Current Developments in Diagnosis of Salivary Gland Tumors: From Structure to Artificial Intelligence
Abstract
:1. Introduction
2. Morphological Diagnosis of Salivary Neoplasms
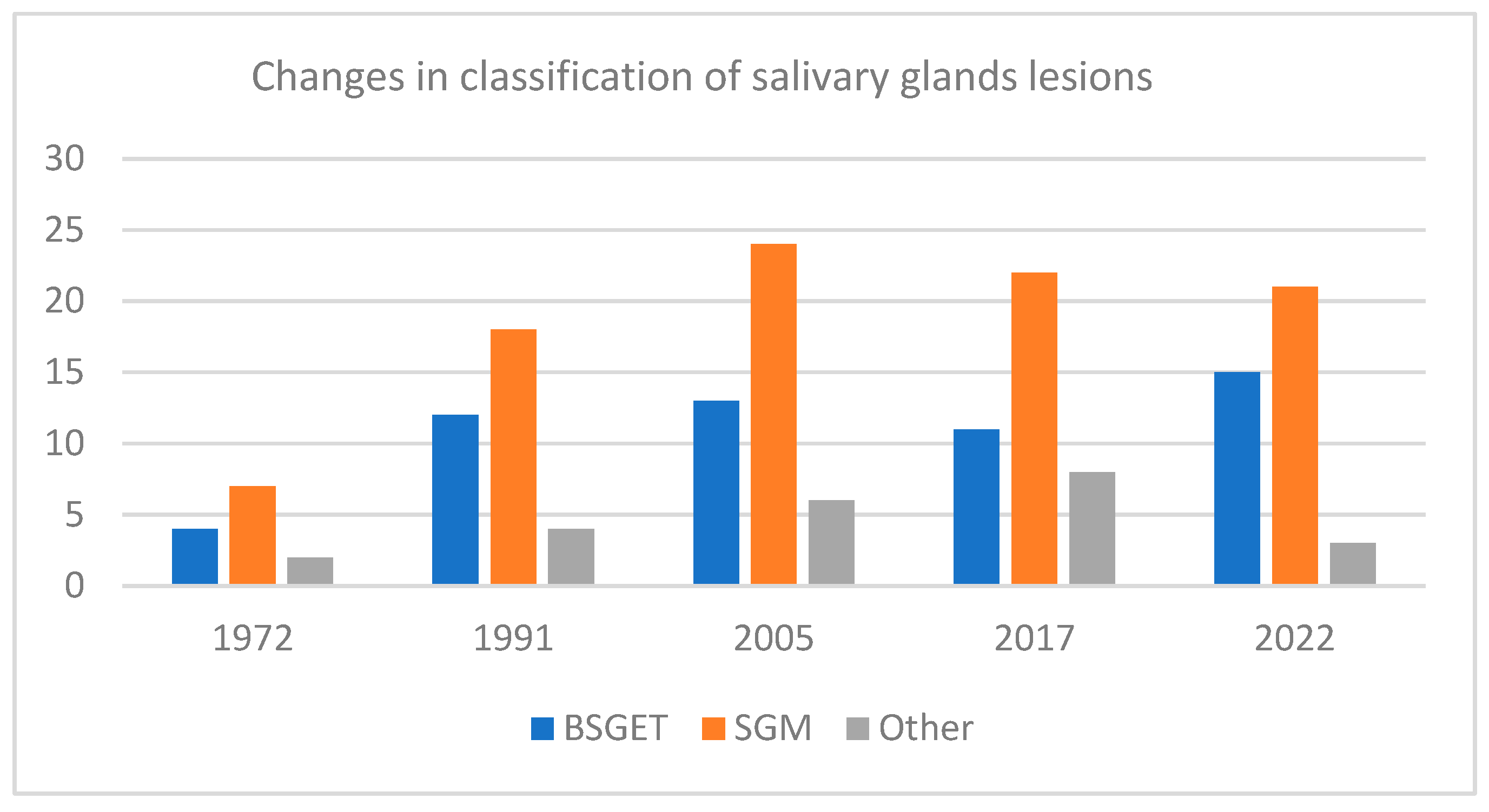
2.1. New Concepts in Classification and Grading of Salivary Gland Tumors
2.2. Advances in the Immunohistochemical Analysis in Salivary Gland Tumors with Morphological Correlations
2.3. Genetic Alterations
2.4. Fine-Needle Aspiration
2.5. Imaging Diagnosis
3. Artificial Intelligence Algorithms in Salivary Gland Tumor Diagnosis
4. Reporting Salivary Gland Malignant Tumors
5. Future Perspectives
5.1. The Tissue-Based Diagnosis
5.2. Treatment
5.3. Artificial Intelligence
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Adeoye, J.; Su, Y.X. Artificial intelligence in salivary biomarker discovery and validation for oral diseases. Oral Dis. 2024, 30, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Kizil, Y.; Aydil, U.; Ekinci, O.; Dilci, A.; Koybașioglu, A.; Duzlu, M.; Inal, E. Salivary gland tumors in Turkey: Demographic featureas and histopathological distribution. Indian J. Otolarygol. Head Neck Surg. 2013, 65 (Suppl. S1), S112–S120. [Google Scholar] [CrossRef] [PubMed]
- Ettl, T.; Schartz-Furlan, S.; Gosau, M.; Reichert, T. Salivary gland carcinomas. Oral Maxillofac. Surg. 2012, 16, 267–283. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.; Hanna, E.Y. Salivary gland cancers: Biology and molecular targets fos therapy. Curr. Oncol. Rep. 2012, 14, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Rossi, E.D.; Baloch, Z.; Barkan, G.; Foschini, M.P.; Kurtycz, D.; Pusztaszeri, M.; Vielh, P.; Faquin, W.C. Second edition of the Milan System for Reporting Salivary Gland Cytopathology: Refining the role of salivary gland FNA. Cytopathology 2024, 35, 188–198. [Google Scholar] [CrossRef] [PubMed]
- De Olivera, F.A.; Duarte, E.C.B.; Taveira, C.T.; Maximo, A.A.; de Aquino, E.C.; Alencar Cassia, R.; Vencio, E.F. Salivary gland tumor: A review of 599 cases in a Brasilian population. Head Neck Pathol. 2009, 3, 271–275. [Google Scholar] [CrossRef]
- Speight, P.M.; Barett, A.W. Prognostic factors in malignant tumours of the salivary glands. Br. J. Oral Maxillofac. Surg. 2009, 47, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Speight, P.; Barett, A.W. Salivary gland tumors: Diagnostic challenges and an update on the latest WHO classification. Diagn. Histopathol. 2020, 26, 147–158. [Google Scholar] [CrossRef]
- Eveson, J.W.; Auclair, P.; Gnepp, D.R.; El-Naggar, A.K. Tumours of the salivary glands: Introduction. In World Health Organization Classification of Tumours. Pathology & Genetics. Head and Neck Tumours; Barnes, L., Eveson, J.W., Reichart, P., Sindranski, D., Eds.; IARC Press: Lyon, France, 2005; Volume 5, pp. 212–215. [Google Scholar]
- Pinkston, J.A.; Cole, P. Incidence rates of salivary gland tumors: Results from a population-based study. Otolaryngol. Head Neck Surg. 1999, 120, 834–840. [Google Scholar] [CrossRef]
- Alves, F.A.; Pires, F.R.; de Almeida, O.P.; Lopes, M.A.; Kovalski, L.P. PCNA, Ki67 and p53 expressions in submandibular salivary gland tumors. Int. J. Oral Maxillofac. Surg. 2004, 33, 593–597. [Google Scholar] [CrossRef]
- Papadogeorgakis, N.; Goutzanis, L.; Petsinis, V.; Alexandridis, C. Management of malignant parotid tumors. Oral Maxillofac. Surg. 2012, 16, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Gontarz, M.; Urbańska-Gąsiorowska, M.; Bargiel, J.; Gąsiorowski, K.; Marecik, T.; Szczurowski, P.; Zapała, J.; Wyszyńska-Pawelec, G. Sublingual gland neoplasms: Clinicopathological study of 8 cases. Med. Oral Patol. Oral Cir. Bucal. 2021, 26, e626–e631. [Google Scholar] [CrossRef] [PubMed]
- Di Villeneuve, L.; Souza, I.L.; Tolentino, F.D.S.; Ferrarotto, R.; Schvartsman, G. Salivary Gland Carcinoma: Novel Targets to Overcome Treatment Resistance in Advanced Disease. Front. Oncol. 2020, 10, 580141, Erratum in Front. Oncol. 2021, 11, 669486. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nishida, H.; Kusaba, T.; Kawamura, K.; Oyama, Y.; Daa, T. Histopathological Aspects of the Prognostic Factors for Salivary Gland Cancers. Cancers 2023, 15, 1236. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Skalova, A.; Hyrcza, M.; Leivo, I. Update from the 5th edition of the World Health Organization Classification of the head and neck Tumors: Salivary glands. Head Neck Pathol. 2022, 16, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Seethala, R.R.; Stenman, G. Update from the 4th Edition of the World Health Organization Clasiffication of Head and Neck Tumours: Tumors of te Salivary Gland. Head Neck Pathol. 2017, 11, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Seethala, R.R.; Altemani, A.; Ferris, R.L.; Fonseca, I.; Gnepp, D.R.; Ha, P.; Nagao, T.; Skalova, A.; Stenman, G.; Thompson, L.D.R. Data Set for the Reporting of Carcinomas of the Major Salivary Glands: Explanations and Recommendations of the Guidelines from the International Collaboration on Cancer Reporting. Arch. Pathol. Lab. Med. 2019, 143, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Żurek, M.; Fus, Ł.; Niemczyk, K.; Rzepakowska, A. Salivary gland pathologies: Evolution in classification and association with unique genetic alterations. Eur. Arch. Otorhinolaryngol. 2023, 280, 4739–4750. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Andreasen, S.; Kiss, K.; Mikkelsen, L.H.; Channir, H.I.; Plaschke, C.C.; Melchior, L.C.; Eriksen, J.G.; Wessel, I. An update on head and neck cancer: New entities and their histopathology, molecular background, treatment, and outcome. Apmis 2019, 127, 240–264. [Google Scholar] [CrossRef] [PubMed]
- Seethala, R.R. An update on grading of salivary gland carcinomas. Head Neck Pathol. 2009, 3, 69–77. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thompson, L.D.; Aslam, M.N.; Stall, J.N.; Udager, A.M.; Chiosea, S.; McHugh, J.B. Clinicopathologic and Immunophenotypic Characterization of 25 Cases of Acinic Cell Carcinoma with High-Grade Transformation. Head Neck Pathol. 2016, 10, 152–160. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, H.; Roh, J.L.; Choi, Y.J.; Choi, J.; Cho, K.J. High Grade Transformation in Mucoepidermoid Carcinoma of the Minor Salivary Gland with Polyploidy of the Rearranged MAML2 Gene. Head Neck Pathol. 2020, 14, 822–827. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baker, A.R.; Ohanessian, S.E.; Adil, E.; Crist, H.S.; Goldenberg, D.; Mani, H. Dedifferentiated epithelial-myoepithelial carcinoma: Analysis of a rare entity based on a case report and literature review. Int. J. Surg. Pathol. 2013, 21, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, I.; Nishida, T.; Miyauchi, M.; Sato, S.; Takata, T. Dedifferentiated malignant myoepithelioma of the parotid gland. Pathol. Int. 2003, 53, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Asai, S.; Sumiyoshi, S.; Yamada, Y.; Tateya, I.; Nagao, T.; Minamiguchi, S.; Haga, H. High-grade salivary gland carcinoma with the ETV6-NTRK3 gene fusion: A case report and literature review of secretory carcinoma with high-grade transformation. Pathol. Int. 2021, 71, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.J.; Weinreb, I.; Perez-Ordonez, B. Low-grade salivary duct carcinoma or low-grade intraductal carcinoma? Review of the literature. Head Neck Pathol. 2013, 7 (Suppl. S1), S59–S67. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brandwein-Gensler, M.S.; Gnepp, D.R. Low Grede Cribriform Cystadenocarcinoma. In World Health Organisation Classification of Tumors: Pathology and Genetics of Head and Neck Tumors; Barnes, L., Eveson, J.W., Reichart, P., Sindransky, D., Eds.; IARC: Lyon, France, 2005; p. 233. [Google Scholar]
- Nagao, T.; Sato, E.; Inuoue, R.; Hisashi, O.; Takahashi, R.H.; Nagai, T.; Yoshida, M.; Suzuki, F.; Obikane, H.; Yamashina, M.; et al. Immunohistochemical analysis of salivary gland tumors: Application for surgical pathology practice. Acta Histochem. Cytochem. 2012, 45, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Bussari, S.; Ganvir, S.M.; Sarode, M.; Jeergal, P.A.; Deshmukh, A.; Srivastava, H. Immunohistochemical Detection of Proliferative Marker Ki-67 in Benign and Malignant Salivary Gland Tumors. J. Contemp. Dent. Pract. 2018, 19, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Cheuck, W.; Chan, J.K.C. Advances in salivary gland pathology. Histopathol. 2007, 51, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Meer, S.; Altini, M. CK7+/CK20-immunoexpesion profile is typical of salivary gland neoplasia. Histopathology 2007, 51, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Nikitakis, N.; Tosios, K.; Papanikolaou, V.; Rivera, H.; Papanicolaou, S.I.; Ioffe, O.B. Immunohistochemical expression of cytokeratins 7 and 20 in malignant salivary gland tumors. Mod. Pathol. 2004, 17, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Singh, J.; Devgan, R.; Utreja, U. An Immunohistochemical Expression of CK5/6, CK7, and CK20 on Cell Blocks in Metastatic Cervical Lymphadenopathy. Int. J. Appl. Basic Med. Res. 2022, 12, 171–176. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schwartz, L.E.; Begum, S.; Westra, W.H.; Bishop, J. GATA3 immunohistochemical expression in salivary gland neoplasms. Head Neck Pathol. 2013, 7, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.; Langhanki, L.; Schutz, A.; Gerstner, A.; Bootz, F.; Wittekind, C.; Tannapfel, A. Expression profiles of p53, p63 and p73 in benign salivary gland tumors. Virchows Arch. 2002, 441, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Curran, A.E.; Allen, C.M.; Beck, F.M.; Damm, D.D.; Murrah, V.A. Distinctive pattern of glial fibrillary acidic protein immunoreactive useful in distinguishing fregmented pleomorphic adenoma, canalicular adenoma and polymorphous lowgrade adenocarcinoma of minor salivary glands. Head Neck Pathol. 2007, 1, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Santos, G.C.; Carvalho, K.C.; Falzoni, R.; Simoes, A.C.; Rocha, R.M.; Lopes, A.; Vassallo, J.; Reis, L.F.; Soares, F.A.; da Cunha, I.W. Glial fibrillary acidic protein in tumor types with cartilaginous differentiation. Mod. Pathol. 2009, 22, 1321–1327. [Google Scholar] [CrossRef] [PubMed]
- López-Janeiro, Á.; Blasco-Santana, L.; Pérez-Pérez, M.; Ruiz-Bravo, E. Diagnostic role of DOG-1, GFAP and B-catenin in Basal cell Adenoma and Cellular Pleomorphic Adenoma of the Salivary Gland. Head Neck Pathol. 2023, 17, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Ghazy, S.E.; Helmy, I.M.; Baghdadi, H.M. Maspin and MCM2 immunoprofiling in salivary gland carcinomas. Diagn. Path 2011, 6, 89. [Google Scholar] [CrossRef] [PubMed]
- Piao, S.; Zhao, S.; Gou, F.; Xue, J.; Yao, G.; Wei, Z.; Huang, Q.; Sun, Y.; Zhang, B. Increased expression of CD147 and MMP-9 is correlated with poor prognosis of salivary duct carcinoma. J. Cancer Res. Clin. Oncol. 2012, 138, 627–635. [Google Scholar] [CrossRef]
- Costa, A.F.; Altemani, A.; Hermsen, M. Current concepts on dedifferentiation/high-grade transformation in salivary gland tumors. Pathol. Res. Int. 2011, 2011, 325965. [Google Scholar] [CrossRef]
- Ohtomo, R.; Mori, T.; Shibata, S.; Tsuta, K.; Maeshima, A.M.; Akazawa, C.; Watanabe, Y.; Honda, K.; Yamada, T.; Yoshimoto, S.; et al. SOX10 is a novel marker of acinus and intercalated duct differentiation in salivary gland tumors: A clue to the histogenesis for tumor diagnosis. Mod. Pathol. 2013, 26, 1041–1050. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.S.; Lee, Y.H.; Chang, Y.L. SOX10-positive salivary gland tumors: A growing list, including mammary analogue secretory carcinoma of the salivary gland, sialoblastoma, low-grade salivary duct carcinoma, basal cell adenoma/adenocarcinoma, and a subgroup of mucoepidermoid carcinoma. Hum. Pathol. 2016, 56, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Jabbarzadeh, M.; Hamblin, M.R.; Pournaghi-Azar, F.; Vakili Saatloo, M.; Kouhsoltani, M.; Vahed, N. Ki-67 expression as a diagnostic biomarker in odontogenic cysts and tumors: A systematic review and meta-analysis. J. Dent. Res. Dent. Clin. Dent. Prospect. 2021, 15, 66–75. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Namboodiripad, P.C. A review: Immunological markers for malignant salivary gland tumors. J. Oral Biol. Craniofac Res. 2014, 4, 127–134. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Scherl, C.; Kato, M.G.; Erkul, E.; Graboyes, E.M.; Nguyen, S.A.; Chi, A.C.; Morgan, P.F.; Day, T.A. Outcomes and prognostic factors for parotid acinic cell Carcinoma: A National Cancer Database study of 2362 cases. Oral Oncol. 2018, 82, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Gomez, D.R.; Katabi, N.; Zhung, J.; Wolden, S.L.; Zelefsky, M.J.; Kraus, D.H.; Shah, J.P.; Wong, R.J.; Ghossein, R.A.; Lee, N.Y. Clinical and pathologic prognostic features in acinic cell carcinoma of the parotid gland. Cancer 2009, 115, 2128–2137. [Google Scholar] [CrossRef] [PubMed]
- Sams, R.N.; Gnepp, D.R. P63 expression can be used in differential diagnosis of salivary gland acinic cell and mucoepidermoid carcinomas. Head Neck Pathol. 2013, 7, 64–68. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zarbo, R.J. Salivary gland neoplasia: A review for the practicing pathologist. Mod. Pathol. 2002, 15, 298–323. [Google Scholar] [CrossRef] [PubMed]
- Bishop, J.A.; Sajed, D.P.; Weinreb, I.; Dickson, B.C.; Bilodeau, E.A.; Agaimy, A.; Franchi, A.; Khurram, S.A.; Da Forno, P.; Robledo, J.; et al. Microsecretory Adenocarcinoma of Salivary Glands: An Expanded Series of 24 Cases. Head Neck Pathol. 2021, 15, 1192–1201. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thway, K.; Fisher, C. Tumors with EWSR1-CREB1 and EWSR1-ATF1 fusions: The current status. Am. J. Surg. Pathol. 2012, 36, e1–e11. [Google Scholar] [CrossRef] [PubMed]
- Kazakov, D.V. Brooke-Spiegler Syndrome and Phenotypic Variants: An Update. Head Neck Pathol. 2016, 10, 125–130. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pusztaszeri, M.; Deschler, D.; Faquin, W.C. The 2021 ASCO guideline on the management of salivary gland malignancy endorses FNA biopsy and the risk stratification scheme proposed by the Milan System for Reporting Salivary Gland Cytopathology. Cancer Cytopathol. 2023, 131, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, H. The Milan System for reporting Salivary Gland Cytopathology and Updates. J. Clin. Transl. Pathol. 2023, 3930, 126–133. [Google Scholar] [CrossRef]
- Sirviö, M.; Aro, K.; Naukkarinen, M.; Mäkitie, A.; Tarkkanen, J.; Kelppe, J.; Atula, T. Clinical decision making when cytology indicates a Warthin tumor. Sci. Rep. 2024, 14, 8832. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cheng, P.C.; Lo, W.C.; Chang, C.M.; Wen, M.H.; Cheng, P.W.; Liao, L.J. Comparisons among the Ultrasonography Prediction Model, Real-Time and Shear Wave Elastography in the Evaluation of Major Salivary Gland Tumors. Diagnostics 2022, 12, 2488. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, Y.; Shu, Z.; Song, G.; Liu, Y.; Pang, P.; Wen, X.; Gong, X. The Role of Preoperative Computed Tomography Radiomics in Distinguishing Benign and Malignant Tumors of the Parotid Gland. Front. Oncol. 2021, 11, 634452. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xia, X.; Feng, B.; Wang, J.; Hua, Q.; Yang, Y.; Sheng, L.; Mou, Y.; Hu, W. Deep Learning for Differentiating Benign from Malignant Parotid Lesions on MR Images. Front. Oncol. 2021, 11, 632104. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Takumi, K.; Nagano, H.; Kikuno, H.; Kumagae, Y.; Fukukura, Y.; Yoshiura, T. Differentiating malignant from benign salivary gland lesions: A multiparametric non-contrast MR imaging approach. Sci. Rep. 2021, 11, 2780. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Minamimoto, R. Proliferation PET/CT Imaging of Salivary Gland Tumor. Diagnostics 2021, 11, 2065. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gu, B.; Xu, X.; Zhang, J.; Ou, X.; Xia, Z.; Guan, Q.; Hu, S.; Yang, Z.; Song, S. The Added Value of 68Ga-FAPI PET/CT in Patients with Head and Neck Cancer of Unknown Primary with 18F-FDG-Negative Findings. J. Nucl. Med. 2022, 63, 875–881. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, R.; Pu, Y.; Huang, S.; Yang, C.; Yang, F.; Pu, Y.; Li, J.; Chen, L.; Huang, Y. FAPI-PET/CT in Cancer Imaging: A Potential Novel Molecule of the Century. Front. Oncol. 2022, 12, 854658. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thoeny, H.C. Imaging of salivary gland tumours. Cancer Imaging 2007, 7, 52–62. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Committeri, U.; Barone, S.; Salzano, G.; Arena, A.; Borriello, G.; Giovacchini, F.; Fusco, R.; Vaira, L.A.; Scarpa, A.; Abbate, V.; et al. Support Tools in the Differential Diagnosis of Salivary Gland Tumors through Inflammatory Biomarkers and Radiomics Metrics: A Preliminary Study. Cancers 2023, 15, 1876. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ibrahim, A.; Gamble, P.; Jaroensri, R.; Abdelsamea, M.M.; Mermel, C.H.; Chen, P.C.; Rakha, E.A. Artificial intelligence in digital breast pathology: Techniques and applications. Breast 2020, 49, 267–273. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- van Rooij, W.; Dahele, M.; Nijhuis, H.; Slotman, B.J.; Verbakel, W.F. Strategies to improve deep learning-based salivary gland segmentation. Radiat. Oncol. 2020, 15, 272. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baxi, V.; Edwards, R.; Montalto, M.; Saha, S. Digital pathology and artificial intelligence in translational medicine and clinical practice. Mod. Pathol. 2022, 35, 23–32. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Felice, F.; Valentini, V.; De Vincentiis, M.; Di Gioia, C.R.T.; Musio, D.; Tummolo, A.A.; Ricci, L.I.; Converti, V.; Mezi, S.; Messineo, D.; et al. Prediction of Recurrence by Machine Learning in Salivary Gland Cancer Patients after Adjuvant (Chemo)Radiotherapy. In Vivo 2021, 35, 3355–3360. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Matsuo, H.; Nishio, M.; Kanda, T.; Kojita, Y.; Kono, A.K.; Hori, M.; Teshima, M.; Otsuki, N.; Nibu, K.; Murakami, T. Diagnostic accuracy of deep-learning with anomaly detection for a small amount of imbalanced data: Discriminating malignant parotid tumors in MRI. Sci. Rep. 2020, 10, 19388. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Kang, K.; Song, Y.; Kim, T.J. Application of Artificial Intelligence in Pathology: Trends and Challenges. Diagnostics 2022, 12, 2794. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pertzborn, D.; Arolt, C.; Ernst, G.; Lechtenfeld, O.J.; Kaesler, J.; Pelzel, D.; Guntinas-Lichius, O.; von Eggeling, F.; Hoffmann, F. Multi-Class Cancer Subtyping in Salivary Gland Carcinomas with MALDI Imaging and Deep Learning. Cancers 2022, 14, 4342. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chang, Y.J.; Huang, T.Y.; Liu, Y.J.; Chung, H.W.; Juan, C.J. Classification of parotid gland tumors by using multimodal MRI and deep learning. NMR Biomed. 2021, 34, e4408. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, T.Y.; Lee, Y. Contrast-enhanced Multi-detector CT Examination of Parotid Gland Tumors: Determination of the Most Helpful Scanning Delay for Predicting Histologic Subtypes. J. Belg. Soc. Radiol. 2019, 103, 2. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aeffner, F.; Zarella, M.D.; Buchbinder, N.; Bui, M.M.; Goodman, M.R.; Hartman, D.J.; Lujan, G.M.; Molani, M.A.; Parwani, A.V.; Lillard, K.; et al. Introduction to Digital Image Analysis in Whole-slide Imaging: A White Paper from the Digital Pathology Association. J. Pathol. Inform. 2019, 10, 9, Erratum in J. Pathol. Inform. 2019, 10, 15. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mroz, P.; Parwani, A.V.; Kulesza, P. Central pathology review for phase III clinical trials: The enabling effect of virtual microscopy. Arch. Pathol. Lab. Med. 2013, 137, 492–495. [Google Scholar] [CrossRef] [PubMed]
- Pell, R.; Oien, K.; Robinson, M.; Pitman, H.; Rajpoot, N.; Rittscher, J.; Snead, D.; Verrill, C.; UK National Cancer Research Institute (NCRI) Cellular-Molecular Pathology (CM-Path) Quality Assurance Working Group. The use of digital pathology and image analysis in clinical trials. J. Pathol. Clin. Res. 2019, 5, 81–90. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nam, S.; Chong, Y.; Jung, C.K.; Kwak, T.Y.; Lee, J.Y.; Park, J.; Rho, M.J.; Go, H. Introduction to digital pathology and computer-aided pathology. J. Pathol. Transl. Med. 2020, 54, 125–134. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, C.H.; Hsu, M.Y.; Jiang, R.S.; Wu, S.H.; Chen, F.J.; Liu, S.A. Shrinkage of head and neck cancer specimens after formalin fixation. J. Chin. Med. Assoc. 2012, 75, 109–113. [Google Scholar] [CrossRef] [PubMed]
- El Hallani, S.; Udager, A.M.; Bell, D.; Fonseca, I.; Thompson, L.D.R.; Assaad, A.; Agaimy, A.; Luvison, A.M.; Miller, C.; Seethala, R.R.; et al. Epithelial-Myoepithelial Carcinoma: Frequent Morphologic and Molecular Evidence of Preexisting Pleomorphic Adenoma, Common HRAS Mutations in PLAG1-intact and HMGA2-intact Cases, and Occasional TP53, FBXW7, and SMARCB1 Alterations in High-grade Cases. Am. J. Surg. Pathol. 2018, 42, 18–27. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cabezas-Camarero, S.; Pérez-Segura, P. Liquid Biopsy in Head and Neck Cancer: Current Evidence and Future Perspective on Squamous Cell, Salivary Gland, Paranasal Sinus and Nasopharyngeal Cancers. Cancers 2022, 14, 2858. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lone, S.N.; Nisar, S.; Masoodi, T.; Singh, M.; Rizwan, A.; Hashem, S.; El-Rifai, W.; Bedognetti, D.; Batra, S.K.; Haris, M.; et al. Liquid biopsy: A step closer to transform diagnosis, prognosis and future of cancer treatments. Mol. Cancer 2022, 21, 79. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bocchetti, M.; Grisolia, P.; Melisi, F.; Ferraro, M.G.; De Luca, P.; Camaioni, A.; Falco, M.; Abate, M.; Misso, G.; Alfano, R.; et al. MicroRNAs’ Crucial Role in Salivary Gland Cancers’ Onset and Prognosis. Cancers 2022, 14, 5304. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Deng, Y.; Cao, Y.; Wang, L.; Ye, D. The Role and Application of Salivary Exosomes in Malignant Neoplasms. Cancer Manag. Res. 2021, 13, 5813–5820. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sama, S.; Komiya, T.; Guddati, A.K. Advances in the Treatment of Mucoepidermoid Carcinoma. World J. Oncol. 2022, 13, 1–7. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mueller, S.K.; Haderlein, M.; Lettmaier, S.; Agaimy, A.; Haller, F.; Hecht, M.; Fietkau, R.; Iro, H.; Mantsopoulos, K. Targeted Therapy, Chemotherapy, Immunotherapy and Novel Treatment Options for Different Subtypes of Salivary Gland Cancer. J. Clin. Med. 2022, 11, 720. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cohen, R.B.; Delord, J.P.; Doi, T.; Piha-Paul, S.A.; Liu, S.V.; Gilbert, J.; Algazi, A.P.; Damian, S.; Hong, R.L.; Le Tourneau, C.; et al. Pembrolizumab for the Treatment of Advanced Salivary Gland Carcinoma: Findings of the Phase 1b KEYNOTE-028 Study. Am. J. Clin. Oncol. 2018, 41, 1083–1088. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cha, C.H.; Luo, S.D.; Chiang, P.L.; Chen, W.C.; Tung, Y.C.; Su, Y.Y.; Lin, W.C. Long-Term Outcomes of Radiofrequency Ablation for Treatment of Cystic Warthin Tumors versus Solid Warthin Tumors. Int. J. Environ. Res. Public Health 2021, 18, 6640. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Quer, M.; Hernandez-Prera, J.C.; Silver, C.E.; Casasayas, M.; Simo, R.; Vander Poorten, V.; Guntinas-Lichius, O.; Bradley, P.J.; Tong-Ng, W.; Rodrigo, J.P.; et al. Current Trends and Controversies in the Management of Warthin Tumor of the Parotid Gland. Diagnostics 2021, 11, 1467. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hu, Z.; Wang, B.; Pan, X.; Cao, D.; Gao, A.; Yang, X.; Chen, Y.; Lin, Z. Using deep learning to distinguish malignant from benign parotid tumors on plain computed tomography images. Front. Oncol. 2022, 12, 919088. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, G.; Zhu, L.; Huang, R.; Xu, Y.; Lu, X.; Chen, Y.; Li, C.; Lei, Y.; Luo, X.; Li, Z.; et al. A deep learning model for the differential diagnosis of benign and malignant salivary gland tumors based on ultrasound imaging and clinical data. Quant. Imaging Med. Surg. 2023, 13, 2989–3000. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Y.; Xie, W.; Huang, S.; Feng, M.; Ke, X.; Zhong, Z.; Tang, L. The Diagnostic Value of Ultrasound-Based Deep Learning in Differentiating Parotid Gland Tumors. J. Oncol. 2022, 2022, 8192999. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gurcan, M.N.; Boucheron, L.E.; Can, A.; Madabhushi, A.; Rajpoot, N.M.; Yener, B. Histopathological image analysis: A review. IEEE Rev. Biomed. Eng. 2009, 2, 147–171. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]



| Type of Tumor | Morphological Aspects | Immunohistochemistry |
|---|---|---|
| PADK | targetoid pattern and streaming of the tumor cells | −p40, +p63, Ki-67 index < 10 +SOX10 |
| PA | round, oval, epithelioid, plasmacytoid and spindle tumor cells and myxoid, chondroid, mucoid and chondroid stroma | +GFAP, +SOX10, +PLAG1, +/−p40, |
| ACC | biphasic tumor with ductal and myoepithelial cells; the tumor cells have angulated nuclei, quantitatively reduced cytoplasm and are arranged in tubular, cribriform or/and solid structures | +p63, p40, +CD117, +MCM-1, +SOX10 and Ki-67 index > 10 |
| MEC | mucous and squamous cells forming solid nests and cystic spaces | Does not have a typical immunohistochemical profile; +CK7, +CK8, +CK18, +CK19, −SOX10, −CD10 |
| CCC | clear cells | +p63 (diffuse), −CD10 |
| OC | radiologically intraosseous clear cell tumor | −CK7, −CK8, + CK19, −CD10, Ki-67 index > 5 |
| IC | limited to the salivary gland duct; bordered by myoepithelial cells | the myoepithelial cells +p63, +calponin, +CK14 and +SMA |
| MC | invasive tumor cells with clear cells, epithelioid, plasmacytoid cytoplasm and splindle cells forming nests, glands or cords | +CK7, +CK14, +vimentin, +S100, +SOX10 +GFAP-15 |
| Ck | CK7 | SMA | Calponin | S100 | GFAP | CEA | EMA | p63 | Vimentin | CD117 | SOX-10 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PA | + | US | + | + | + | + | US | US | + | −/+ | + | + |
| BA | + | + | +/− | US | + | − | + | + | + | + | −/+ | + |
| WT | US | US | − | US | − | − | US | US | US | − | US | US |
| Onc | + | US | + | + | US | + | US | US | + | US | US | − |
| Myo | US | US | + | + | −/+ | −/+ | US | US | + | US | US | US |
| DP | US | + | − | US | −/+ | US | + | + | US | − | US | − |
| SP | + | + | US | US | + | US | + | + | US | + | US | US |
| IDA | US | + | US | US | + | US | US | US | US | US | US | US |
| SDA | US | + | − | US | + | US | US | US | −/+ | US | US | US |
| CA | + | US | − | − | + | − | US | US | − | − | + | US |
| Ck | CK7+/ CK20− | CK8 | CK19 | EMA | SMA | Vim | S100 | GFAP | AR | PR | ER | p63 | PSA | CD117 | Ki-67 | CEA | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MEC | + | YES | + | + | + | −/+ | −/+ | −/+ | −/+ | − | US | − | + | US | US | US | −/+ |
| AclC | + | YES (with some CK7-/CK20+) | + | US | + | − | US | −/+ (foc) | US | US | US | US | − | US | US | >5% | + |
| ACC | +(L) | YES | US | + | +(L) | +(NL) | + | + | −/+ | −/+ | −/+ | −/+ | +(în NL) | US | + | >10% 13.6–34.7%) | +(L) |
| PADK | + | US | US | US | + | +/− | + | + | −/+ | US | US | US | + | US | US | <10% | + |
| BCADK | + | US | + | + | + (foc) | −/+ (foc) | + | + (foc) | US | + | US | US | US | US | + | US | + (foc) |
| SDC | + | YES | US | US | + | − | −/+ | −/rarely + | + | + | − | − | − | −/+ | US | >10% (2.7–50% of cases) | + |
| CCC | + | − | − | + | + | − | − | −/+ | − | US | US | US | + | US | US | US | + |
| EMC | +(L) | US | US | US | + | + | + | + (myo cells) | US | +(L) | US | US | + | US | + | −/+ | − |
| MC | + | + | US | US | −/+ | +/− | + | + | + | US | US | US | −/+ | US | −/+ | >10% | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faur, A.C.; Buzaș, R.; Lăzărescu, A.E.; Ghenciu, L.A. Current Developments in Diagnosis of Salivary Gland Tumors: From Structure to Artificial Intelligence. Life 2024, 14, 727. https://doi.org/10.3390/life14060727
Faur AC, Buzaș R, Lăzărescu AE, Ghenciu LA. Current Developments in Diagnosis of Salivary Gland Tumors: From Structure to Artificial Intelligence. Life. 2024; 14(6):727. https://doi.org/10.3390/life14060727
Chicago/Turabian StyleFaur, Alexandra Corina, Roxana Buzaș, Adrian Emil Lăzărescu, and Laura Andreea Ghenciu. 2024. "Current Developments in Diagnosis of Salivary Gland Tumors: From Structure to Artificial Intelligence" Life 14, no. 6: 727. https://doi.org/10.3390/life14060727
APA StyleFaur, A. C., Buzaș, R., Lăzărescu, A. E., & Ghenciu, L. A. (2024). Current Developments in Diagnosis of Salivary Gland Tumors: From Structure to Artificial Intelligence. Life, 14(6), 727. https://doi.org/10.3390/life14060727







