S.; et al. Reference values of skeletal muscle area for diagnosis of sarcopenia using chest computed tomography in Asian general population. J. Cachexia Sarcopenia Muscle 2022, 13, 955–965. [Google Scholar] [CrossRef]
- Moon, S.W.; Choi, J.S.; Lee, S.H.; Jung, K.S.; Jung, J.Y.; Kang, Y.A.; Park, M.S.; Kim, Y.S.; Chang, J.; Kim, S.Y. Thoracic skeletal muscle quantification: Low muscle mass is related with worse prognosis in idiopathic pulmonary fibrosis patients. Respir. Res. 2019, 20, 35. [Google Scholar] [CrossRef]
- Mathur, S.; Rodrigues, N.; Mendes, P.; Rozenberg, D.; Singer, L.G. Computed Tomography–Derived Thoracic Muscle Size as an Indicator of Sarcopenia in People With Advanced Lung Disease. Cardiopulm. Phys. Ther. J. 2017, 28, 99–105. [Google Scholar] [CrossRef]
- McDonald, M.-L.N.; Diaz, A.A.; Ross, J.C.; San Jose Estepar, R.; Zhou, L.; Regan, E.A.; Eckbo, E.; Muralidhar, N.; Come, C.E.; Cho, M.H.; et al. Quantitative Computed Tomography Measures of Pectoralis Muscle Area and Disease Severity in Chronic Obstructive Pulmonary Disease. A Cross-Sectional Study. Ann. Am. Thorac. Soc. 2014, 11, 326–334. [Google Scholar] [CrossRef]
- Tanimura, K.; Sato, S.; Fuseya, Y.; Hasegawa, K.; Uemasu, K.; Sato, A.; Oguma, T.; Hirai, T.; Mishima, M.; Muro, S. Quantitative Assessment of Erector Spinae Muscles in Patients with Chronic Obstructive Pulmonary Disease. Novel Chest Computed Tomography–derived Index for Prognosis. Ann. Am. Thorac. Soc. 2015, 13, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Ha, J.; Ko, Y.; Kim, K.W.; Park, T.; Lee, J.; You, M.W.; Yoon, K.H.; Park, J.Y.; Kee, Y.J.; et al. Reliability of Skeletal Muscle Area Measurement on CT with Different Parameters: A Phantom Study. Korean J. Radiol. 2021, 22, 624–633. [Google Scholar] [CrossRef]
- Studenski, S.A.; Peters, K.W.; Alley, D.E.; Cawthon, P.M.; McLean, R.R.; Harris, T.B.; Ferrucci, L.; Guralnik, J.M.; Fragala, M.S.; Kenny, A.M.; et al. The FNIH Sarcopenia Project: Rationale, Study Description, Conference Recommendations, and Final Estimates. J. Gerontol. Ser. A 2014, 69, 547–558. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A Short Physical Performance Battery Assessing Lower Extremity Function: Association with Self-Reported Disability and Prediction of Mortality and Nursing Home Admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef]
- Scott, D.; Park, M.S.; Kim, T.N.; Ryu, J.Y.; Hong, H.C.; Yoo, H.J.; Baik, S.H.; Jones, G.; Choi, K.M. Associations of low muscle mass and the metabolic syndrome in Caucasian and Asian middle-aged and older adults. J. Nutr. Health Aging 2016, 20, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, I.H. Sarcopenia: Origins and Clinical Relevance1. J. Nutr. 1997, 127, 990S–991S. [Google Scholar] [CrossRef]
- Newman, A.B.; Haggerty, C.L.; Goodpaster, B.; Harris, T.; Kritchevsky, S.; Nevitt, M.; Miles, T.P.; Visser, M.; The Health, Aging, and Body Composition Research Group. Strength and Muscle Quality in a Well-Functioning Cohort of Older Adults: The Health, Aging and Body Composition Study. J. Am. Geriatr. Soc. 2003, 51, 323–330. [Google Scholar] [CrossRef]
- Fabiano Di, M.; Silvia, T.; Maria Adelaide, R.; Alice, C.R.; Giulia, M.P.; Anna, V.; Pierachille, S.; Antonio, E.P.; Stefano, C. Physical Activity Impairment in Depressed COPD Subjects. Respir. Care 2014, 59, 726. [Google Scholar] [CrossRef]
- Koehler, J.; Boirie, Y.; Bensid, L.; Pereira, B.; Ghelis, N.; Dupuis, C.; Tournadre, A.; Boyer, L.; Cassagnes, L. Thoracic sarcopenia as a predictive factor of SARS-CoV2 evolution. Clin. Nutr. 2022, 41, 2918–2923. [Google Scholar] [CrossRef]
- Mourtzakis, M.; Prado, C.M.M.; Lieffers, J.R.; Reiman, T.; McCargar, L.J.; Baracos, V.E. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl. Physiol. Nutr. Metab. 2008, 33, 997–1006. [Google Scholar] [CrossRef]
- Belger, E.; Truhn, D.; Weber, C.D.; Neumann, U.P.; Hildebrand, F.; Horst, K. The Impact of Body Mass Composition on Outcome in Multiple Traumatized Patients—Results from the Fourth Thoracic and Third Lumbar Vertebrae: A Single-Center Retrospective Observational Study. J. Clin. Med. 2023, 12, 2520. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, E.Y.; Kang, S.M.; Ahn, H.K.; Kim, H.S. Single cross-sectional area of pectoralis muscle by computed tomography–correlation with bioelectrical impedance based skeletal muscle mass in healthy subjects. Clin. Physiol. Funct. Imaging 2017, 37, 507–511. [Google Scholar] [CrossRef]
- Yokosuka, R.; Imai, R.; Ro, S.; Murakami, M.; Okafuji, K.; Kitamura, A.; Tomishima, Y.; Jinta, T.; Nishimura, N.; Tamura, T.; et al. Pectoralis Muscle Mass on Chest CT at Admission Predicts Prognosis in Patients with Pneumonia. Can. Respir. J. 2021, 2021, 3396950. [Google Scholar] [CrossRef]
- Herlitz, J.; Ekström, L.; Wennerblom, B.; Axelsson, Å.; Bång, A.; Holmberg, S. Prognosis Among Survivors of Prehospital Cardiac Arrest. Ann. Emerg. Med. 1995, 25, 58–63. [Google Scholar] [CrossRef]
- López-Herce, J.; García, C.; Rodríguez-Núñez, A.; Domínguez, P.; Carrillo, A.; Calvo, C.; Delgado, M.A. Long-term outcome of paediatric cardiorespiratory arrest in Spain. Resuscitation 2005, 64, 79–85. [Google Scholar] [CrossRef]
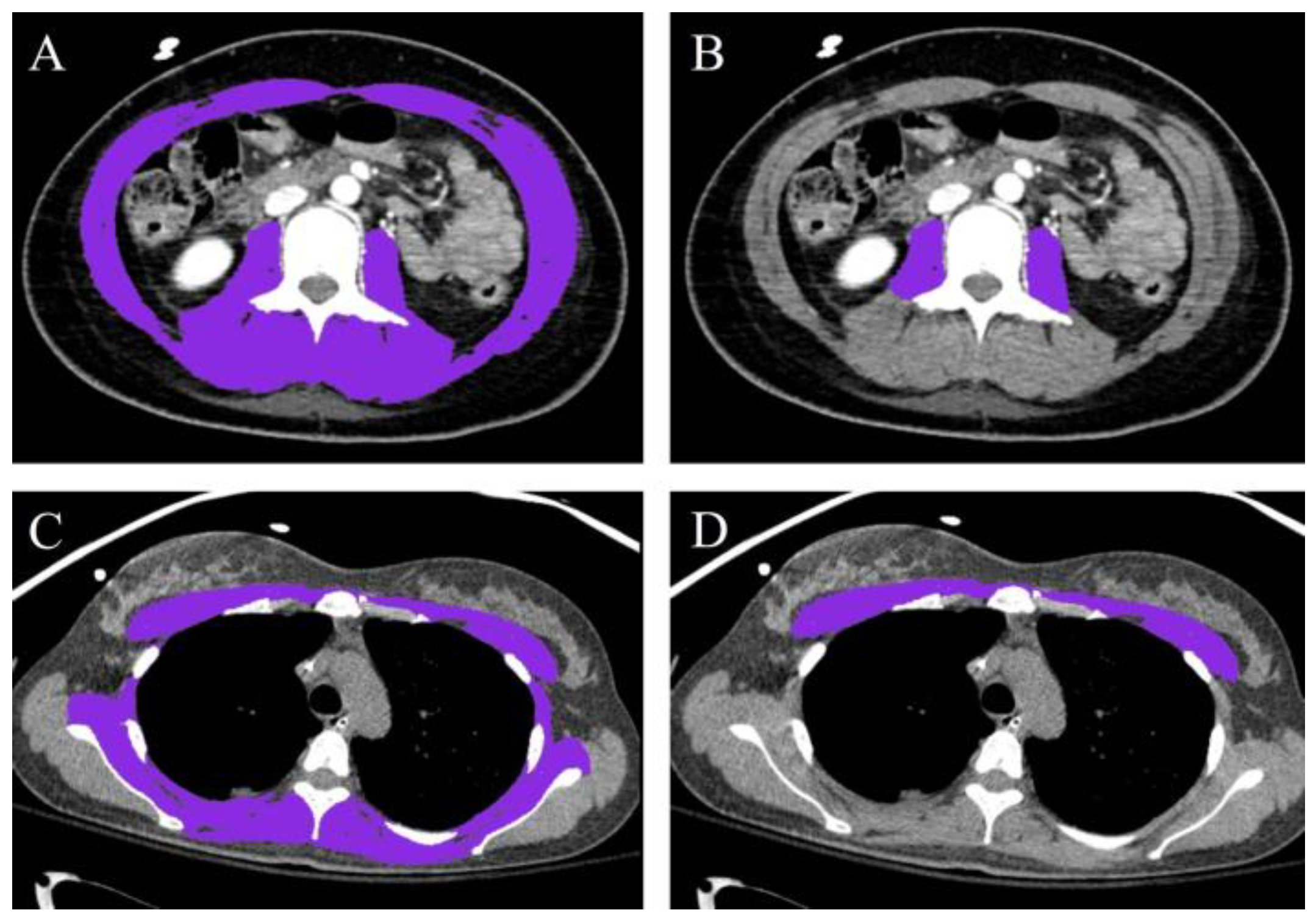
| Abdominal CT Group (n = 78) | ||||||
|---|---|---|---|---|---|---|
| Good Neurological Outcome (n = 18) | Poor Neurological Outcome (n = 60) | p Value | Survival (n = 41) | Death (n = 37) | p Value | |
| Age | 58.5 [48.0–66.0] | 65.0 [52.0–74.0] | 0.267 | 62.0 [47.0–69.0] | 65.0 [60.0–75.0] | 0.064 |
| Men | 15 (83.3) | 39 (65.0) | 0.235 | 32 (78.0) | 22 (59.5) | 0.126 |
| BMI (kg/m2) | 23.0 ± 3.9 | 24.1 ± 4.1 | 0.310 | 23.4 ± 4.0 | 24.3 ± 4.2 | 0.336 |
| Smoking | 0.357 | 0.710 | ||||
| Current | 6 (33.3) | 12 (20.0) | 11 (26.8) | 7 (18.9) | ||
| Ex-smoker | 3 (16.7) | 7 (11.7) | 5 (12.2) | 5 (13.5) | ||
| Non-smoker | 9 (50.0) | 41 (68.3) | 25 (61.0) | 25 (67.6) | ||
| CCI | 2.5 [1.0–4.0] | 3.5 [2.0–6.5] | 0.146 | 3.0 [1.0–5.0] | 4.0 [2.0–8.0] | 0.146 |
| Witnessed arrest | 14 (77.8) | 38 (63.3) | 0.392 | 33 (80.5) | 19 (51.4) | 0.013 |
| Location | 0.007 | 0.376 | ||||
| Residence | 3 (16.7) | 34 (56.7) | 17 (41.5) | 20 (54.1) | ||
| Other | 15 (83.3) | 26 (43.3) | 24 (58.5) | 17 (45.9) | ||
| Bystander CPR | 14 (77.8) | 35 (58.3) | 0.223 | 26 (63.4) | 23 (62.2) | 1.000 |
| Shockable rhythm | 11 (61.1) | 9 (15.0) | <0.001 | 15 (36.6) | 5 (13.5) | 0.038 |
| Cardiogenic cause | 14 (77.8) | 17 (28.3) | <0.001 | 19 (46.3) | 12 (32.4) | 0.307 |
| Arrest time a (mins) | 22.5 [13.0–41.0] | 35.5 [22.5–44.0] | 0.100 | 32.0 [21.0–42.0] | 33.0 [18.0–44.0] | 0.984 |
| PCI | 7 (38.9) | 6 (10.0) | 0.012 | 9 (22.0) | 4 (10.8) | 0.311 |
| ECMO | 0.114 | 0.272 | ||||
| ECPR | 0 (0.0) | 1 (1.7) | 1 (2.4) | 0 (0.0) | ||
| after ROSC | 3 (16.7) | 2 (3.3) | 4 (9.8) | 1 (2.7) | ||
| No ECMO | 15 (83.3) | 57 (95.0) | 36 (87.8) | 36 (97.3) | ||
| Target body temperature | 0.545 | 0.955 | ||||
| 33 °C | 4 (22.2) | 20 (33.3) | 12 (29.3) | 12 (32.4) | ||
| 36 °C | 14 (77.8) | 40 (66.7) | 29 (70.7) | 25 (67.6) | ||
| NSE | 37.7 [17.0–50.9] | 198.5 [55.4–300.0] | 0.001 | 61.0 [37.7–300.0] | 198.5 [47.6–300.0] | 0.350 |
| Initial PaO2 (mmHg) | 137.7 [62.8–235.6] | 102.5 [72.5–162.1] | 0.294 | 106.4 [75.7–187.4] | 105.6 [64.8–177.5] | 0.503 |
| PaO2 after 4 h (mmHg) | 159.2 [73.6–179.5] | 121.7 [89.1–241.8] | 0.631 | 164.2 [94.0–238.5] | 116.0 [83.0–217.0] | 0.376 |
| Initial PaCO2 (mmHg) | 46.0 ± 19.5 | 72.4 ± 28.7 | <0.001 | 57.3 [41.1–80.6] | 67.0 [52.5–84.8] | 0.596 |
| PaCO2 after 4 h (mmHg) | 37.4 [33.7–43.2] | 47.9 [36.8–56.6] | 0.029 | 41.0 [33.7–51.9] | 45.4 [39.3–55.6] | 0.211 |
| Time from arrest to CT (days) | 0.0 [0.0–0.0] | 0.0 [0.0–0.0] | 0.077 | 0.0 [0.0–0.0] | 0.0 [0.0–0.0] | 0.625 |
| Chest CT Group (n = 162) | ||||||
| Good Neurological Outcome (n = 41) | Poor Neurological Outcome (n = 121) | pValue | Survival (n = 91) | Death (n = 71) | pValue | |
| Age | 59.0 [47.0–66.0] | 64.0 [49.0–74.0] | 0.085 | 59.0 [43.5–70.5] | 65.0 [55.0–74.0] | 0.040 |
| Men | 36 (87.8) | 77 (63.6) | 0.007 | 68 (74.7) | 45 (63.4) | 0.165 |
| BMI (kg/m2) | 23.3 [22.0–26.0] | 23.5 [21.1–26.2] | 0.767 | 23.9 ± 4.1 | 23.3 ± 4.6 | 0.357 |
| Smoking | 0.317 | 0.174 | ||||
| Current | 14 (34.1) | 29 (24.0) | 29 (31.9) | 14 (19.7) | ||
| Ex-smoker | 7 (17.1) | 17 (14.0) | 14 (15.4) | 10 (14.1) | ||
| Non-smoker | 20 (48.8) | 75 (62.0) | 48 (52.7) | 47 (66.2) | ||
| CCI | 2.0 [1.0–4.0] | 4.0 [1.0–6.0] | 0.013 | 3.0 [0.5–5.0] | 4.0 [2.0–7.5] | 0.004 |
| Witnessed arrest | 33 (80.5) | 73 (60.3) | 0.031 | 70 (76.9) | 36 (50.7) | 0.001 |
| Location | 0.002 | 0.115 | ||||
| Residence | 9 (22.0) | 63 (52.1) | 35 (38.5) | 37 (52.1) | ||
| Other | 32 (78.0) | 58 (47.9) | 56 (61.5) | 34 (47.9) | ||
| Bystander CPR | 34 (82.9) | 73 (60.3) | 0.014 | 68 (74.7) | 39 (54.9) | 0.013 |
| Shockable rhythm | 29 (70.7) | 19 (15.7) | <0.001 | 40 (44.0) | 8 (11.3) | <0.001 |
| Cardiogenic cause | 33 (80.5) | 43 (35.5) | <0.001 | 52 (57.1) | 24 (33.8) | 0.005 |
| Arrest time a (mins) | 20.0 [13.0–28.0] | 33.0 [20.0–44.0] | 0.001 | 25.0 [15.0–39.0] | 37.0 [21.0–45.0] | 0.005 |
| PCI | 15 (36.6) | 9 (7.4) | <0.001 | 18 (19.8) | 6 (8.5) | 0.073 |
| ECMO | 0.278 | 0.454 | ||||
| ECPR | 1 (2.4) | 1 (0.8) | 2 (2.2) | 0 (0.0) | ||
| after ROSC | 4 (9.8) | 5 (4.1) | 5 (5.5) | 4 (5.6) | ||
| No ECMO | 36 (87.8) | 115 (95.0) | 84 (92.3) | 67 (94.4) | ||
| Target body temperature | 0.035 | 0.960 | ||||
| 33 °C | 7 (17.1) | 44 (36.4) | 28 (30.8) | 23 (32.4) | ||
| 36 °C | 34 (82.9) | 77 (63.6) | 63 (69.2) | 48 (67.6) | ||
| NSE | 24.6 [17.6–37.7] | 206.5 [48.5–300.0] | <0.001 | 44.8 [27.1–221.1] | 235.6 [55.4–300.0] | 0.003 |
| Initial PaO2 (mmHg) | 106.4 [68.3–212.2] | 99.4 [64.8–160.8] | 0.289 | 92.4 [65.8–184.9] | 115.1 [67.5–178.4] | 0.510 |
| PaO2 after 4 h (mmHg) | 134.9 [87.5–193.3] | 135.8 [88.2–230.4] | 0.739 | 140.2 [88.0–204.3] | 123.9 [85.6–229.0] | 0.939 |
| Initial PaCO2 (mmHg) | 44.6 [30.3–59.4] | 71.4 [52.9–86.4] | <0.001 | 58.0 [41.4–78.8] | 68.8 [52.0–87.0] | 0.038 |
| PaCO2 after 4 h (mmHg) | 41.0 [34.3–48.7] | 46.0 [36.7–55.1] | 0.051 | 45.8 [36.3–52.5] | 45.1 [35.6–54.9] | 0.991 |
| Time from arrest to CT (days) | 0.0 [0.0–0.0] | 0.0 [0.0–0.0] | 0.077 | 0.0 [0.0–0.0] | 0.0 [0.0–0.0] | 0.625 |
| Good Neurological Outcome | Survival | |||
|---|---|---|---|---|
| Cutoff Value | Cutoff Value | |||
| Men | Women | Men | Women | |
| L3/BMI | 5.8994 | 3.5512 | 6.489 | 4.6331 |
| Psoas/BMI | 0.7703 | 0.3152 | 0.7703 | 0.5832 |
| T4/BMI | 5.4658 | 4.9739 | 5.7717 | 4.9545 |
| Pectoralis/BMI | 2.1648 | 1.531 | 2.2767 | 1.7563 |
| Good Neurological Outcome (n = 18) | Poor Neurological Outcome (n = 60) | p Value | Survival (n = 41) | Death (n = 37) | p Value | |
|---|---|---|---|---|---|---|
| Low L3/BMI | 6 (33.3) | 28 (46.7) | 0.466 | 25 (61.0) | 29 (78.4) | 0.156 |
| Low psoas/BMI | 6 (33.3) | 29 (48.3) | 0.394 | 22 (53.7) | 30 (81.1) | 0.020 |
| Good Neurological Outcome (n = 41) | Poor Neurological Outcome (n = 121) | p Value | Survival (n = 91) | Death (n = 71) | p Value | |
| Low T4/BMI | 6 (14.6) | 68 (56.2) | <0.001 | 35 (38.5) | 48 (67.6) | <0.001 |
| Low pectoralis/BMI | 14 (34.1) | 78 (64.5) | 0.001 | 49 (53.8) | 53 (74.6) | 0.011 |
| Good Neurological Outcome | Survival | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Adjusted OR (95% CI) | p Value | Adjusted OR (95% CI) | p Value | Adjusted OR (95% CI) | p Value | Adjusted OR (95% CI) | p Value | Adjusted OR (95% CI) | p Value | |
| Low psoas/BMI | 0.38 (0.11–1.19) | 0.102 | ||||||||
| Low T4/BMI | 0.22 (0.05–0.76) | 0.021 | 0.56 (0.24–1.31) | 0.182 | ||||||
| Low pectoralis/ BMI | 0.49 (0.15–1.55) | 0.222 | 0.80 (0.33–1.96) | 0.620 | ||||||
| Age | 1.00 (0.95–1.04) | 0.937 | 1.00 (0.96–1.05) | 0.926 | 0.98 (0.95–1.01) | 0.300 | 1.00 (0.97–1.03) | 0.795 | 1.00 (0.97–1.03) | 0.886 |
| Men | 1.15 (0.28–5.09) | 0.845 | 1.94 (0.55–7.82) | 0.318 | 1.79 (0.58–5.71) | 0.315 | 0.99 (0.41–2.36) | 0.981 | 1.13 (0.49–2.64) | 0.769 |
| CCI | 0.97 (0.72–1.28) | 0.818 | 0.91 (0.68–1.19) | 0.498 | 0.84 (0.68–1.02) | 0.089 | 0.81 (0.66–0.99) | 0.039 | ||
| Witnessed arrest | 1.85 (0.51–7.37) | 0.359 | 1.93 (0.57–7.14) | 0.302 | 3.42 (1.18–10.68) | 0.027 | 3.13 (1.38–7.38) | 0.007 | 3.40 (1.52–7.94) | 0.004 |
| Location (Residence) | 0.47 (0.14–1.44) | 0.193 | 0.46 (0.14–1.40) | 0.182 | ||||||
| Bystander CPR | 5.15 (1.46–22.66) | 0.018 | 4.76 (1.40–19.97) | 0.020 | 2.09 (0.94–4.71) | 0.071 | 2.02 (0.92–4.53) | 0.082 | ||
| Shockable rhythm | 3.31 (0.93–12.70) | 0.068 | 3.20 (0.91–12.09) | 0.074 | 2.34 (0.70–8.71) | 0.179 | 3.72 (1.15–12.95) | 0.032 | 3.77 (1.18–12.94) | 0.029 |
| Cardiogenic cause | 2.72 (0.65–11.57) | 0.166 | 2.82 (0.69–11.51) | 0.142 | 1.29 (0.50–3.34) | 0.597 | 1.28 (0.50–3.28) | 0.603 | ||
| Arrest time | 0.96 (0.93–0.99) | 0.023 | 0.96 (0.93–0.99) | 0.020 | 0.97 (0.95–0.99) | 0.011 | 0.97 (0.95–0.99) | 0.011 | ||
| PCI | 4.20 (1.04–19.75) | 0.053 | 3.84 (0.96–17.76) | 0.067 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, Y.; Kim, E.N.; You, J.S.; Han, M.; Park, Y.S. Association between Muscle Mass Index and Neurological Outcomes of Patients with Out-of-Hospital Cardiac Arrest. Life 2024, 14, 680. https://doi.org/10.3390/life14060680
Cho Y, Kim EN, You JS, Han M, Park YS. Association between Muscle Mass Index and Neurological Outcomes of Patients with Out-of-Hospital Cardiac Arrest. Life. 2024; 14(6):680. https://doi.org/10.3390/life14060680
Chicago/Turabian StyleCho, Yongtak, Eung Nam Kim, Je Sung You, Minkyung Han, and Yoo Seok Park. 2024. "Association between Muscle Mass Index and Neurological Outcomes of Patients with Out-of-Hospital Cardiac Arrest" Life 14, no. 6: 680. https://doi.org/10.3390/life14060680
APA StyleCho, Y., Kim, E. N., You, J. S., Han, M., & Park, Y. S. (2024). Association between Muscle Mass Index and Neurological Outcomes of Patients with Out-of-Hospital Cardiac Arrest. Life, 14(6), 680. https://doi.org/10.3390/life14060680






