Beyond the Bile: Exploring the Microbiome and Metabolites in Cholangiocarcinoma
Abstract
:1. Introduction
2. Methods
2.1. Patient Selection and Comparative Analysis of Bile Microbiome
2.2. Bile Fluid Collection during ERCP
2.3. Extraction of DNA from Bile Samples
2.4. Amplification of the V3–V4 Variable Region of the Bacterial 16S rRNA Gene
2.5. Data Analysis
2.6. Nuclear Magnetic Resonance Spectroscopy (NMR) Metabolomics
2.7. Cell Culture
2.8. CFSE Proliferation Assay
2.9. Annexin V/PI Apoptosis Assay
2.10. Ethics Statement
3. Results
3.1. Characteristics of Patients
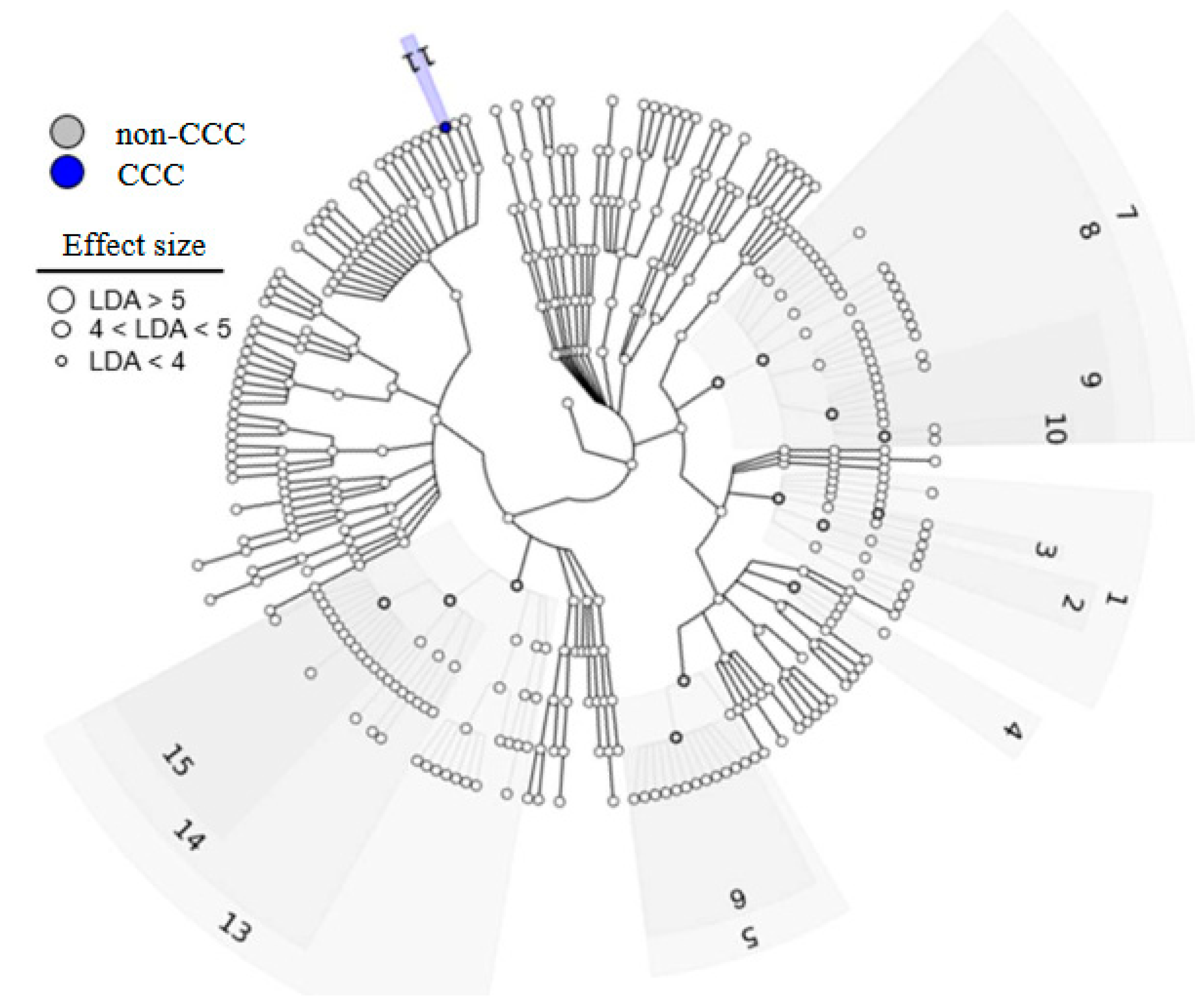
3.2. Bile Microbiome Taxonomic Analysis
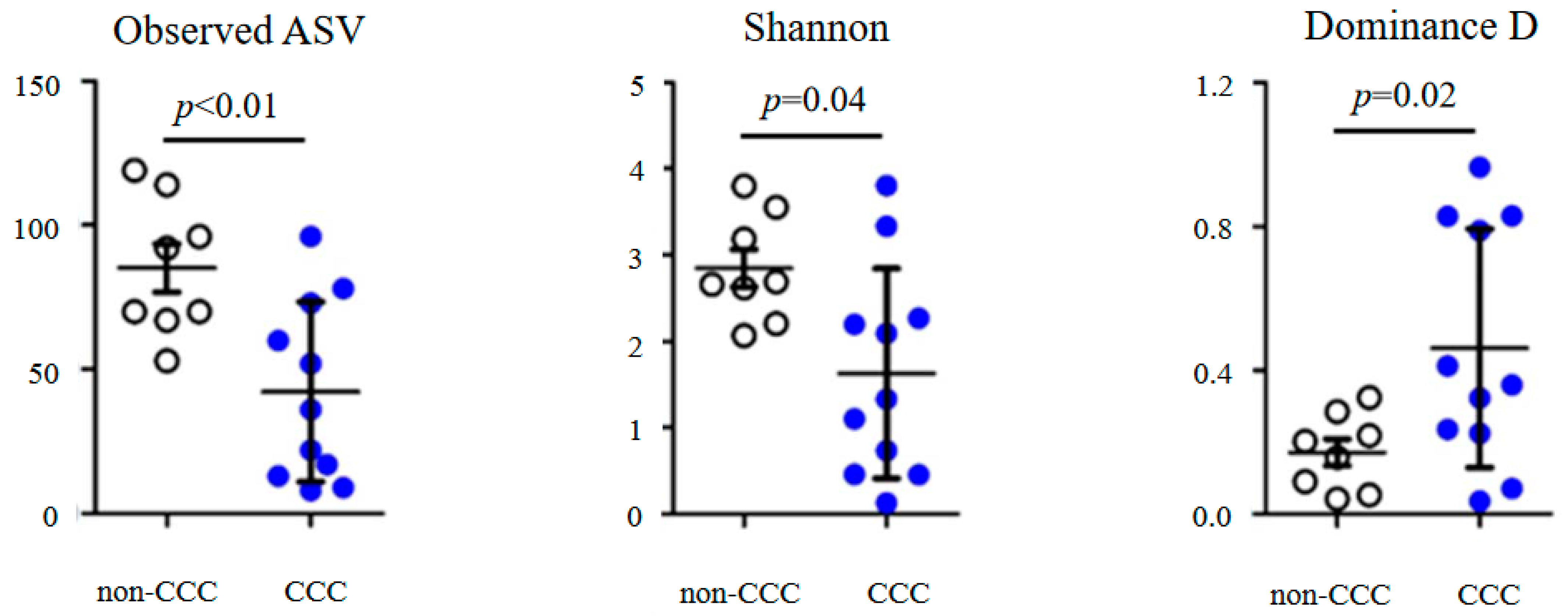
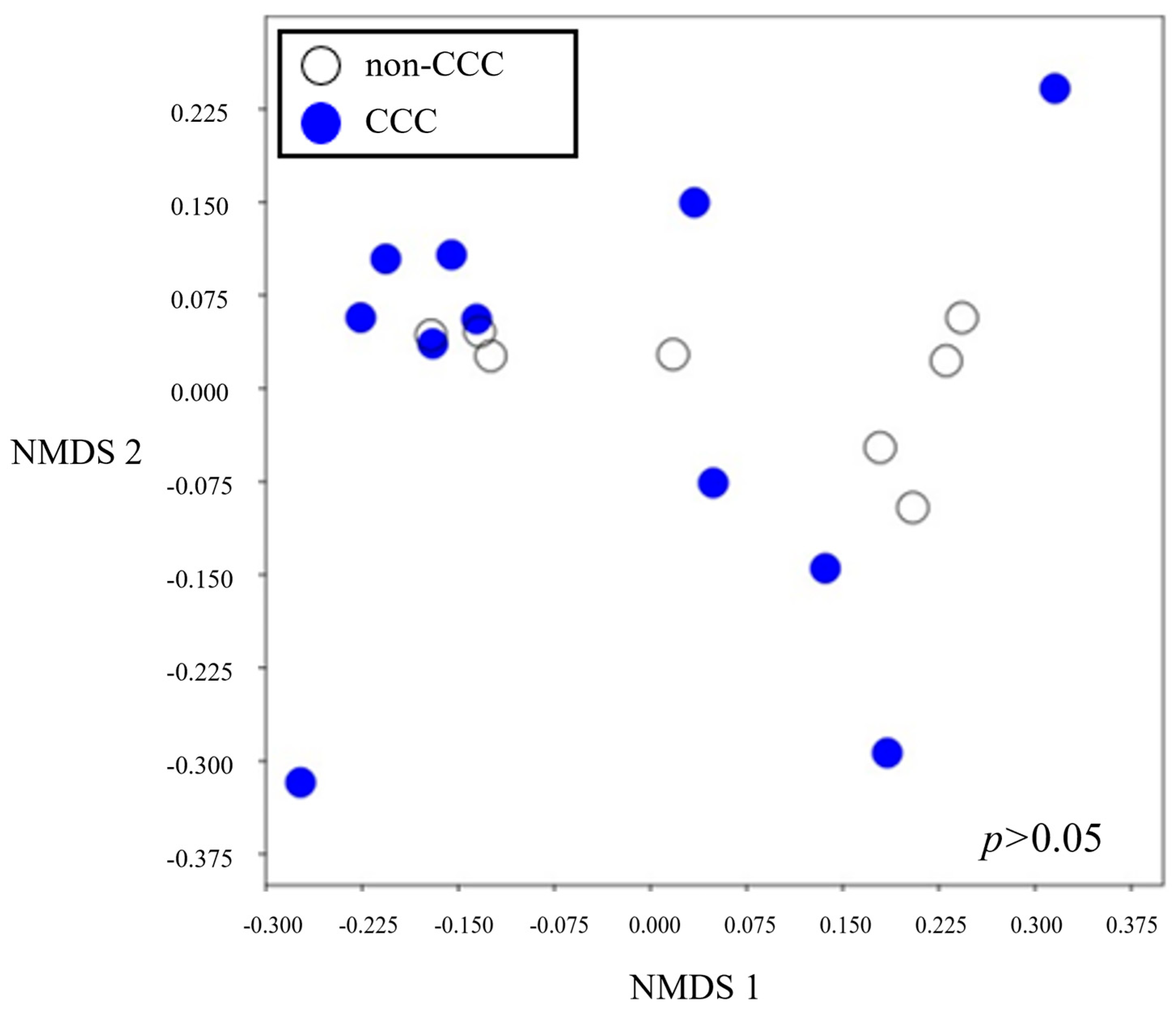
3.3. Alpha and Beta Diversities
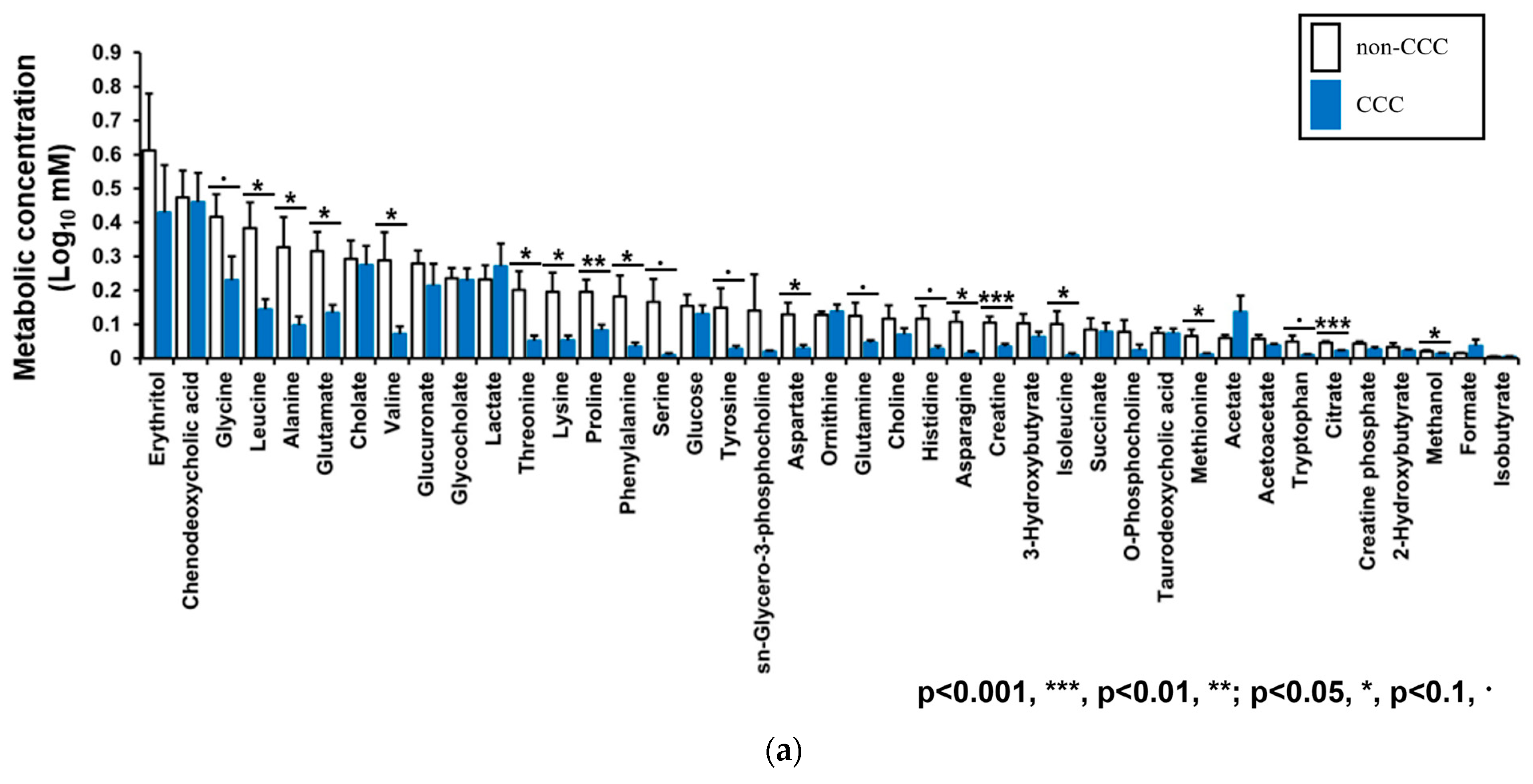
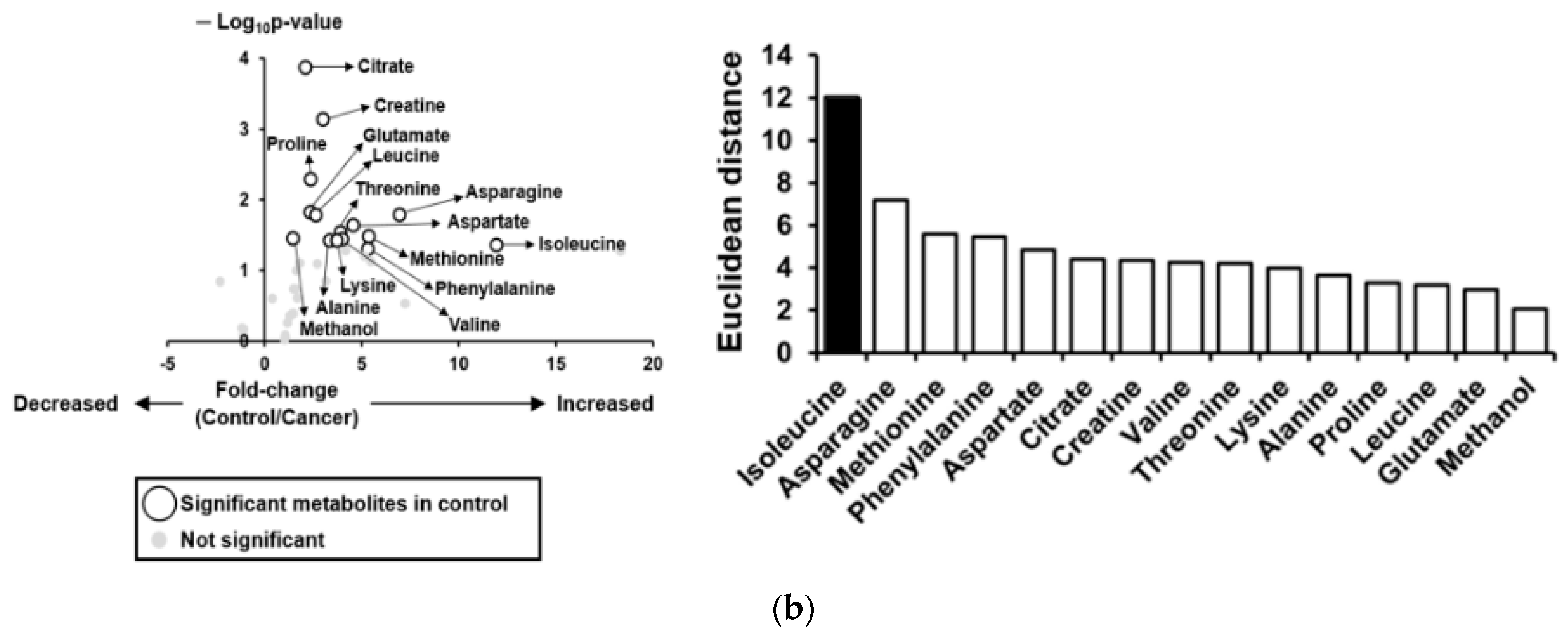
3.4. Metabolic Profiles of Bile Fluid as Determined by NMR
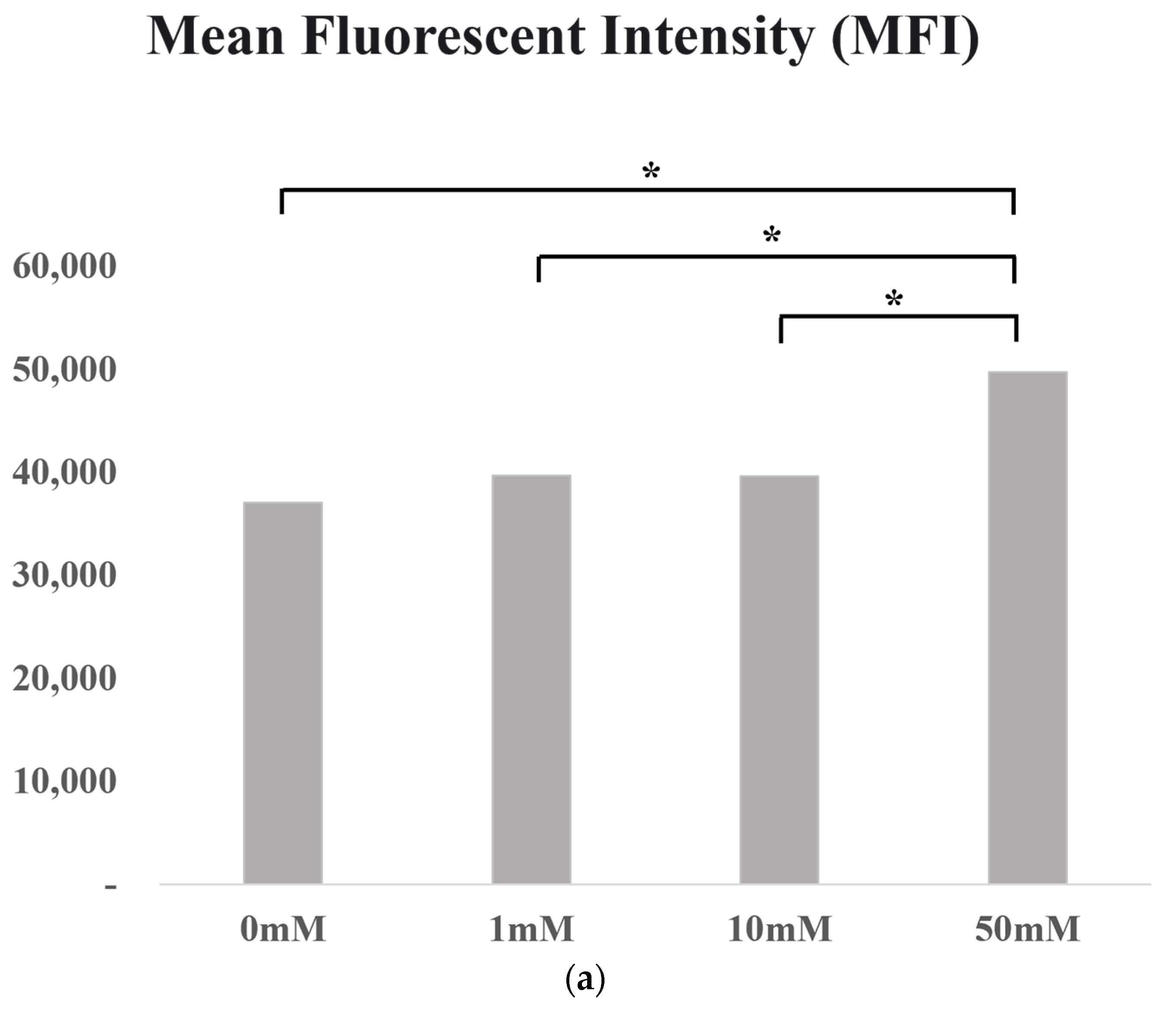
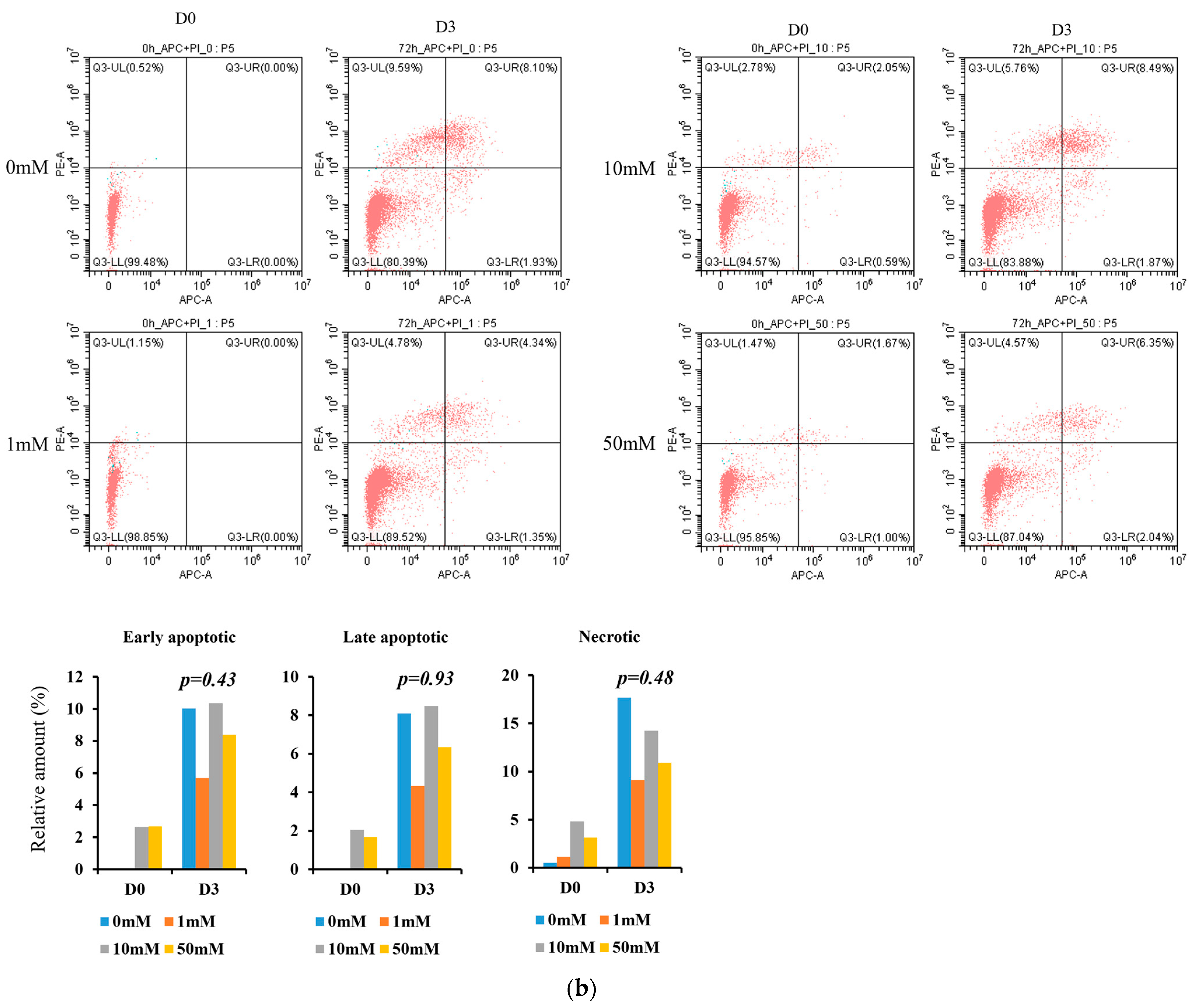
3.5. In Vitro Experiment
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Razumilava, N.; Gores, G.J. Cholangiocarcinoma. Lancet 2014, 383, 2168–2179. [Google Scholar] [CrossRef]
- Rizvi, S.; Khan, S.A.; Hallemeier, C.L.; Kelley, R.K.; Gores, G.J. Cholangiocarcinoma—Evolving concepts and therapeutic strategies. Nat. Rev. Clin. Oncol. 2018, 15, 95–111. [Google Scholar]
- Banales, J.M.; Marin, J.J.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef] [PubMed]
- Patel, T. Cholangiocarcinoma—Controversies and challenges. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 189–200. [Google Scholar] [CrossRef] [PubMed]
- NCCN. Biliary Tract Cancers. In NCCN Clinical Practice Guidelines in Oncology, Version 1; NCCN: Plymouth Meeting, PA, USA, 2023; pp. 1–93. [Google Scholar]
- Park, J.; Kim, M.-H.; Kim, K.-P.; Park, D.H.; Moon, S.-H.; Song, T.J.; Eum, J.; Lee, S.S.; Seo, D.W.; Lee, S.K. Natural history and prognostic factors of advanced cholangiocarcinoma without surgery, chemotherapy, or radiotherapy: A large-scale observational study. Gut Liver 2009, 3, 298. [Google Scholar] [CrossRef] [PubMed]
- Tyson, G.L.; El-Serag, H.B. Risk factors for cholangiocarcinoma. Hepatology 2011, 54, 173–184. [Google Scholar] [CrossRef]
- Csendes, A.; Burdiles, P.; Maluenda, F.; Diaz, J.C.; Csendes, P.; Mitru, N. Simultaneous bacteriologic assessment of bile from gallbladder and common bile duct in control subjects and patients with gallstones and common duct stones. Arch. Surg. 1996, 131, 389–394. [Google Scholar] [CrossRef]
- Maki, T. Pathogenesis of calcium bilirubinate gallstone: Role of E. coli, beta-glucuronidase and coagulation by inorganic ions, polyelectrolytes and agitation. Ann. Surg. 1966, 164, 90–100. [Google Scholar] [CrossRef]
- Grigor’eva, I.N.; Romanova, T.I. Gallstone Disease and Microbiome. Microorganisms 2020, 8, 835. [Google Scholar] [CrossRef]
- Ye, F.; Shen, H.; Li, Z.; Meng, F.; Li, L.; Yang, J.; Chen, Y.; Bo, X.; Zhang, X.; Ni, M. Influence of the Biliary System on Biliary Bacteria Revealed by Bacterial Communities of the Human Biliary and Upper Digestive Tracts. PLoS ONE 2016, 11, e0150519. [Google Scholar] [CrossRef]
- Lee, J.; Park, J.-S.; Bae, J.; Lee, S.; Hwang, Y. Bile Microbiome in Patients with Recurrent Common Bile Duct Stones and Correlation with the Duodenal Microbiome. Life 2022, 12, 1540. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, S.; Jin, C.; Lin, Z.; Deng, T.; Xie, X.; Deng, L.; Li, X.; Ma, J.; Ding, X. A predictive model based on the gut microbiota improves the diagnostic effect in patients with cholangiocarcinoma. Front. Cell. Infect. Microbiol. 2021, 11, 751795. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Morine, Y.; Mawatari, K.; Chiba, A.; Yamada, S.; Saito, Y.; Ishibashi, H.; Takahashi, A.; Shimada, M. Bile metabolites and risk of carcinogenesis in patients with pancreaticobiliary maljunction: A pilot study. Anticancer. Res. 2021, 41, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, H.K.; Min, S.K.; Lee, W.H. 16S rDNA microbiome composition pattern analysis as a diagnostic biomarker for biliary tract cancer. World J. Surg. Oncol. 2020, 18, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef] [PubMed]
- Murata, K.; Moriyama, M. Isoleucine, an essential amino acid, prevents liver metastases of colon cancer by antiangiogenesis. Cancer Res. 2007, 67, 3263–3268. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Liu, X.; Cheng, C.; Yu, W.; Yi, P. Metabolism of amino acids in cancer. Front. Cell Dev. Biol. 2021, 8, 603837. [Google Scholar] [CrossRef]
- Malczewski, A.B.; Navarro, S.; Coward, J.I.; Ketheesan, N. Microbiome-derived metabolome as a potential predictor of response to cancer immunotherapy. J. Immunother. Cancer 2020, 8, e001383. [Google Scholar] [CrossRef]
- Lin, Y.; Ma, C.; Liu, C.; Wang, Z.; Yang, J.; Liu, X.; Shen, Z.; Wu, R. NMR-based fecal metabolomics fingerprinting as predictors of earlier diagnosis in patients with colorectal cancer. Oncotarget 2016, 7, 29454. [Google Scholar] [CrossRef] [PubMed]
- Lukey, M.J.; Katt, W.P.; Cerione, R.A. Targeting amino acid metabolism for cancer therapy. Drug Discov. Today 2017, 22, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Ternes, D.; Tsenkova, M.; Pozdeev, V.I.; Meyers, M.; Koncina, E.; Atatri, S.; Schmitz, M.; Karta, J.; Schmoetten, M.; Heinken, A. The gut microbial metabolite formate exacerbates colorectal cancer progression. Nat. Metab. 2022, 4, 458–475. [Google Scholar] [CrossRef] [PubMed]
- Lieu, E.L.; Nguyen, T.; Rhyne, S.; Kim, J. Amino acids in cancer. Exp. Mol. Med. 2020, 52, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Cotton, C.A.; Bernhardsgrütter, I.; He, H.; Burgener, S.; Schulz, L.; Paczia, N.; Dronsella, B.; Erban, A.; Toman, S.; Dempfle, M. Underground isoleucine biosynthesis pathways in E. coli. Elife 2020, 9, e54207. [Google Scholar] [CrossRef]
- Wang, H.; Chen, S.; Kang, W.; Ding, B.; Cui, S.; Zhou, L.; Zhang, N.; Luo, H.; Wang, M.; Zhang, F. High dose isoleucine stabilizes nuclear PTEN to suppress the proliferation of lung cancer. Discov. Oncol. 2023, 14, 25. [Google Scholar] [CrossRef]

| Variables | Total (n = 24) | Cholangiocarcinoma (n = 11) | Non-Cholangiocarcinoma (n = 13) | * p-Value |
|---|---|---|---|---|
| Age (years) § | 73 (42–88) | 75 (58–88) | 72 (42–84) | 0.69 |
| Sex, male, n (%) | 17 (70.8) | 8 (72.7) | 9 (69.2) | 0.61 |
| Hypertension, n (%) | 12 (50) | 4 (36.4) | 8 (61.5) | 0.41 |
| DM, n (%) | 4 (16.7) | 3 (27.3) | 1 (7.7) | 0.30 |
| Dyslipidemia, n (%) | 10 (41.7) | 6 (54.5) | 4 (30.8) | 0.41 |
| WBC (/uL) § | 9618 (3640–29,130) | 7428 (3640–14,720) | 11,471 (5410–29,130) | 0.12 |
| CRP, mg/dL § | 3.9 (0.1–28.7) | 4.3 (0.1–16.8) | 3.5 (0.1–28.7) | 0.77 |
| T.bil, mg/dL § | 5.9 (0.2–27.9) | 8.7 (0.3–27.9) | 3.5 (0.2–23.9) | 0.12 |
| AST, IU/L § | 167.6 (19.0–663.0) | 206.3 (23.0–625.0) | 134.8 (19.0–663.0) | 0.40 |
| ALT, IU/L § | 136.3 (10.0–688.0) | 131.1 (14.0–380.0) | 140.8 (10.0–688.0) | 0.89 |
| ALP, IU/L § | 424.1 (45.0–2781.0) | 742.8 (109.0–2781.0) | 154.4 (45.0–630.0) | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Kim, H.; Park, J.-S. Beyond the Bile: Exploring the Microbiome and Metabolites in Cholangiocarcinoma. Life 2024, 14, 698. https://doi.org/10.3390/life14060698
Lee J, Kim H, Park J-S. Beyond the Bile: Exploring the Microbiome and Metabolites in Cholangiocarcinoma. Life. 2024; 14(6):698. https://doi.org/10.3390/life14060698
Chicago/Turabian StyleLee, Jungnam, Hanul Kim, and Jin-Seok Park. 2024. "Beyond the Bile: Exploring the Microbiome and Metabolites in Cholangiocarcinoma" Life 14, no. 6: 698. https://doi.org/10.3390/life14060698





