Downstream Target Analysis for miR-365 among Oral Squamous Cell Carcinomas Reveals Differential Associations with Chemoresistance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines and Tissue Culture
- SCC4 CRL-1624 Age: 55 years old Sex: Male
- Short tandem repeat (STR) confirmation: 92% Oral squamous cell carcinoma (OSCC)
- STR analysis:
- vWA: 15,17 TPOX: 8 TH01: 9.3 Penta_E: 14
- Penta_D: 12 FGA: 21,22 D8S1179: 14 D7S820: 9,11
- D5S818: 13 D3S1358: 18 D2S1338: 16,24 D21S11: 32.2
- D19S433: 12,14 D18S51: 15 D16S539: 12 D13S317: 11,13
- CSF1PO: 11 Amelogenin: X,Y
- SCC9 CRL-1629 Age: 25 years old Sex: Male
- STR confirmation: 100% (OSCC)
- STR analysis:
- vWA: 17 TPOX: 9,11 TH01: 8,9 Penta_E: 11
- Penta_D: 9 FGA: 20,25 D8S1179: 13 D7S820: 8
- D5S818: 12 D3S1358: 15 D2S1338: 19,21 D21S11: 28
- D19S433: 12,14 D18S51: 12,14 D16S539: 10,11 D13S317: 9
- CSF1PO: 11 Amelogenin: X,Y
- SCC15 CRL-1623 Age: 55 years old Sex: Male
- STR confirmation: 95% (OSCC)
- STR analysis:
- vWA: 15,17 TPOX: 8 TH01: 9,9.3 Penta_E: 7,13
- Penta_D: 9,13 FGA: 19,24 D8S1179: 10,13 D7S820: 10,11
- D5S818: 12 D3S1358: 1 D2S1338: 16,23 D21S11: 30,31.2
- D19S433: 15 D18S51: 16 D16S539: 12,15 D13S317: 9,14
- CSF1PO: 10,13 Amelogenin: X,Y
- SCC25 CRL-1628 Age: 70 years old Sex: Male
- STR confirmation: 100% (OSCC)
- STR analysis:
- vWA: 17,19 TPOX: 8,12 TH01: 8 Penta_E: 14,15
- Penta_D: 13 FGA: 20,24 D8S1179: 13 D7S820: 12
- D5S818: 12 D3S1358: 17 D2S1338: 17,19 D21S11: 30
- D19S433: 13,14 D18S51: 16 D16S539: 11,12 D13S317: 13
- CSF1PO: 10 Amelogenin: X
- CAL27 CRL-2095 Age: 56 years old Sex: Male
- STR confirmation: 93% (OSCC)
- STR analysis
- vWA: 14,17 TPOX: 8 TH01: 6,9.3 Penta_E: 7
- Penta_D: 9,10 FGA: 25 D8S1179: 13,15 D7S820: 10
- D5S818: 11,12 D3S1358: 16 D2S1338: 23,24 D21S11: 28,29
- D19S433: 14,15.2 D18S51: 13 D16S539: 11,12 D13S317: 10,11
- CSF1PO: 10,12 Amelogenin: X
2.2. Growth Assays
2.3. RNA Extraction and cDNA Synthesis
2.4. qPCR Screening
- Positive controls
- GAPDH forward, 5-′ATCTTCCAGGAGCGAGATCC-3′
- GAPDH reverse, 5′-ACCACTGACACGTTGGCAGT-3′
- Beta actin forward, 5′-GTGGGGTCCTGTGGTGTG-3′
- Beta actin reverse, 5′-GAAGGGGACAGGCAGTGA-3′
- Screening primers
- miR-16 forward, 5′-TAGCAGCACGTAAATATTGGCG-3′
- miR-16 reverse, 5′-TGCGTGTCGTGGAGTC-3′
- miR-365 forward, 5′-ATAGGATCCTGAGGTCCCTTTCGTG-3′
- miR-365 reverse, 5′-GCGAAGCTTAAAAACAGCGGAAGAGTTT-3′
- Downstream targets
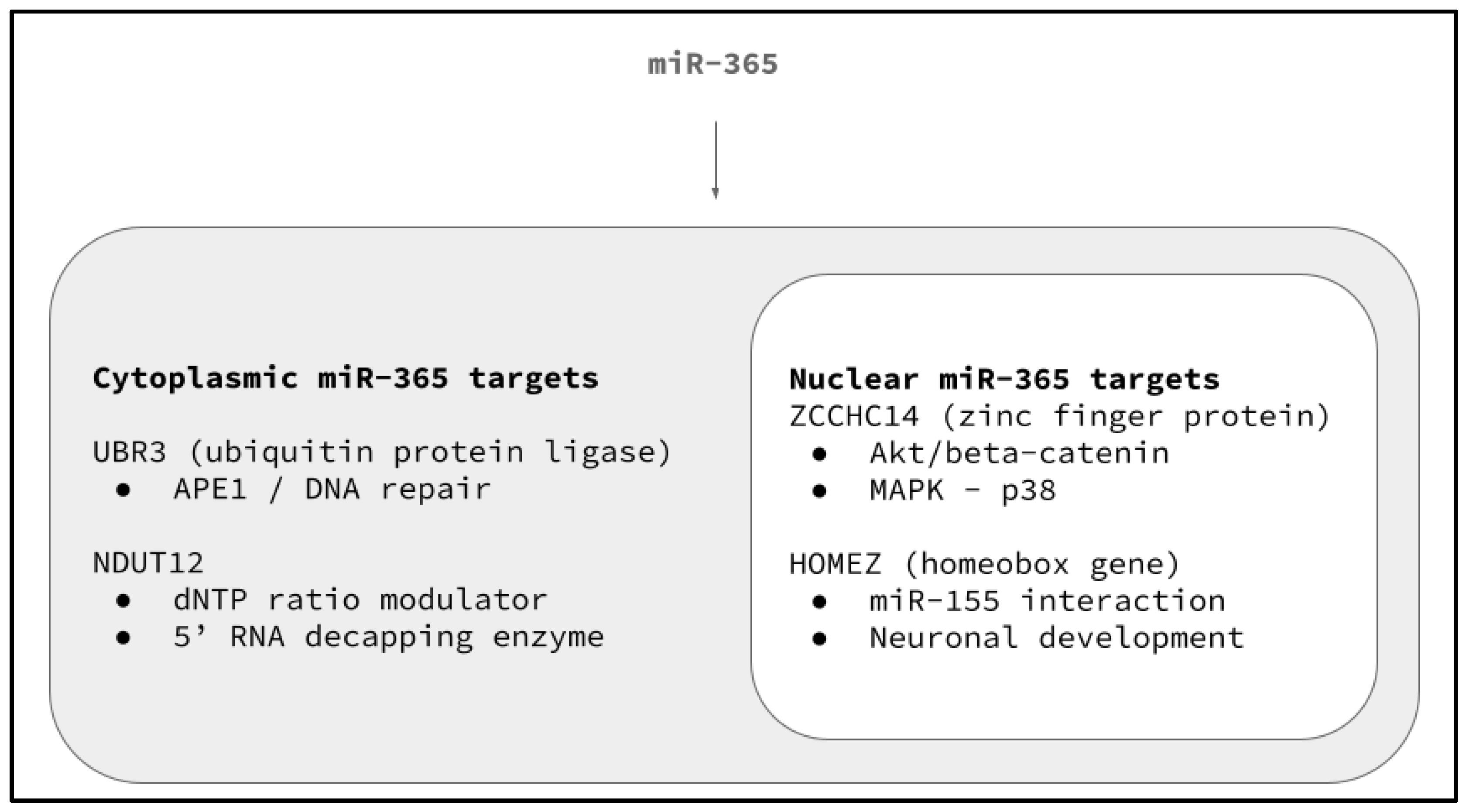
- UBR3 (target score 95)
- UBR3 forward, 5′-TGGCTGTTCAAGGTTTCATAGG-3′
- UBR3 reverse, 5′-GGTGCCACTGCTTAGTTTTACC-3′
- NUDT12 (target score 93)
- NUDT12 forward, 5′-ATCCCTTGGTTACTCTAGGTGG-3′
- NUDT12 reverse, 5′-GGCTGGGCCAAATAATCCTTT-3′
- MOCS2 (target score 93)
- MOCS2 forward, 5′-AGTGCTGAAATAACAGGAGTTCG-3′
- MOCS2 reverse, 5′-TCCAAGCTCGACATATTCTTGAC-3′
- EPOR (target score 92)
- EPOR forward, 5′-TGGAGGACTTGGTGTGTTTCT-3′
- EPOR reverse, 5′-GCAACTCTAGGGGCACGAA-3′
- ZCCHC14 (target score 92)
- ZCCHC14 forward, 5′-CGGACGCATTTTATGTGGAGC-3′
- ZCCHC14 reverse, 5′-TCTGCGAGGACGGGATACC-3′
- IQCK (target score 91)
- IQCK forward, 5′-CTGCTCTACAGACTCGTCGTT-3′
- IQCK reverse, 5′-AGATTCTTGCTTGACGGCTCG-3′
- HOMEZ (target score 91)
- HOMEZ forward, 5′-CTGGACTGCGCTATCTCTGAA-3′
- HOMEZ reverse, 5′-CTGAAGGTTTTGAGCAGGTGT-3
- CPA3 (target score 91)
- CPA3 forward, 5′-GGGTTTGATTGCTACCACTCTT-3′
- CPA3 reverse, 5′-GCCAAGTCCTTTATGATGTCTGC-3′
- SLC24A3 (target score 90)
- SLC24A3 forward, 5′-TTGACCTCATGGACCTCGTAG-3′
- SLC24A3 reverse, 5′-GGCATCTTCGGAAGTCAGAGT-3′
- CCDC47 (target score 90)
- CCDC47 forward, 5′-TCTCCTCAACGGGTCATAATCA-3′
- CCDC47 reverse, 5′-GGTTCACTCTCAGTATCTCCCT-3′
2.5. Transfection and Overexpression of miR-365
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2019 Lip, Oral, and Pharyngeal Cancer Collaborators; Cunha, A.R.D.; Compton, K.; Xu, R.; Mishra, R.; Drangsholt, M.T.; Antunes, J.L.F.; Kerr, A.R.; Acheson, A.R.; Lu, D.; et al. The Global, Regional, and National Burden of Adult Lip, Oral, and Pharyngeal Cancer in 204 Countries and Territories: A Systematic Analysis for the Global Burden of Disease Study 2019. JAMA Oncol. 2023, 9, 1401–1416. [Google Scholar] [CrossRef] [PubMed]
- Global Burden of Disease 2019 Cancer Collaboration; Kocarnik, J.M.; Compton, K.; Dean, F.E.; Fu, W.; Gaw, B.L.; Harvey, J.D.; Henrikson, H.J.; Lu, D.; Pennini, A.; et al. Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life Years for 29 Cancer Groups From 2010 to 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. JAMA Oncol. 2022, 8, 420–444. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.S.; Ramirez, R.J.; Lee, J.J.; Valenzuela, C.V.; Zevallos, J.P.; Mazul, A.L.; Puram, S.V.; Doering, M.M.; Pipkorn, P.; Jackson, R.S. Survival of Young Versus Old Patients With Oral Cavity Squamous Cell Carcinoma: A Meta-Analysis. Laryngoscope 2021, 131, 1310–1319. [Google Scholar] [CrossRef] [PubMed]
- Haynes, D.A.; Vanison, C.C.; Gillespie, M.B. The Impact of Dental Care in Head and Neck Cancer Outcomes: A Systematic Review and Meta-Analysis. Laryngoscope 2022, 132, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, N.A.; Kedia, S.K.; Ward, K.D.; Pichon, L.C.; Chen, W.; Dillon, P.J.; Navaparia, H. Effectiveness of Interventions to Improve Oral Cancer Knowledge: A Systematic Review. J. Cancer Educ. 2022, 37, 479–498. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.; Macey, R.; Kerr, A.R.; Lingen, M.W.; Ogden, G.R.; Warnakulasuriya, S. Diagnostic tests for oral cancer and potentially malignant disorders in patients presenting with clinically evident lesions. Cochrane Database Syst. Rev. 2021, 7, CD010276. [Google Scholar] [CrossRef] [PubMed]
- Worthington, H.V.; Bulsara, V.M.; Glenny, A.M.; Clarkson, J.E.; Conway, D.I.; Macluskey, M. Interventions for the treatment of oral cavity and oropharyngeal cancers: Surgical treatment. Cochrane Database Syst. Rev. 2023, 8, CD006205. [Google Scholar] [CrossRef] [PubMed]
- Draghini, L.; Lancellotta, V.; Fionda, B.; De Angeli, M.; Cornacchione, P.; Massaccesi, M.; Trippa, F.; Kovács, G.; Morganti, A.G.; Bussu, F.; et al. Can interventional radiotherapy (brachytherapy) be an alternative to surgery in early-stage oral cavity cancer? A systematic review. Strahlenther. Onkol. 2024, 200, 367–376. [Google Scholar] [CrossRef]
- Pombo Lopes, J.; Rodrigues, I.; Machado, V.; Botelho, J.; Bandeira Lopes, L. Chemotherapy and Radiotherapy Long-Term Adverse Effects on Oral Health of Childhood Cancer Survivors: A Systematic Review and Meta-Analysis. Cancers 2023, 16, 110. [Google Scholar] [CrossRef]
- Atashi, F.; Vahed, N.; Emamverdizadeh, P.; Fattahi, S.; Paya, L. Drug resistance against 5-fluorouracil and cisplatin in the treatment of head and neck squamous cell carcinoma: A systematic review. J. Dent. Res. Dent. Clin. Dent. Prospect. 2021, 15, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Winquist, E.; Agbassi, C.; Meyers, B.M.; Yoo, J.; Chan, K.K.W.; Head and Neck Disease Site Group. Systemic therapy in the curative treatment of head and neck squamous cell cancer: A systematic review. J. Otolaryngol. Head Neck Surg. 2017, 46, 29. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, R.; Polpaya, K.; Kunale, M.; Kodiveri Muthukaliannan, G.; Shetty, S.; Baxi, S.; Mani, R.R.; Paranjothy, C.; Purushothaman, V.; Kayarohanam, S.; et al. Clinical Investigation of Chemotherapeutic Resistance and miRNA Expressions in Head and Neck Cancers: A Thorough PRISMA Compliant Systematic Review and Comprehensive Meta-Analysis. Genes 2022, 13, 2325. [Google Scholar] [CrossRef] [PubMed]
- Yap, T.; Pruthi, N.; Seers, C.; Belobrov, S.; McCullough, M.; Celentano, A. Extracellular Vesicles in Oral Squamous Cell Carcinoma and Oral Potentially Malignant Disorders: A Systematic Review. Int. J. Mol. Sci. 2020, 21, 1197. [Google Scholar] [CrossRef] [PubMed]
- Palaia, G.; Pippi, R.; Rocchetti, F.; Caputo, M.; Macali, F.; Mohsen, A.; Del Vecchio, A.; Tenore, G.; Romeo, U. Liquid biopsy in the assessment of microRNAs in oral squamous cell carcinoma: A systematic review. J. Clin. Exp. Dent. 2022, 14, e875–e884. [Google Scholar] [CrossRef]
- Maleki, M.; Golchin, A.; Javadi, S.; Khelghati, N.; Morovat, P.; Asemi, Z.; Alemi, F.; Vaghari-Tabari, M.; Yousefi, B.; Majidinia, M. Role of exosomal miRNA in chemotherapy resistance of Colorectal cancer: A systematic review. Chem. Biol. Drug Des. 2023, 101, 1096–1112. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, R.; Nayagam, S.G.; Kar, A.; Sathyakumar, S.; Mohammed, H.; Smiti, M.; Sabarimurugan, S.; Kumarasamy, C.; Priyadharshini, T.; Gothandam, K.M.; et al. Clinical Theragnostic Relationship between Drug-Resistance Specific miRNA Expressions, Chemotherapeutic Resistance, and Sensitivity in Breast Cancer: A Systematic Review and Meta-Analysis. Cells 2019, 8, 1250. [Google Scholar] [CrossRef] [PubMed]
- Lucidi, A.; Buca, D.; Ronsini, C.; Tinari, S.; Bologna, G.; Buca, D.; Leombroni, M.; Liberati, M.; D’Antonio, F.; Scambia, G.; et al. Role of Extracellular Vesicles in Epithelial Ovarian Cancer: A Systematic Review. Int. J. Mol. Sci. 2020, 21, 8762. [Google Scholar] [CrossRef] [PubMed]
- Jahani, S.; Nazeri, E.; Majidzadeh-A, K.; Jahani, M.; Esmaeili, R. Circular RNA; a new biomarker for breast cancer: A systematic review. J. Cell. Physiol. 2020, 235, 5501–5510. [Google Scholar] [CrossRef]
- Chen, H.H.; Zhang, T.N.; Zhang, F.Y.; Zhang, T. Non-coding RNAs in drug and radiation resistance of bone and soft-tissue sarcoma: A systematic review. Elife 2022, 11, e79655. [Google Scholar] [CrossRef]
- Coon, J.; Kingsley, K. Assessment of MicroRNA (miR)-365 Effects on Oral Squamous Carcinoma Cell Line Phenotypes. Biomolecules 2021, 11, 874. [Google Scholar] [CrossRef] [PubMed]
- Coon, J.; Kingsley, K.; Howard, K.M. miR-365 (microRNA): Potential Biomarker in Oral Squamous Cell Carcinoma Exosomes and Extracellular Vesicles. Int. J. Mol. Sci. 2020, 21, 5317. [Google Scholar] [CrossRef] [PubMed]
- Huni, K.C.; Cheung, J.; Sullivan, M.; Robison, W.T.; Howard, K.M.; Kingsley, K. Chemotherapeutic Drug Resistance Associated with Differential miRNA Expression of miR-375 and miR-27 among Oral Cancer Cell Lines. Int. J. Mol. Sci. 2023, 24, 1244. [Google Scholar] [CrossRef] [PubMed]
- Belnap, C.; Divis, T.; Kingsley, K.; Howard, K.M. Differential Expression of MicroRNA MiR-145 and MiR-155 Downstream Targets in Oral Cancers Exhibiting Limited Chemotherapy Resistance. Int. J. Mol. Sci. 2024, 25, 2167. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Jang, T.H.; Tung, S.L.; Yen, T.C.; Chan, S.H.; Wang, L.H. A novel miR-365-3p/EHF/keratin 16 axis promotes oral squamous cell carcinoma metastasis, cancer stemness and drug resistance via enhancing β5-integrin/c-met signaling pathway. J. Exp. Clin. Cancer Res. 2019, 38, 89. [Google Scholar] [CrossRef] [PubMed]
- Orlandella, F.M.; Imperlini, E.; Pane, K.; Luciano, N.; Braile, M.; De Stefano, A.E.; Iervolino, P.L.C.; Ruocco, A.; Orrù, S.; Franzese, M.; et al. miR-331-5p Affects Motility of Thyroid Cancer Cell Lines and Regulates BID Expression. Biomedicines 2024, 12, 658. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Qiu, Y.; Zheng, X.; Dai, Y.; Xu, T. The miR-199a-5p/PD-L1 axis regulates cell proliferation, migration and invasion in follicular thyroid carcinoma. BMC Cancer 2022, 22, 756. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Sun, Z.; Wang, D.; Du, T. MiR-106b-5p regulates esophageal squamous cell carcinoma progression by binding to HPGD. BMC Cancer 2022, 22, 308. [Google Scholar] [CrossRef]
- Dai, F.; Liu, T.; Zheng, S.; Liu, Q.; Yang, C.; Zhou, J.; Chen, Y.; Sheyhidin, I.; Lu, X. MiR-106b promotes migration and invasion through enhancing EMT via downregulation of Smad 7 in Kazakh’s esophageal squamous cell carcinoma. Tumour Biol. 2016, 37, 14595–14604. [Google Scholar] [CrossRef]
- Wang, Z.Z.; Luo, Y.R.; Du, J.; Yu, Y.; Yang, X.Z.; Cui, Y.J.; Jin, X.F. MiR-296-5p inhibits cell invasion and migration of esophageal squamous cell carcinoma by downregulating STAT3 signaling. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 5206–5214. [Google Scholar] [CrossRef]
- Liu, C.; Tian, X.; Sun, H.B.; Wang, Z.F.; Jiang, L.F.; Li, Z.X. MiR-601 inhibits the proliferation and metastasis of esophageal squamous cell carcinoma (ESCC) by targeting HDAC6. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Zhu, J.F.; Liu, J.Y.; Li, Y.L.; Dong, X.; Huang, H.; Shan, L. miR-133b inhibits cell proliferation migration invasion of esophageal squamous cell carcinoma by targeting EGFR. Biomed. Pharmacother. 2019, 111, 476–484. [Google Scholar] [CrossRef]
- Yin, Y.; Du, L.; Li, X.; Zhang, X.; Gao, Y. miR-133a-3p suppresses cell proliferation, migration, and invasion and promotes apoptosis in esophageal squamous cell carcinoma. J. Cell. Physiol. 2019, 234, 12757–12770. [Google Scholar] [CrossRef] [PubMed]
- Al Rawi, N.; Elmabrouk, N.; Abu Kou, R.; Mkadmi, S.; Rizvi, Z.; Hamdoon, Z. The role of differentially expressed salivary microRNA in oral squamous cell carcinoma. A systematic review. Arch. Oral Biol. 2021, 125, 105108. [Google Scholar] [CrossRef] [PubMed]
- Menini, M.; De Giovanni, E.; Bagnasco, F.; Delucchi, F.; Pera, F.; Baldi, D.; Pesce, P. Salivary Micro-RNA and Oral Squamous Cell Carcinoma: A Systematic Review. J. Pers. Med. 2021, 11, 101. [Google Scholar] [CrossRef]
- Bauman, J.; Langer, C.; Quon, H.; Algazy, K.; Lin, A.; Desai, A.; Mutale, F.; Weiss, J. Induction chemotherapy with cetuximab, carboplatin and paclitaxel for the treatment of locally advanced squamous cell carcinoma of the head and neck. Exp. Ther. Med. 2013, 5, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Meisenberg, C.; Tait, P.S.; Dianova, I.I.; Wright, K.; Edelmann, M.J.; Ternette, N.; Tasaki, T.; Kessler, B.M.; Parsons, J.L.; Kwon, Y.T.; et al. Ubiquitin ligase UBR3 regulates cellular levels of the essential DNA repair protein APE1 and is required for genome stability. Nucleic Acids Res. 2012, 40, 701–711. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, H.; Guo, L.; Zhao, Y.; Chen, F. Reconstructing the coding and non-coding RNA regulatory networks of miRNAs and mRNAs in breast cancer. Gene 2014, 548, 6–13. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, J.; Yang, Q.; Zhu, L.; Zhao, W.; Wang, X.; Yao, J.; Zhou, Y.; Shao, S. CircRNA_15430 reduced by Helicobacter pylori infection and suppressed gastric cancer progression via miR-382-5p/ZCCHC14 axis. Biol. Direct 2023, 18, 51. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, W.; Yang, Y.; Dai, E.; Bai, Y. Diagnostic and prognostic value of microRNA-2355-3p and contribution to the progression in lung adenocarcinoma. Bioengineered 2021, 12, 4747–4756. [Google Scholar] [CrossRef]
- Shi, X.; Han, X.; Cao, Y.; Li, C.; Cao, Y. ZCCHC14 regulates proliferation and invasion of non-small cell lung cancer through the MAPK-P38 signalling pathway. J. Cell Mol. Med. 2021, 25, 1406–1414. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhang, J.; Chen, C.; Luo, J.; Yan, H.; Jiang, W. The Akt/GSK3β/β-catenin signaling regulated by ZCCHC14 is responsible for accelerating the proliferation of hepatocellular carcinoma. J. BUON 2021, 26, 1958–1963. [Google Scholar] [PubMed]
- Bayarsaihan, D.; Enkhmandakh, B.; Makeyev, A.; Greally, J.M.; Leckman, J.F.; Ruddle, F.H. Homez, a homeobox leucine zipper gene specific to the vertebrate lineage. Proc. Natl. Acad. Sci. USA 2003, 100, 10358–10363. [Google Scholar] [CrossRef] [PubMed]
- Ghimouz, R.; Bar, I.; Hanotel, J.; Minela, B.; Keruzore, M.; Thelie, A.; Bellefroid, E.J. The homeobox leucine zipper gene Homez plays a role in Xenopus laevis neurogenesis. Biochem. Biophys. Res. Commun. 2011, 415, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Slezak-Prochazka, I.; Kluiver, J.; de Jong, D.; Smigielska-Czepiel, K.; Kortman, G.; Winkle, M.; Rutgers, B.; Koerts, J.; Visser, L.; Diepstra, A.; et al. Inhibition of the miR-155 target NIAM phenocopies the growth promoting effect of miR-155 in B-cell lymphoma. Oncotarget 2016, 7, 2391–2400. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Grudzien-Nogalska, E.; Hamilton, K.; Jiao, X.; Yang, J.; Tong, L.; Kiledjian, M. Mammalian Nudix proteins cleave nucleotide metabolite caps on RNAs. Nucleic Acids Res. 2020, 48, 6788–6798. [Google Scholar] [CrossRef] [PubMed]
- Doamekpor, S.K.; Sharma, S.; Kiledjian, M.; Tong, L. Recent insights into noncanonical 5’ capping and decapping of RNA. J. Biol. Chem. 2022, 298, 102171. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Cheng, X.; Zhang, F.; Chen, Q.; Chen, X.; Shen, Y.; Lai, C.; Kota, V.G.; Sun, W.; Huang, Q.; et al. Integrated multi-omics data analyses for exploring the co-occurring and mutually exclusive gene alteration events in colorectal cancer. Hum. Mutat. 2020, 41, 1588–1599. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, L.; Kong, D.; Hu, G.; Wei, B. Construction of Novel DNA Methylation-Based Prognostic Model to Predict Survival in Glioblastoma. J. Comput. Biol. 2020, 27, 718–728. [Google Scholar] [CrossRef]
- Mu, G.; Wang, J.; Liu, Z.; Zhang, H.; Zhou, S.; Xiang, Q.; Cui, Y. Association between smokeless tobacco use and oral cavity cancer risk in women compared with men: A systematic review and meta-analysis. BMC Cancer 2021, 21, 960. [Google Scholar] [CrossRef]
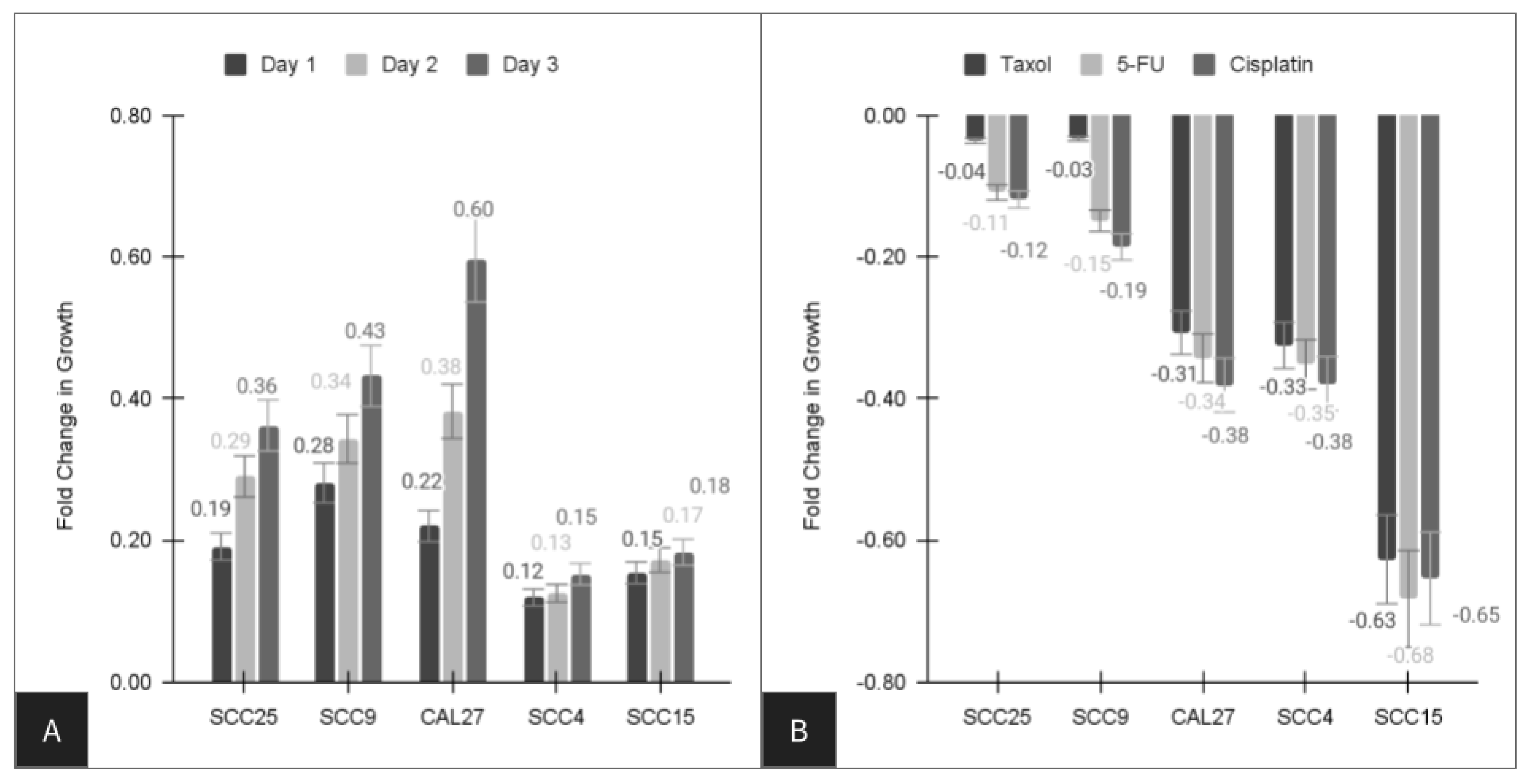
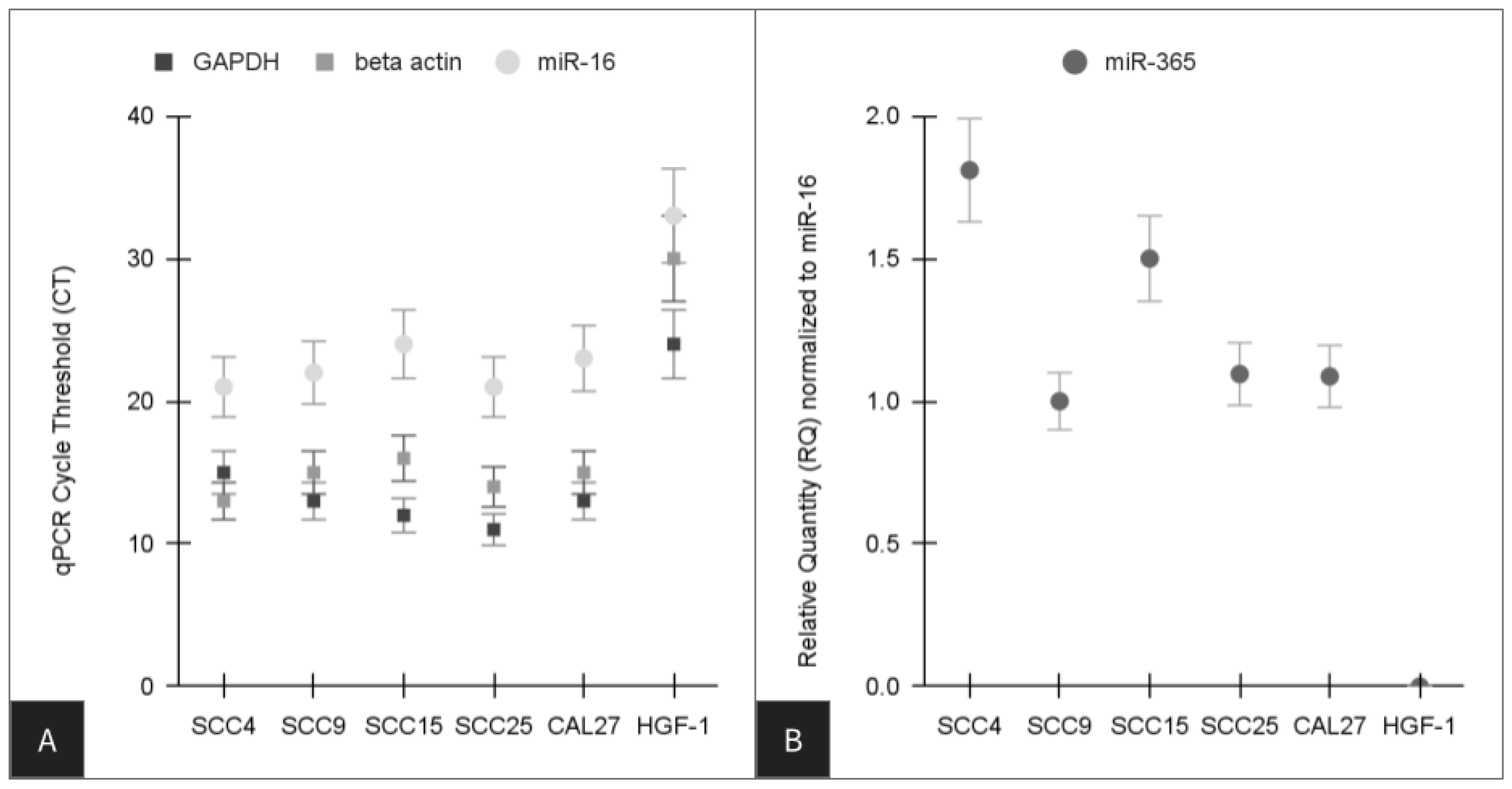
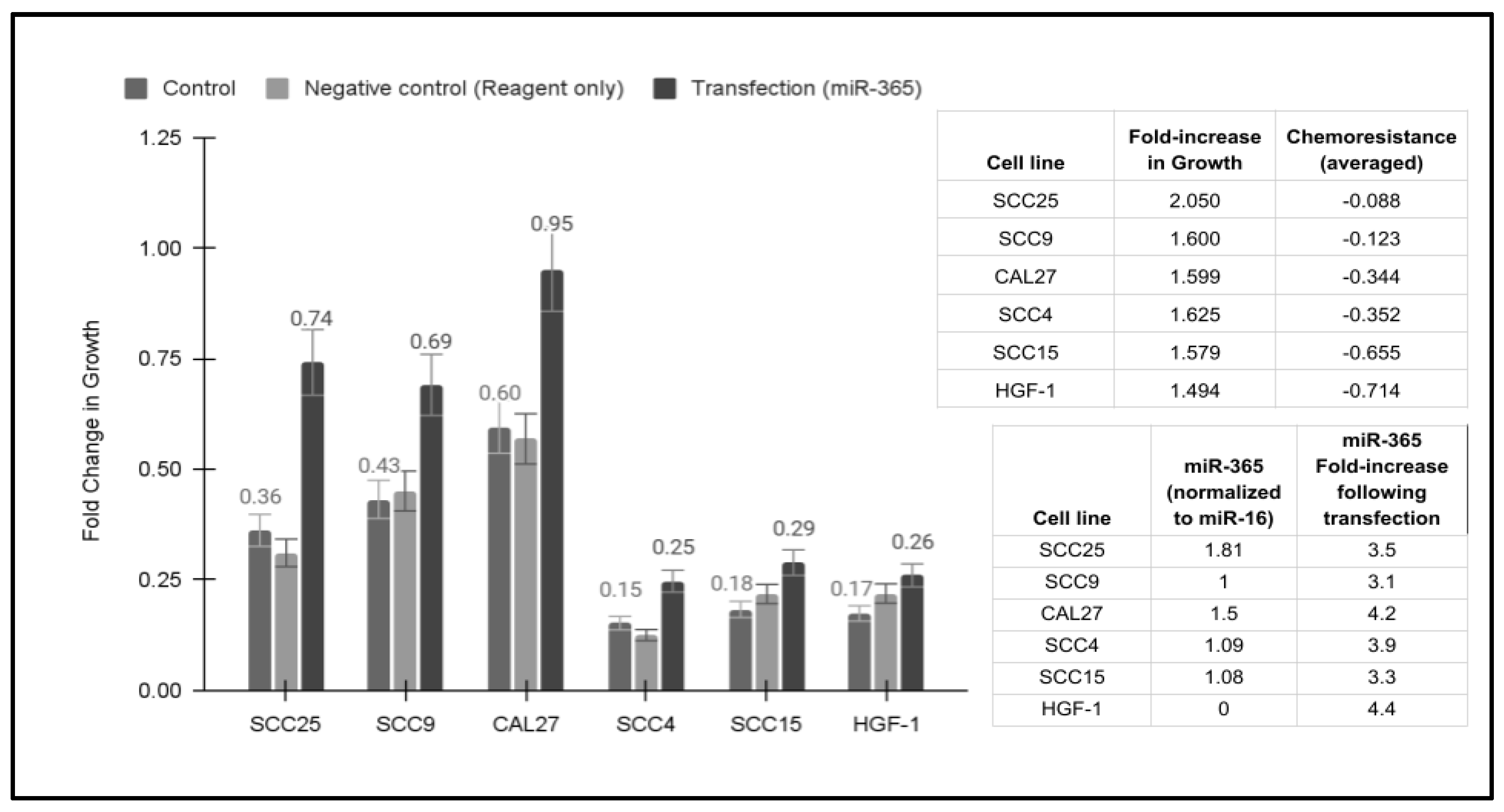


| Cell Line | Paclitaxel (Taxol) | Fluorouracil 5-FU | Cisplatin |
|---|---|---|---|
| SCC4 | −32.5% | −35.2% | −37.9% |
| SCC9 | −3.3% * | −14.9% | −18.6% |
| SCC15 | −62.7% | −68.3% | −65.4% |
| SCC25 | −3.6% * | −10.9% | −11.9% |
| CAL27 | −30.7% | −34.3% | −38.1% |
| Average | −34.4% | −32.7% | −26.6% |
| Range | −3.3% to −62.7% | −10.9% to −68.3% | −11.9% to −65.4% |
| Cell Line | RNA Analysis [ng/uL] | cDNA Analysis [ng/uL] | A260:A280 Analysis |
|---|---|---|---|
| SCC4 | 224.3 ng/uL | 1557 ng/uL | 1.80 |
| SCC9 | 164.4 ng/uL | 1461 ng/uL | 1.91 |
| SCC15 | 294.4 ng/uL | 1252 ng/uL | 1.88 |
| SCC25 | 234.2 ng/uL | 1151 ng/uL | 1.79 |
| CAL27 | 155.4 ng/uL | 1257 ng/uL | 1.92 |
| Average | 214.5 ng/uL | 1335.6 ng/uL | 1.86 |
| Range | 155–294 ng/uL | 1151–1557 ng/uL | 1.80–1.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, B.; Kruse, N.; Howard, K.M.; Kingsley, K. Downstream Target Analysis for miR-365 among Oral Squamous Cell Carcinomas Reveals Differential Associations with Chemoresistance. Life 2024, 14, 741. https://doi.org/10.3390/life14060741
Yu B, Kruse N, Howard KM, Kingsley K. Downstream Target Analysis for miR-365 among Oral Squamous Cell Carcinomas Reveals Differential Associations with Chemoresistance. Life. 2024; 14(6):741. https://doi.org/10.3390/life14060741
Chicago/Turabian StyleYu, Brendon, Nathaniel Kruse, Katherine M. Howard, and Karl Kingsley. 2024. "Downstream Target Analysis for miR-365 among Oral Squamous Cell Carcinomas Reveals Differential Associations with Chemoresistance" Life 14, no. 6: 741. https://doi.org/10.3390/life14060741






