Optimizing Soil Health and Sorghum Productivity through Crop Rotation with Quinoa
Abstract
1. Introduction
2. Materials and Methods
2.1. Site and Experiment Design
2.2. Field Preparation
2.3. Soil Analysis
2.4. Measurement of Yield Traits
2.5. Statistical Analysis
3. Results
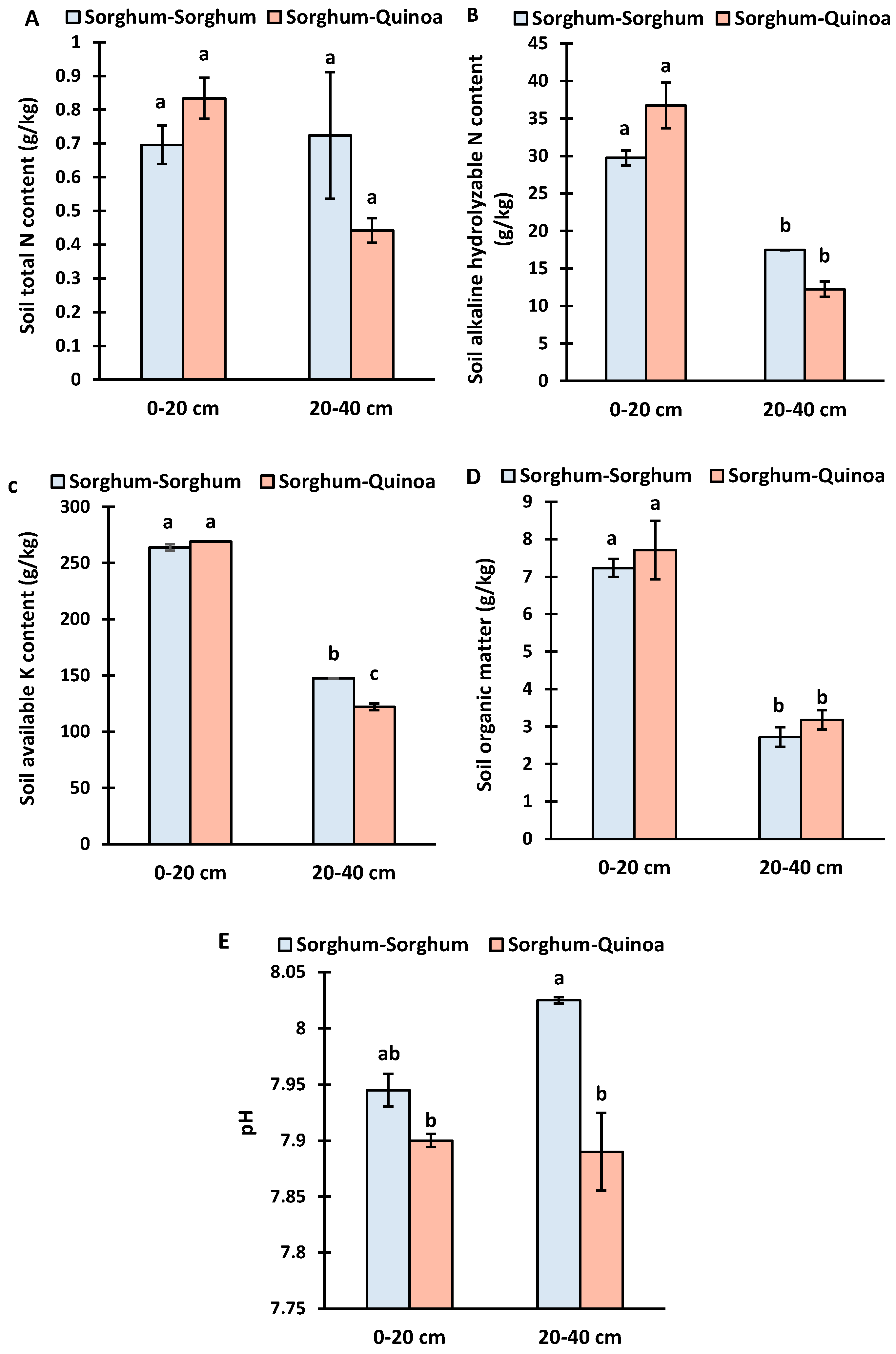
3.1. Soil Properties at Different Soil Depths under Continuous Cropping and Crop Rotation
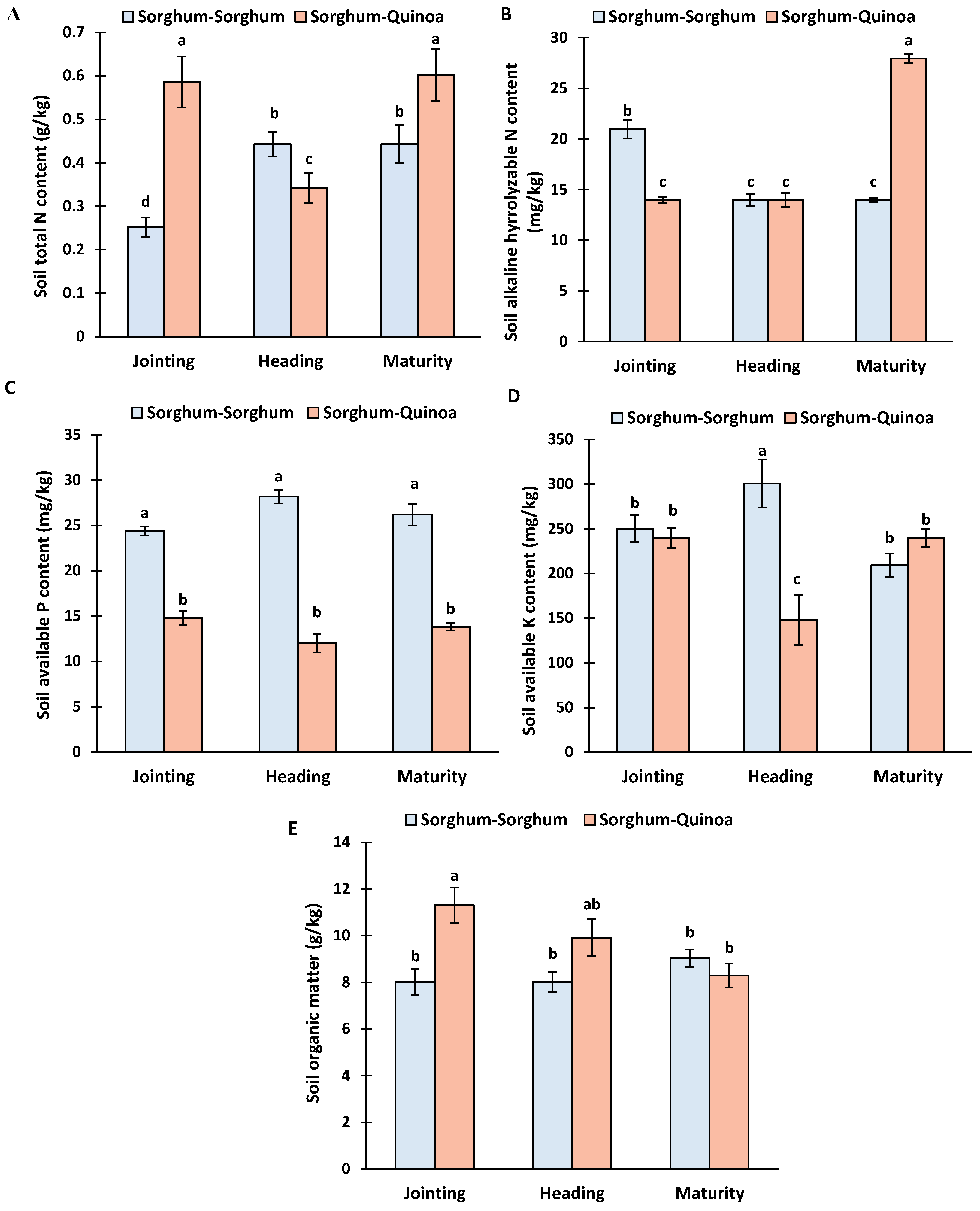
3.2. Soil Properties at Different Growth Stages of Sorghum Continuous Cropping and Crop Rotation
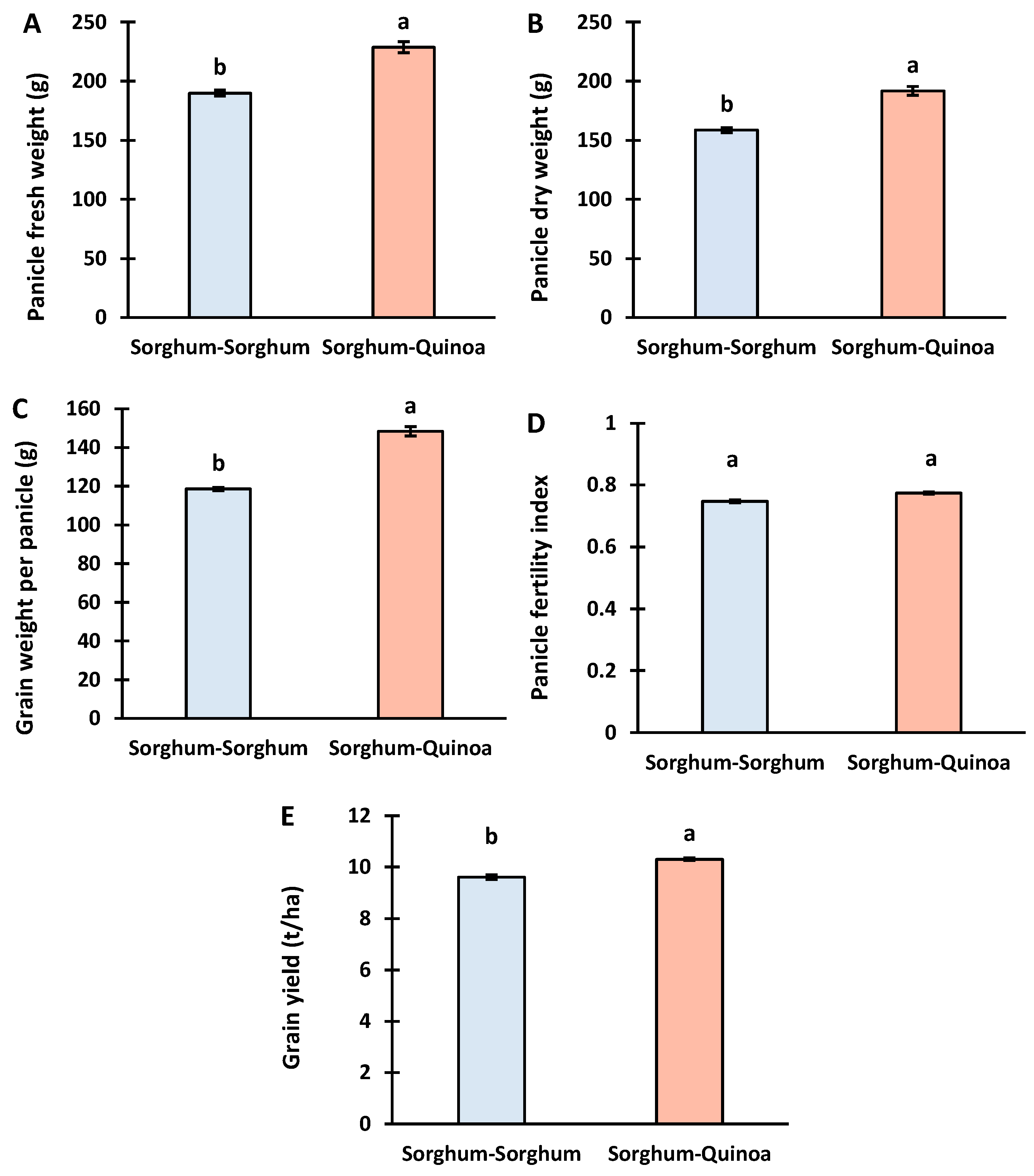
3.3. Yield of Sorghum under Sorghum Continuous Cropping and Crop Rotation with Quinoa
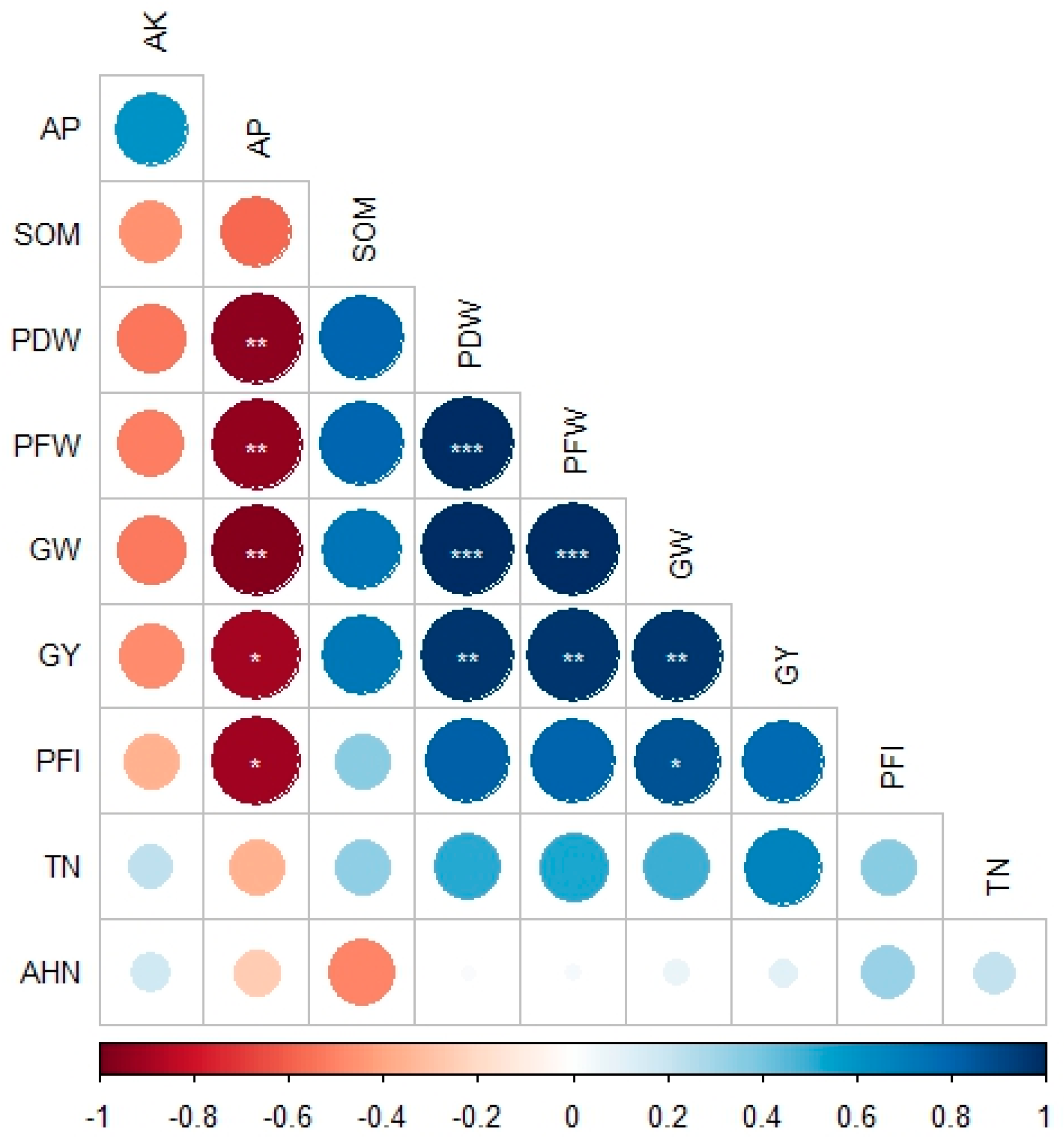
3.4. Correlation of Soil Nutrients and Sorghum Yield Traits
4. Discussion
4.1. Soil Properties at Different Soil Depths under Continuous Cropping and Crop Rotation
4.2. Soil Properties at Different Growth Stages under Continuous Cropping and Crop Rotation
4.3. Yield of Sorghum under Continuous Cropping and Crop Rotation with Quinoa
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kerr, R.B.; Postigo, J.C.; Smith, P.; Cowie, A.; Singh, P.K.; Rivera-Ferre, M.; Tirado-von der Pahlen, M.C.; Campbell, D.; Neufeldt, H. Agroecology as a transformative approach to tackle climatic, food, and ecosystemic crises. Curr. Opin. Environ. Sustain. 2023, 62, 101275. [Google Scholar] [CrossRef]
- Deguine, J.P.; Aubertot, J.N.; Bellon, S.; Côte, F.; Lauri, P.E.; Lescourret, F.; Ratnadass, A.; Scopel, E.; Andrieu, N.; Bàrberi, P.; et al. Agroecological crop protection for sustainable agriculture. Adv. Agron. 2023, 178, 1–59. [Google Scholar]
- Kherif, O.; Keskes, M.I.; Pansu, M.; Ouaret, W.; Rebouh, Y.N.; Dokukin, P.; Kucher, D.; Latati, M. Agroecological modeling of nitrogen and carbon transfers between decomposer micro-organisms, plant symbionts, soil and atmosphere in an intercropping system. Ecol. Model. 2021, 440, 109390. [Google Scholar] [CrossRef]
- León Araya, A. Monocrops. J. Peasant Stud. 2023, 50, 797–808. [Google Scholar] [CrossRef]
- Salaheen, S.; Biswas, D. Organic farming practices: Integrated culture versus monoculture. In Safety and Practice for Organic Food; Academic Press: Cambridge, MA, USA, 2019; pp. 23–32. [Google Scholar]
- Li, H.; Li, C.; Song, X.; Liu, Y.; Gao, Q.; Zheng, R.; Li, J.; Zhang, P.; Liu, X. Impacts of continuous and rotational cropping practices on soil chemical properties and microbial communities during peanut cultivation. Sci. Rep. 2022, 12, 2758. [Google Scholar] [CrossRef] [PubMed]
- Kaye, N.M.; Mason, S.C.; Jackson, D.S.; Galusha, T.D. Crop rotation and soil amendment alters sorghum grain quality. Crop Sci. 2007, 47, 722–727. [Google Scholar] [CrossRef]
- Ma, Z.; Guan, Z.; Liu, Q.; Hu, Y.; Liu, L.; Wang, B.; Huang, L.; Li, H.; Yang, Y.; Han, M.; et al. Obstacles in continuous cropping: Mechanisms and control measures. Adv. Agron. 2023, 179, 205–256. [Google Scholar]
- Rusch, A.; Bommarco, R.; Jonsson, M.; Smith, H.G.; Ekbom, B. Flow and stability of natural pest control services depend on complexity and crop rotation at the landscape scale. J. Appl. Ecol. 2013, 50, 345–354. [Google Scholar] [CrossRef]
- Oberholster, T.; Vikram, S.; Cowan, D.; Valverde, A. Key microbial taxa in the rhizosphere of sorghum and sunflower grown in crop rotation. Sci. Total Environ. 2018, 624, 530–539. [Google Scholar] [CrossRef]
- Waha, K.; Dietrich, J.P.; Portmann, F.T.; Siebert, S.; Thornton, P.K.; Bondeau, A.; Herrero, M. Multiple cropping systems of the world and the potential for increasing cropping intensity. Glob. Environ. Chang. 2020, 64, 102131. [Google Scholar] [CrossRef]
- Malobane, M.E.; Nciizah, A.D.; Nyambo, P.; Mudau, F.N.; Wakindiki, I.I. Microbial biomass carbon and enzyme activities as influenced by tillage, crop rotation and residue management in a sweet sorghum cropping system in marginal soils of South Africa. Heliyon 2020, 6, e05513. [Google Scholar] [CrossRef]
- Gaba, S.; Lescourret, F.; Boudsocq, S.; Enjalbert, J.; Hinsinger, P.; Journet, E.P.; Navas, M.L.; Wery, J.; Louarn, G.; Malézieux, E.; et al. Multiple cropping systems as drivers for providing multiple ecosystem services: From concepts to design. Agron. Sustain. Dev. 2015, 35, 607–623. [Google Scholar] [CrossRef]
- Simão, L.M.; Peterson, D.; Roozeboom, K.L.; Rice, C.W.; Du, J.; Lin, X.; Lollato, R.P. Crop rotation and tillage impact yield performance of soybean, sorghum, and wheat. Agron. J. 2023, 115, 658–673. [Google Scholar] [CrossRef]
- da Silva, P.C.G.; Tiritan, C.S.; Echer, F.R.; dos Santos Cordeiro, C.F.; Rebonatti, M.D.; dos Santos, C.H. No-tillage and crop rotation increase crop yields and nitrogen stocks in sandy soils under agroclimatic risk. Field Crop. Res. 2020, 258, 107947. [Google Scholar] [CrossRef]
- Ouda, S.; Zohry, A.E.H.; Noreldin, T.; Ouda, S.; Zohry, A.; Noreldin, T. Crop rotation maintains soil sustainability. In Crop Rotation: An Approach to Secure Future Food; Springer: Berlin/Heidelberg, Germany, 2018; pp. 55–76. [Google Scholar]
- Chen, S.; Zhou, X.; Yu, H.; Wu, F. Root exudates of potato onion are involved in the suppression of clubroot in a Chinese cabbage-potato onion-Chinese cabbage crop rotation. Eur. J. Plant Pathol. 2018, 150, 765–777. [Google Scholar] [CrossRef]
- Schlegel, A.J.; Assefa, Y.; Haag, L.A.; Thompson, C.R.; Stone, L.R. Yield and overall productivity under long-term wheat-based crop rotations: 2000 through 2016. Agron. J. 2019, 111, 264–274. [Google Scholar] [CrossRef]
- Zohry, A.; Ouda, S. Crop Rotation Defeats Pests and Weeds. In Crop Rotation; Springer: Cham, Switzerland, 2018; pp. 77–88. [Google Scholar] [CrossRef]
- Jalali, M.; Eslami, S.V.; Mahmoodi, S.; Aien, A. Effect of Weeds Control on Crop Growth and Yield in Additive Quinoa (Chenopodium quinoa Willd) and Potato (Solanum tubersum L.) Intercropping. Iran. J. Field Crop. Res. 2020, 18, 451–464. [Google Scholar]
- Ding, Y.; Huang, X.; Li, Y.; Liu, H.; Zhang, Q.; Liu, X.; Xu, J.; Di, H. Nitrate leaching losses mitigated with intercropping of deep-rooted and shallow-rooted plants. J. Soils Sediments 2021, 21, 364–375. [Google Scholar] [CrossRef]
- Mugi-Ngenga, E.; Bastiaans, L.; Anten, N.P.R.; Zingore, S.; Baijukya, F.; Giller, K.E. The role of inter-specific competition for water in maize-legume intercropping systems in northern Tanzania. Agric. Syst. 2023, 207, 103619. [Google Scholar] [CrossRef]
- Gao, S.; Wang, Y.; Li, G. Sorghum breeding and production in China. In Cereals in China; He, Z., Bonjean, A.P.A., Eds.; CIMMYT: Mexico City, Mexico, 2010; pp. 97–108. [Google Scholar]
- Yang, S.; Malaga, J. Measuring and forecasting Chinese domestic supply and demand for grain sorghum. Agric. Sci. 2020, 11, 71. [Google Scholar] [CrossRef]
- Fu, H.; Chen, Y.; Yang, X.; Di, J.; Xu, M.; Zhang, B. Water resource potential for large-scale sweet sorghum production as bioenergy feedstock in Northern China. Sci. Total Environ. 2019, 653, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Su, P.; Shan, L.; Ma, J. Yield, quality and irrigation water use efficiency of sweet sorghum [Sorghum bicolor (Linn.) Moench] under different land types in arid regions. Aust. J. Crop Sci. 2012, 6, 10–16. [Google Scholar]
- Li, J.; Lei, S.; Gong, H.; Liu, Z.; Zhang, Y.; Ouyang, Z. Field performance of sweet sorghum in salt-affected soils in China: A quantitative synthesis. Environ. Res. 2023, 222, 115362. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Cui, W.; Feng, X.; Zhao, J.; Lu, G. Sorghum insect problems and Management. J. Integr. Plant Biol. 2010, 53, 178–192. [Google Scholar] [CrossRef] [PubMed]
- Wesley, R.A.; Elmore, C.D.; Spurlock, S.R. Deep tillage and crop rotation effects on cotton, soybean, and grain sorghum on clayey soils. Agron. J. 2001, 93, 170–178. [Google Scholar] [CrossRef]
- Godsey, C.B.; Pierzynski, G.M.; Mengel, D.B.; Lamond, R.E. Changes in soil pH, organic carbon, and extractable aluminum from crop rotation and tillage. Soil Sci. Soc. Am. J. 2007, 71, 1038–1044. [Google Scholar] [CrossRef]
- Deng, Y.; Sun, X.; Zhang, Q.; Anwar, S.; Lu, J.; Guo, H.; Qin, L.; Zhang, L.; Wang, C. comprehensive evaluation and physiological response of quinoa genotypes to low nitrogen. Agronomy 2023, 13, 1597. [Google Scholar] [CrossRef]
- Ahmadi, S.H.; Solgi, S.; Sepaskhah, A.R. Quinoa: A super or pseudo-super crop? Evidences from evapotranspiration, root growth, crop coefficients, and water productivity in a hot and semi-arid area under three planting densities. Agric. Water Manag. 2019, 225, 105784. [Google Scholar] [CrossRef]
- Koca, Y.O. Determination of the forage yield and growth parameters of maize (Zea mays L.) with quinoa (Chenopodium quinoa) intercropping at different plant mixtures. Turk. J. Field Crop. 2021, 26, 44–53. [Google Scholar] [CrossRef]
- Vahidi, H.; Mahmoodi, S.; Parsa, S.; Fallahi, H.R. Evaluation the yield and intercropping indices of millet (Panicum miliaceaum L.) and quinoa (Chenopodium quinoa Willd.) under effect of plant density and cultivation ratios in Birjand Region. J. Agroecol. 2021, 13, 471–488. [Google Scholar]
- Tekin, S.; Yazar, A.; Barut, H. Comparison of wheat-based rotation systems vs monocroppingunder dryland Mediterranean condition. Int. J. Agric. Biol. Eng. 2017, 10, 203. [Google Scholar]
- Simon, B.; Tolner, L.; Rékási, M.; Michéli, E. Soil acidity investigation by potentiometric titrations. Cereal Res. Commun. 2006, 34, 283–286. [Google Scholar] [CrossRef]
- Flowers, T.H.; Bremner, J.M. A rapid dichromate procedure for routine estimation of total nitrogen in soils. Commun. Soil Sci. Plant Anal. 1991, 22, 1409–1416. [Google Scholar] [CrossRef]
- Dodor, D.E.; Tabatabai, M.A. A simple alkaline hydrolysis method for estimating nitrogen mineralization potentially of soils. West. Afr. J. Appl. Ecol. 2019, 27, 16–31. [Google Scholar]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, L.A. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; USDA. Circular 939; US Government Printing Office: Washington, DC, USA, 1954. [Google Scholar]
- Bao, S.D. Soil Agricultural Chemical Analysis, 3rd ed.; China Agricultural Press: Beijing, China, 2000; pp. 265–267. [Google Scholar]
- Walkley, A.J.; Black, I.A. Estimation of soil organic carbon by the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Pradhan, S.; Babar, M.A.; Robbins, K.; Bai, G.; Mason, R.E.; Khan, J.; Shahi, D.; Avci, M.; Guo, J.; Maksud Hossain, M.; et al. Understanding the genetic basis of spike fertility to improve grain number, harvest index, and grain yield in wheat under high temperature stress environments. Front. Plant Sci. 2019, 10, 1481. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Li, C.; Zhao, W.; Stringer, L.C.; Fu, B. Spatial distributions of soil nutrients affected by land use, topography and their interactions, in the Loess Plateau of China. Int. Soil Water Conser Res. 2024, 12, 227–239. [Google Scholar] [CrossRef]
- Neugschwandtner, R.W.; Liebhard, P.; Kaul, H.P.; Wagentristl, H. Soil chemical properties as affected by tillage and crop rotation in a long-term field experiment. Plant Soil Environ. 2014, 60, 57–62. [Google Scholar] [CrossRef]
- Wang, Z.G.; Bao, X.G.; Li, X.F.; Jin, X.; Zhao, J.H.; Sun, J.H.; Christie, P.; Li, L. Intercropping maintains soil fertility in terms of chemical properties and enzyme activities on a timescale of one decade. Plant Soil 2015, 391, 265–282. [Google Scholar] [CrossRef]
- Pellegrino, E.; Bene, C.; Tozzini, C.; Bonari, E. Impact on soil quality of a 10-year-old short-rotation coppice poplar stand compared with intensive agricultural and uncultivated systems in a Mediterranean area. Agric. Ecosyst. Environ. 2011, 140, 245–254. [Google Scholar] [CrossRef]
- D’Acunto, L.; Andrade, J.F.; Poggio, S.L.; Semmartin, M. Diversifying crop rotation increased metabolic soil diversity and activity of the microbial community. Agric. Ecosyst Environ. 2018, 257, 159–164. [Google Scholar] [CrossRef]
- Dodor, D.E.; Kamara, M.S.; Asamoah-Bediako, A.; Adiku, S.G.; MacCarthy, D.S.; Kumahor, S.K.; Neina, D. Evaluation of alkaline hydrolyzable organic nitrogen as an index of nitrogen mineralization potential of some coastal Savannah soils of Ghana. Nitrogen 2022, 3, 652–662. [Google Scholar] [CrossRef]
- Zhang, T.; Wan, S.; Kang, Y.; Feng, H. Urease activity and its relationships to soil physiochemical properties in a highly saline-sodic soil. J. Soil Sci. Plant Nutr. 2014, 14, 304–315. [Google Scholar] [CrossRef]
- Xia, H.Y.; Zhao, J.H.; Sun, J.H.; Xue, Y.F.; Eagling, T.; Bao, X.G.; Zhang, F.S.; Li, L. Maize grain concentrations and above-ground shoot acquisition of micronutrients as affected by intercropping with turnip, faba bean, chickpea, and soybean. Sci. China-Life Sci. 2013, 56, 823–834. [Google Scholar] [CrossRef]
- Heineck, G.C.; Ehlke, N.J.; Altendorf, K.R.; Denison, R.F.; Jungers, J.M.; Lamb, E.G.; Watkins, E. Relationships and influence of yield components on spaced-plant and sward seed yield in perennial ryegrass. Grass Forage Sci. 2020, 75, 424–437. [Google Scholar] [CrossRef]
- Agomoh, I.V.; Drury, C.F.; Yang, X.; Phillips, L.A.; Reynolds, W.D. Crop rotation enhances soybean yields and soil health indicators. Soil Sci. Soc. Am. J. 2021, 85, 1185–1195. [Google Scholar] [CrossRef]

| Soil Traits | Value |
|---|---|
| pH | 8.98 |
| Total salt content (%) | 0.042 |
| Available phosphorus (mg kg−1) | 8.7 |
| Available potassium (mg kg−1) | 159 |
| Organic matter (g kg−1) | 6.66 |
| Total nitrogen (g kg−1) | 0.511 |
| Total potassium (g kg−1) | 19.6 |
| Total phosphorous (g kg−1) | 0.68 |
| Cation exchange capacity (cmol kg−1) | 18.9 |
| Alkalinity (%) | 13.6 |
| Mineral nitrogen (mg kg−1) | 35.2 |
| Soluble carbon (g kg−1) | 0.175 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Ren, A.; Anwar, S.; Shi, L.; Bai, W.; Zhang, Y.; Gao, Z. Optimizing Soil Health and Sorghum Productivity through Crop Rotation with Quinoa. Life 2024, 14, 745. https://doi.org/10.3390/life14060745
Li G, Ren A, Anwar S, Shi L, Bai W, Zhang Y, Gao Z. Optimizing Soil Health and Sorghum Productivity through Crop Rotation with Quinoa. Life. 2024; 14(6):745. https://doi.org/10.3390/life14060745
Chicago/Turabian StyleLi, Guang, Aixia Ren, Sumera Anwar, Lijuan Shi, Wenbin Bai, Yali Zhang, and Zhiqiang Gao. 2024. "Optimizing Soil Health and Sorghum Productivity through Crop Rotation with Quinoa" Life 14, no. 6: 745. https://doi.org/10.3390/life14060745
APA StyleLi, G., Ren, A., Anwar, S., Shi, L., Bai, W., Zhang, Y., & Gao, Z. (2024). Optimizing Soil Health and Sorghum Productivity through Crop Rotation with Quinoa. Life, 14(6), 745. https://doi.org/10.3390/life14060745






