Delivery Room Lung Ultrasound—Feasibility, Normal Patterns, and Predictive Value for Respiratory Support in Term and Near-Term Neonates: A Monocentric Study
Abstract
:1. Introduction
- To characterize the lung ultrasound patterns noted immediately after delivery in term and near-term neonates.
- To verify whether there is a correlation between the lung ultrasound patterns determined in the delivery room and the patterns observed at one hour of life.
- To check if the lung ultrasound patterns observed immediately after delivery could anticipate the occurrence of respiratory distress, and the need for respiratory support in a sample of term and premature (≥33 weeks gestational age) neonates.
2. Materials and Methods
2.1. Lung Ultrasound
- -
- The first examination was performed in the delivery room, after the delivery and stabilization of the neonate on the radiant warmer at 1–5 min after delivery.
- -
- The second examination was performed with the same device at 1 h of life.
- -
- Right lung anterior;
- -
- Right lung lateral;
- -
- Left lung anterior;
- -
- Left lung lateral.

2.2. Other Variables
- -
- Gestational age and birth weight.
- -
- The delivery room blood gas values.
- -
- The need for positive-pressure ventilation in the delivery room.
- -
- The presence of respiratory distress.
- -
- The need for mechanical ventilation or CPAP.
- -
- Presence of maternal diabetes or pregnancy-induced hypertension;
- -
- Smoking in the mother;
- -
- Mode of delivery—cesarean section versus spontaneous vaginal delivery;
- -
- Risk of materno-fetal infection (positive vaginal cultures of the mother or urinary tract infection in the mother during pregnancy);
- -
- Color of the amniotic fluid at delivery;
- -
- Use of antibiotics in the neonate;
- -
- Need of IV line in the neonate (glucose or parenteral nutrition);
- -
- Duration of stay in hospital of the neonate.
2.3. Statistical Analysis
3. Results
3.1. Characterization of Lung Ultrasound Scores and Patterns in the Delivery Room and at 1 h of Age
3.1.1. LUS Score
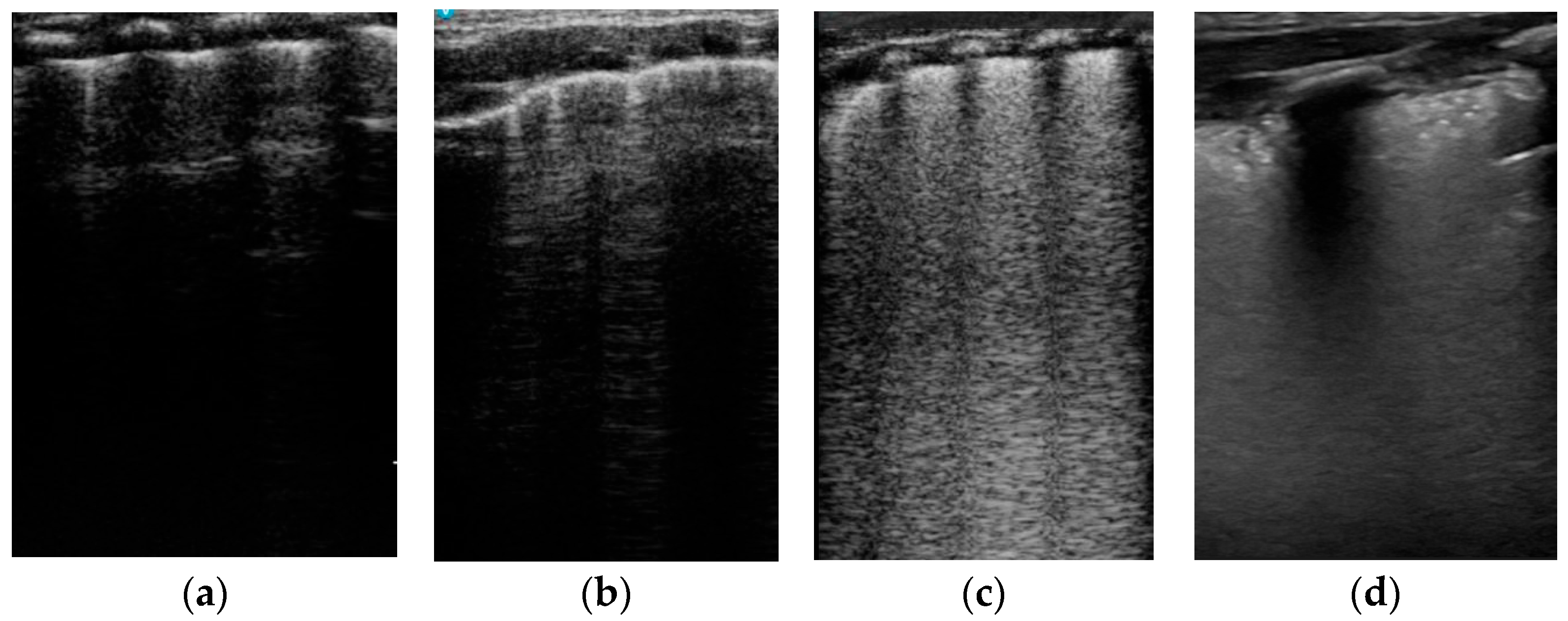
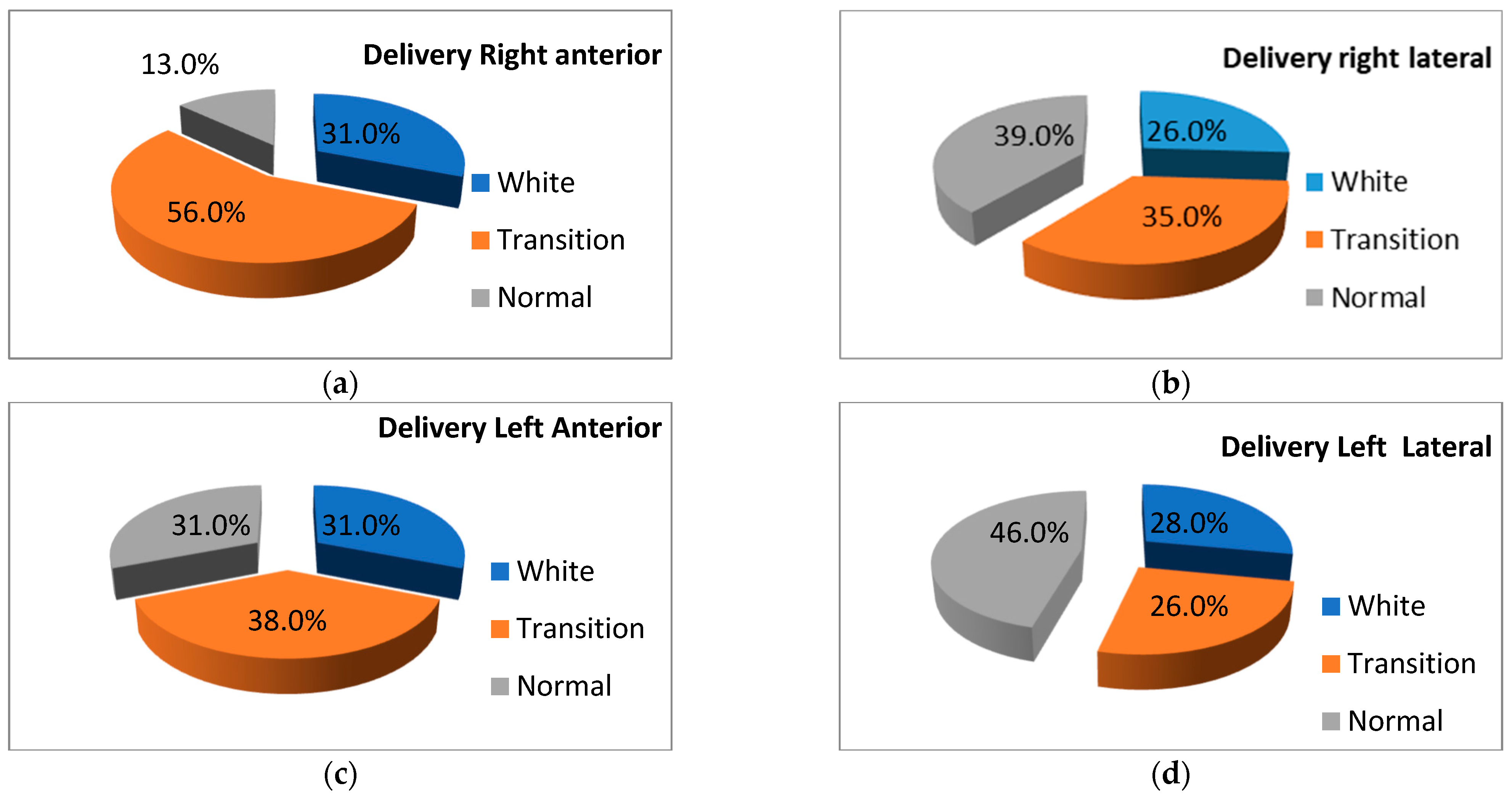
3.1.2. LUS Patterns
3.2. Association of the LUS Scores and Patterns with Different Conditions
3.3. Correlation between LUS Scores and LUS Patterns and Respiratory Conditions: Prediction of Respiratory Support
4. Discussion
- -
- To investigate whether the LUS pattern differences noted at delivery are not due to different moments of examination by concomitant examination with two devices of different lungs and lung regions in the same patient.
- -
- To take into account in a future delivery room LUS study other variables that could influence the clearance of lung fluid in different regions—position of the baby, presence of secretions in the airways, and aspiration of the secretions.
- -
- To assess whether there are statistically significant differences between neonates born vaginally and by cesarean section by recruiting a larger cohort.
- -
- To assess whether the LUS scores and LUS patterns at delivery are also good predictors for mechanical ventilation and indications for surfactant replacement therapy.
- -
- To investigate whether the use of LUS as an adjuvant for decision making in these situations could result in a better prognosis for infants with respiratory distress.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sweet, D.G.; Carnielli, V.; Greisen, G.; Hallman, M.; Ozek, E.; Te Pas, A.; Plavka, R.; Roehr, C.C.; Saugstad, O.D.; Simeoni, U.; et al. European Consensus Guidelines on the Management of Respiratory Distress Syndrome—2019 Update. Neonatology 2019, 115, 432–450. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://nightingale.vtoxford.org/reports.aspx (accessed on 28 February 2024).
- Van Kaam, A.; Niemarkt, H.J.; Onland, W. Timing of surfactant treatment in respiratory distress syndrome. Semin. Fetal Neonatal Med. 2023, 28, 101495. [Google Scholar] [CrossRef] [PubMed]
- Sweet, D.G.; Carnielli, V.P.; Greisen, G.; Hallman, M.; Klebermass-Schrehof, K.; Ozek, E.; Te Pas, A.; Plavka, R.; Roehr, C.; Saugstad, O.D.; et al. European Consensus Guidelines on the Management of Respiratory Distress Syndrome: 2022 Update. Neonatology 2023, 120, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, D.A.; Mauriat, P. Lung Ultrasound in the Critically Ill Neonate. Curr. Pediatr. Rev. 2012, 8, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, F.; Yousef, N.; Migliaro, F.; Capasso, L.; De Luca, D. Point of care lung ultrasound in neonatology: Classification into descriptive and functional applications. Pediatr. Res. 2021, 90, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Cattarossi, L. Lung Ultrasound: Its role in neonatology and paediatrics. Early Hum. Dev. 2013, 89 (Suppl. S1), S17–S19. [Google Scholar] [CrossRef] [PubMed]
- Volpicelli, G.; Elbarbary, M.; Blaivas, M.; Lichtenstein, D.A.; Mathis, G.; Kirkpatrick, A.W.; Melniker, L.; Gargani, L.; Nobile, V.E.; Dean, A.; et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012, 38, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Capetti, R.; Cattarossi, L. The ‘Double Lung Point’: An Ultrasound Sign Diagnostic of Transient Tachypnea of the Newborn. Neonatology 2007, 91, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Capetti, R.; Cattarossi, L.; Macagno, F.; Violino, M.; Furlan, R. Lung Ultrasound in Respiratory Distress Syndrome: A Useful Tool for Early Diagnosis. Neonatology 2008, 94, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, F.; Liu, Y.; Wang, H.W.; Feng, Z.C. Lung Ultrasonography for the Diagnosis of Severe Neonatal Pneumonia. Chest 2014, 146, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Piastra, M.; Yousef, N.; Brat, R.; Manzoni, P.; Mokhtari, M.; De Luca, D. Lung ultrasound findings in meconium aspiration syndrome. Early Hum. Dev. 2014, 90 (Suppl. S2), S41–S43. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, D.; Menu, Y. A Bedside Ultrasound Sign Ruling Out Pnemothorax in the Critically Ill. Chest 1995, 108, 1345–1348. [Google Scholar] [CrossRef]
- Lichtenstein, D.A.; Lascols, N.; Prin, S.; Meziere, G. The “lung pulse”: An early ultrasound sing of complete atelectasis. Intensive Care Med. 2003, 29, 2187–2192. [Google Scholar] [CrossRef] [PubMed]
- Kurepa, D.; Zaghoul, N.; Watkins, L.; Liu, J. Neonatal lung ultrasound exam guidelines. J. Perinatol. 2018, 38, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, F.; Migliaro, F.; Sodano, A.; Umbaldo, A.; Romano, A.; Vallone, G.; Capasso, L. Can neonatal lung ultrasound monitor fluid clearance and predict the need of respiratory support? Crit. Care 2012, 16, R220. [Google Scholar] [CrossRef] [PubMed]
- Brat, R.; Yousef, N.; Klifa, R.; Reynaud, S.; Shankar Aguilera, S.; De Luca, D. Lung Ultrasonography Score to Evaluate Oxygenation and Surfactant Need in Neonates Treated with Continuous Positive Airway Pressure. JAMA Pediatr. 2015, 169, e151797. [Google Scholar] [CrossRef] [PubMed]
- Toma, A.I.; Cuzino, I.; Cozinov, A.; Olteanu, R. Pulmonary ultrasound—Applications in neonatology. Arch Dis. Child 2014, 99 (Suppl. S2), A174. [Google Scholar] [CrossRef]
- Raimondi, F.; Migliaro, F.; Corsini, I.; Meneghin, F.; Pierri, L.; Salomè, S.; Perri, A.; Aversa, S.; Nobile, S.; Lama, S.; et al. Neonatal Lung Ultrasound and Surfactant Administration. A Pragmatic Multicenter Study. Chest 2021, 160, 2178–2186. [Google Scholar] [CrossRef] [PubMed]
- Te Pas, A.B.; Davis, P.G.; Hooper, S.B.; Morley, C.J. From liquid to air: Breathing after Birth. J. Pediatr. 2008, 152, 607–611. [Google Scholar] [CrossRef]
- Greenough, A.; Milner, A.D.; Hannarn, S.; Fox, G.F.; TUrowski, C.; Davenport, M.M. Pulmonary disease of the newborn. In Rennie & Roberton s Textbook of Neonatology, 5th ed.; Rennie, J.M., Ed.; Churchil Livingston: London, UK; Elsevier: Amsterdam, The Netherlands, 2005; pp. 448–615. [Google Scholar]
- Blank DARogerson, S.R.; Kamlin, C.O.F.; Fox, L.M.; Lorenz, L.; Kane, S.C.; Polglase, G.M.; Hopper, S.B.; Davis, P.G. Lung ultrasound during the initiation of breathing in healthy term and late preterm infants immediately after birth, a prospective, observational study. Resuscitation 2017, 114, 59–65. [Google Scholar] [CrossRef]
- Blank, D.A.; Kamlin, C.O.F.; Rogerson, S.R.; Fox, L.M.; Lorenz, L.; Kane, S.C.; Polglase, G.M.; Hopper, S.B.; Davis, P.G. Lung ultrasound immediately after birth to describe normal neonatal transition: An observational study. Arch Dis. Child Fetal. Neonatal. Ed. 2017, 103, F157–F162. [Google Scholar] [CrossRef] [PubMed]
- Badurdeen, S.; Kamlin, C.O.F.; Rogerson, S.R.; Kane, S.X.; Polglase, G.R.; Hopper, S.P.; Davis, P.G.; Blank, D.A. Lung ultrasound during newborn resuscitation predicts the need for surfactant therapy in very- and extremely preterm infants. Resuscitation 2021, 162, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Shankar, H.; Pagel, P.S. Potential Adverse Effects of Ultrasound- related Biological Effects. A Critical Review. Anesthesiology 2011, 115, 1109–1124. [Google Scholar] [CrossRef] [PubMed]
- Fowlkes, J.B.; Bioeffects Committee of the American Institute of Ultrasound in Medicine. American Institute of Ultrasound in Medicine consensus report on potential bioeffects of diagnostic ultrasound: Executive summary. J. Ultrasound Med. 2008, 27, 503–515. [Google Scholar] [PubMed]
- Yamada, N.; Szyld, E.; Strand, M.; Finan, E.; Illuzzi, J.L.; Kamath-Rayne, B.; Kapadia, V.S.; Niermeyer, S.; Schmölzer, G.M.; Williams, A.; et al. 2023 American Heart Association and American Academy of Pediatrics Focused Update on Neonatal Resuscitation: An Update to the American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2024, 149, e157–e166. [Google Scholar] [CrossRef] [PubMed]
- Madar, J.; Roehr, C.C.; Ainsworth, S.; Morley, C.; Rüdiger, M.; Skåre, C.; Szczapa, T.; Te Pas, A.; Trevisanuto, D.; Urlesberger, B.; et al. European Resuscitation Council Guidelines 2021: Newborn resuscitation and support of transition of infants at birth. Resuscitation 2021, 161, 291–326. [Google Scholar] [CrossRef] [PubMed]
- Boiculese, L.V.; Dascalu, C. Informatica Medicala; Editura Venus: Bucuresti, Romania, 2001. [Google Scholar]
- Swinscow, T.D.V. Statistics at Square One, 9th ed.; BMJ Publishing Group: London, UK, 1997. [Google Scholar]
- Poerio, A.; Galletti, S.; Baldazzi, M.; Martini, S.; Rollo, A.; Spinedi, S.; Raimondi, F.; Zompatori, M.; Corvaglia, L.; Aceti, A. Lung ultrasound features predict admission to the neonatal intensive care unit in infants with transient neonatal tachypnoea or respiratory distress syndrome born by caesarean section. Eur. J. Pediatr. 2021, 180, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Hooper, S.B.; Kitchen, M.J.; Wallace, M.J.; Yagi, N.; Uesugi, K.; Morgan, M.J.; Hall, C.; Karen Siu, K.K.W.; Williams, I.M.; Siew, M.; et al. Imaging lung aeration and lung liquid clearance at birth. FASEB J. 2007, 21, 3329–3337. [Google Scholar] [CrossRef] [PubMed]
- Siew, M.L.; Te Pas, A.B.; Wallace, M.J.; Marcus JKitchen, M.J.; Lewis, R.A.; Fouras, A.; Morley, C.J.; Davis, P.G.; Yagi, N.; Uesugi, K.; et al. Positive end-expiratory pressure enhanced development of a functional residual capacity in preterm rabbits ventilated from birth. J. Appl. Physiol. 2009, 106, 1487–1493. [Google Scholar] [CrossRef]
- Raschetti, R.; Yousef, N.; Vigo, G.; Marseglia, G.; Centorrino, R.; Ben-Ammar, R.; Shankar-Aguilera, S.; De Luca, D. Echography-Guided Surfactant Therapy to Improve Timeliness of Surfactant Replacement: A Quality Improvement Project. J. Pediatr. 2019, 212, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Patnaik, S.; Verma, A.; Garegrat, R.; Maheshwari, R.; Suryawanshi, P. Diagnostic utility of lung ultrasound in predicting the need for surfactant therapy in preterm neonates with respiratory distress. Front. Pediatr. 2023, 11, 1307761. [Google Scholar] [CrossRef] [PubMed]
- Tataranno, M.; De Bernardo, G.; Trevisanuto, D.; Sordino, D.; Riccitelli, M.; Buonocore, G.; Perrone, S. Differences between umbilical blood gas in term and preterm newborns. Electron Physician 2019, 11, 7529–7535. [Google Scholar] [CrossRef] [PubMed]
- Carter, A.M. Placental Gas Exchange and the Oxygen Supply to the Fetus. Compr. Physiol. 2015, 5, 1381–1403. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.J.; Olver, R.E.; Ramsden CAStrang, L.B.; Walters, D.V. Effects of adrenaline and of spontaneouslabour on the secretion and absorption of lung liquid in the fetal lamb. J. Physiol. 1983, 344, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Carlton, D.P. Regulation of Liquid Secretion and Absorption by the Fetal and Neonatal Lung. In Fetal and Neonatal Physiology, 4th ed.; Polin, R.A., Fox, W.W., Abman, S.H., Eds.; Elsevier: Amsterdam, The Netherlands; Saunders: Philadelphia, PA, USA, 2011; pp. 907–919. [Google Scholar]
- Harding, R.; Liggins, G.C. Changes in thoracic dimensions induced by breathing movements in fetal sheep. Reprod. Fertil. Dev. 1996, 8, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.G.; Johns, D.P.; Harding, R. Postnatal development of respiratory function in lambs studied serially between birth and 8 weeks. Respir. Physiol. 1998, 113, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Stocks, J. Respiratory physiology during early life. Monaldi Arch. Chest. Dis. 1999, 54, 358–364. [Google Scholar] [PubMed]
- Hedstrom, A.B.; Faino, A.V.; Batra, M. The Silverman Andersen respiratory severity score in the delivery room predicts subsequent intubation in very preterm neonates. Acta Paediatr 2020. epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Hedstrom, A.; Faino, A.V.; Batra, M. Utility of lung ultrasound and respiratory severity score for detection of respiratory distress syndrome in the delivery room. Acta Paediatr. 2020, 110, 1683. [Google Scholar] [CrossRef] [PubMed]
- Filip, C.; Zonda, G.I.; Vasilache, I.-A.; Scripcariu, I.S.; Vicoveanu, P.; Dima, V.; Socolov, D.; Paduraru, L. Neonatal Cerebral Sinovenous Thrombosis and the Main Perinatal Risk Factors—A Retrospective Unicentric Study. Children 2022, 9, 1182. [Google Scholar] [CrossRef] [PubMed]


| Condition | Number | Percentage |
|---|---|---|
| Gestational diabetes (mother) | 11 | 11% |
| Pregnancy-induced hypertension (mother) | 4 | 4% |
| Smoking (mother) | 19 | 19% |
| Risk of materno-fetal infection | 38 | 38% |
| Meconial amniotic fluid | 7 | 7% |
| Delivery by cesarean section/vaginal | 93/7 | 93%/7% |
| IV lines in the neonate | 24 | 24% |
| Antibiotic treatment in the neonate | 19 | 19% |
| Respiratory distress | 13 | 13% |
| Administration of oxygen | 10 | 10% |
| CPAP | 9 | 9% |
| Mechanical ventilation | 3 | 3% |
| Mean | Standard Deviation | Median | Variance | Minimum/Maximum | |
|---|---|---|---|---|---|
| Delivery room | 8.05 | 1.95 | 8 | 3.81 | 5/12 |
| 1 h | 6.4 | 1.75 | 7 | 3.06 | 3/14 |
| LUSbirth | LUS 1h | pH | pCO2 | pO2 | BE | HCO3− | ||
|---|---|---|---|---|---|---|---|---|
| LUS score | Pearson Correlation | 1 | 0.473 ** | −0.082 | 0.059 | −0.104 | 0.018 | −0.010 |
| Birth | Sig. (2-tailed) | 0.000 | 0.418 | 0.557 | 0.306 | 0.862 | 0.922 | |
| N | 100 | 100 | 100 | 100 | 99 | 100 | 100 | |
| LUS score | Pearson Correlation | 0.473 ** | 1 | 0.008 | −0.111 | −0.021 | −0.130 | −0.119 |
| 1h | Sig. (2-tailed) | 0.000 | 0.940 | 0.272 | 0.838 | 0.196 | 0.239 | |
| N | 100 | 100 | 100 | 100 | 99 | 100 | 100 | |
| pH | Pearson Correlation | −0.082 | 0.008 | 1 | −0.598 ** | 0.339 ** | 0.353 ** | 0.621 ** |
| Sig. (2-tailed) | 0.418 | 0.940 | 0.000 | 0.001 | 0.000 | 0.000 | ||
| N | 100 | 100 | 100 | 100 | 99 | 100 | 100 | |
| pCO2 | Pearson Correlation | 0.059 | −0.111 | −0.598 ** | 1 | −0.388 ** | 0.397 ** | 0.020 |
| Sig. (2-tailed) | 0.557 | 0.272 | 0.000 | 0.000 | 0.000 | 0.844 | ||
| N | 100 | 100 | 100 | 100 | 99 | 100 | 100 | |
| pO2 | Pearson Correlation | −0.104 | −0.021 | 0.339 ** | −0.388 ** | 1 | −0.013 | 0.251 * |
| Sig. (2-tailed) | 0.306 | 0.838 | 0.001 | 0.000 | 0.900 | 0.012 | ||
| N | 99 | 99 | 99 | 99 | 99 | 99 | 99 | |
| BE | Pearson Correlation | 0.018 | −0.130 | 0.353 | 0.397 ** | −0.013 | 1 | 0.909 ** |
| Sig. (2-tailed) | 0.862 | 0.196 | 0.000 | 0.000 | 0.900 | 0.000 | ||
| N | 100 | 100 | 100 | 100 | 99 | 100 | 100 | |
| HCO3− | Pearson Correlation | −0.010 | −0.119 | 0.621 ** | 0.020 | 0.251 * | 0.909 ** | 1 |
| Sig. (2-tailed) | 0.922 | 0.239 | 0.000 | 0.844 | 0.012 | 0.000 | ||
| N | 100 | 100 | 100 | 100 | 99 | 100 | 100 | |
| Parameters | LUS Score Birth | LUS Score 1 h | ||
|---|---|---|---|---|
| Mean Rank | p | Mean Rank | p | |
| Maternal diabetes | 63.32 48.92 | 0.115 | 51.27 50.40 | 0.924 |
| Pregnancy-induced hypertension | 69.00 49.73 | 0.204 | 37.63 51.04 | 0.379 |
| Risk of maternofetal infection | 48.74 51.58 | 0.629 | 54.78 47.88 | 0.238 |
| Amniotic fluid—meconial | 44.93 50.92 | 0.598 | 48.79 50.63 | 0.868 |
| Male Female | 50.18 50.94 | 0.896 | 48.09 53.83 | 0.317 |
| Antibiotics yes/no | 66.58 46.73 | 0.006 | 63.26 47.51 | 0.029 |
| LUS Score | CPAP | Non-CPAP | * p | ||||
|---|---|---|---|---|---|---|---|
| Mean ± SD | Median | Limits | Mean ± SD | Median | Limits | ||
| Delivery room | 11.44 ± 1.33 | 11 | 8–12 | 7.71 ± 1.66 | 8 | 5–11 | 0.001 |
| One hour | 8.33 ± 3.00 | 8 | 3–14 | 6.29 ± 1.48 | 6 | 3–10 | 0.001 |
| * p | 0.004 | 0.001 | |||||
| Pattern | |||||
|---|---|---|---|---|---|
| Region | White | Transition | Normal | p Value | |
| Delivery room | Right anterior | 9 (100%) | - | - | 0.001 |
| Right lateral | 7 (77.8%) | 1 (11.1%) | 1 (11.1%) | 0.003 | |
| Left anterior | 8 (88.9%) | 1 (11.1%) | - | 0.001 | |
| Left lateral | 7 (77.8%) | 1 (11.1%) | 1 (11.1%) | 0.004 | |
| One hour | Right anterior | 1 (11.1%) | 8 (88.9%) | - | 0.013 |
| Right lateral | 4 (44.4%) | 5 (55.6%) | - | 0.001 | |
| Left anterior | 1 (11.1%) | 6 (66.7%) | 2 (22.2%) | 0.081 | |
| Left lateral | 4 (44.4%) | 3 (33.3%) | 2 (22.2%) | 0.005 | |
| LUS Score | Oxygen Needs | No Oxygen | * p | ||||
|---|---|---|---|---|---|---|---|
| Mean ± SD | Median | Limits | Mean ± SD | Median | Limits | ||
| Delivery room | 10.50 ± 1.43 | 10 | 8–12 | 7.78 ± 1.81 | 8 | 5–12 | 0.001 |
| One hour | 7.70 ± 2.83 | 8 | 3–14 | 6.33 ± 1.55 | 6 | 3–10 | 0.018 |
| * p | 0.002 | 0.001 | |||||
| LUS Score | Respiratory Distress | No Respiratory Distress | * p | ||||
|---|---|---|---|---|---|---|---|
| Mean ± SD | Median | Limits | Mean ± SD | Median | Limits | ||
| Delivery room | 11.00 ± 1.41 | 11 | 8–12 | 7.61 ± 1.61 | 8 | 5–12 | 0.001 |
| One hour | 7.85 ± 2.73 | 8 | 3–14 | 6.269 ± 1.47 | 6 | 3–10 | 0.002 |
| * p | 0.001 | 0.001 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toma, A.I.; Dima, V.; Fieraru, A.; Arghirescu, A.; Andrășoaie, L.N.; Chirap, R.; Coandă, A.A.; Bujdei, T.; Marinescu, A.N.; Isam, A.J. Delivery Room Lung Ultrasound—Feasibility, Normal Patterns, and Predictive Value for Respiratory Support in Term and Near-Term Neonates: A Monocentric Study. Life 2024, 14, 732. https://doi.org/10.3390/life14060732
Toma AI, Dima V, Fieraru A, Arghirescu A, Andrășoaie LN, Chirap R, Coandă AA, Bujdei T, Marinescu AN, Isam AJ. Delivery Room Lung Ultrasound—Feasibility, Normal Patterns, and Predictive Value for Respiratory Support in Term and Near-Term Neonates: A Monocentric Study. Life. 2024; 14(6):732. https://doi.org/10.3390/life14060732
Chicago/Turabian StyleToma, Adrian Ioan, Vlad Dima, Alina Fieraru, Alexandra Arghirescu, Larisa Nicoleta Andrășoaie, Răzvan Chirap, Anelise Alina Coandă, Teodora Bujdei, Andreea Nicoleta Marinescu, and Al Jashi Isam. 2024. "Delivery Room Lung Ultrasound—Feasibility, Normal Patterns, and Predictive Value for Respiratory Support in Term and Near-Term Neonates: A Monocentric Study" Life 14, no. 6: 732. https://doi.org/10.3390/life14060732






