Temporal Progression of Entry Factors into the Vicious Circle of Dry Eye in Untreated Sufferers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample
2.3. Procedures
2.3.1. Tear Meniscus Height
2.3.2. Lipid Layer Pattern
2.3.3. Bulbar Hyperemia
2.4. Statistical Analysis
3. Results
3.1. Analysis of Differences between Sessions in the 8-Year Follow-Up Group
3.2. Analysis of Differences between Sessions in the 6-Year Follow-Up Group
3.3. Analysis of Differences between Sessions in the 4-Year Follow-Up Group
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef]
- Akowuah, P.K.; Kobia-Acquah, E. Prevalence of Dry Eye Disease in Africa: A Systematic Review and Meta-analysis. Optom. Vis. Sci. Off. Publ. Am. Acad. Optom. 2020, 97, 1089–1098. [Google Scholar] [CrossRef]
- Cai, Y.; Wei, J.; Zhou, J.; Zou, W. Prevalence and incidence of dry eye disease in Asia: A systematic review and meta-analysis. Ophthalmic Res. 2022, 65, 647–658. [Google Scholar] [CrossRef]
- Galor, A.; Britten-Jones, A.C.; Feng, Y.; Ferrari, G.; Goldblum, D.; Gupta, P.K.; Merayo-Lloves, J.; Na, K.S.; Naroo, S.A.; Nichols, K.K.; et al. TFOS Lifestyle: Impact of lifestyle challenges on the ocular surface. Ocul. Surf. 2023, 28, 262–303. [Google Scholar] [CrossRef] [PubMed]
- García-Ayuso, D.; Di Pierdomenico, J.; Moya-Rodríguez, E.; Valiente-Soriano, F.J.; Galindo-Romero, C.; Sobrado-Calvo, P. Assessment of dry eye symptoms among university students during the COVID-19 pandemic. Clin. Exp. Optom. 2021, 105, 507–513. [Google Scholar] [CrossRef]
- Garcia-Queiruga, J.; Pena-Verdeal, H.; Sabucedo-Villamarin, B.; Giraldez, M.J.; Garcia-Resua, C.; Yebra-Pimentel, E. Meibomian gland secretion quality association with ocular parameters in university students during COVID-19 restrictions. Int. Ophthalmol. 2023, 43, 2349–2362. [Google Scholar] [CrossRef]
- Garcia-Queiruga, J.; Pena-Verdeal, H.; Sabucedo-Villamarin, B.; Giraldez, M.J.; Garcia-Resua, C.; Yebra-Pimentel, E. A cross-sectional study of non-modifiable and modifiable risk factors of dry eye disease states. Contact Lens Anterior Eye 2023, 46, 101800. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.; Efron, N.; Bandamwar, K.; Barnett, M.; Jacobs, D.S.; Jalbert, I.; Pult, H.; Rhee, M.K.; Sheardown, H.; Shovlin, J.P.; et al. TFOS Lifestyle: Impact of contact lenses on the ocular surface. Ocul. Surf. 2023, 29, 175–219. [Google Scholar] [CrossRef] [PubMed]
- Wolffsohn, J.S.; Arita, R.; Chalmers, R.; Djalilian, A.; Dogru, M.; Dumbleton, K.; Gupta, P.K.; Karpecki, P.; Lazreg, S.; Pult, H.; et al. TFOS DEWS II Diagnostic Methodology report. Ocul. Surf. 2017, 15, 539–574. [Google Scholar] [CrossRef]
- Bron, A.J.; de Paiva, C.S.; Chauhan, S.K.; Bonini, S.; Gabison, E.E.; Jain, S.; Knop, E.; Markoulli, M.; Ogawa, Y.; Perez, V.; et al. TFOS DEWS II pathophysiology report. Ocul. Surf. 2017, 15, 438–510. [Google Scholar] [CrossRef]
- Baudouin, C.; Messmer, E.M.; Aragona, P.; Geerling, G.; Akova, Y.A.; Benítez-del-Castillo, J.; Boboridis, K.G.; Merayo-Lloves, J.; Rolando, M.; Labetoulle, M. Revisiting the vicious circle of dry eye disease: A focus on the pathophysiology of meibomian gland dysfunction. Br. J. Ophthalmol. 2016, 100, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Rolando, M.; Merayo-Lloves, J. Management Strategies for Evaporative Dry Eye Disease and Future Perspective. Curr. Eye Res. 2022, 47, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Vehof, J.; Utheim, T.P.; Bootsma, H.; Hammond, C.J. Advances, limitations and future perspectives in the diagnosis and management of dry eye in Sjogren’s syndrome. Clin. Exp. Rheumatol. 2020, 38 (Suppl. S126), 301–309. [Google Scholar] [PubMed]
- Barabino, S.; Benitez-Del-Castillo, J.M.; Fuchsluger, T.; Labetoulle, M.; Malachkova, N.; Meloni, M.; Utheim, T.P.; Rolando, M. Dry eye disease treatment: The role of tear substitutes, their future, and an updated classification. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 8642–8652. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Angelina, A.; Marrone, M.; Stark, W.J.; Akpek, E.K. Autologous serum eye drops for dry eye. Cochrane Database Syst. Rev. 2017, 2, CD009327. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cao, K.; Wei, Z.; Baudouin, C.; Labbe, A.; Liang, Q. Autologous Serum Eye Drops versus Artificial Tear Drops for Dry Eye Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Ophthalmic Res. 2020, 63, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Sabucedo-Villamarin, B.; Pena-Verdeal, H.; Garcia-Queiruga, J.; Giraldez, M.J.; Garcia-Resua, C.; Yebra-Pimentel, E. Longitudinal analysis of variation in status and diagnostic stability of untreated dry eye disease. Ocul. Surf. 2023, 29, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Wolffsohn, J.S.; Wang, M.T.M.; Vidal-Rohr, M.; Menduni, F.; Dhallu, S.; Ipek, T.; Acar, D.; Recchioni, A.; France, A.; Kingsnorth, A.; et al. Demographic and lifestyle risk factors of dry eye disease subtypes: A cross-sectional study. Ocul. Surf. 2021, 21, 58–63. [Google Scholar] [CrossRef]
- McMonnies, C.W. Conjunctival Tear Layer Temperature, Evaporation, Hyperosmolarity, Inflammation, Hyperemia, Tissue Damage, and Symptoms: A Review of an Amplifying Cascade. Curr. Eye Res. 2017, 42, 1574–1584. [Google Scholar] [CrossRef]
- Talens-Estarelles, C.; Cervino, A.; Garcia-Lazaro, S.; Fogelton, A.; Sheppard, A.; Wolffsohn, J.S. The effects of breaks on digital eye strain, dry eye and binocular vision: Testing the 20-20-20 rule. Contact Lens Anterior Eye 2023, 46, 101744. [Google Scholar] [CrossRef]
- Schiffman, R.M.; Christianson, M.D.; Jacobsen, G.; Hirsch, J.D.; Reis, B.L. Reliability and validity of the Ocular Surface Disease Index. Arch. Ophthalmol. 2000, 118, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Beltran, F.; Betancourt, N.R.; Martinez, J.; Valdes, C.S.; Babayan, A.; Ramírez-Assad, C.; Juarez, E.M.; Hernandez-Quintela, E.; Mexican Ocular Surface Disease Study Group. Transcultural validation of ocular surface disease index (osdi) questionnaire for mexican population. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6050. [Google Scholar]
- Gomes, J.A.P.; Azar, D.T.; Baudouin, C.; Bitton, E.; Chen, W.; Hafezi, F.; Hamrah, P.; Hogg, R.E.; Horwath-Winter, J.; Kontadakis, G.A.; et al. TFOS Lifestyle: Impact of elective medications and procedures on the ocular surface. Ocul. Surf. 2023, 29, 331–385. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Qu, J.H.; Zhang, X.Y.; Sun, X.G. Repeatability and Reproducibility of Noninvasive Keratograph 5M Measurements in Patients with Dry Eye Disease. J. Ophthalmol. 2016, 2016, 8013621. [Google Scholar] [CrossRef] [PubMed]
- Guillon, J.P. Use of the Tearscope Plus and attachments in the routine examination of the marginal dry eye contact lens patient. Adv. Exp. Med. Biol. 1998, 438, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Sabucedo-Villamarin, B.; Pena-Verdeal, H.; Garcia-Queiruga, J.; Giraldez, M.J.; Garcia-Resua, C.; Yebra-Pimentel, E. Categorization of the Aqueous Deficient Dry Eye by a Cut-Off Criterion of TMH Measured with Tearscope. Life 2022, 12, 2007. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Resua, C.; Pena-Verdeal, H.; Minones, M.; Giraldez, M.J.; Yebra-Pimentel, E. Interobserver and intraobserver repeatability of lipid layer pattern evaluation by two experienced observers. Contact Lens Anterior Eye 2014, 37, 431–437. [Google Scholar] [CrossRef]
- Guillon, J.-P. Non-invasive tearscope plus routine for contact lens fitting. Contact Lens Anterior Eye 1998, 21, S31–S40. [Google Scholar] [CrossRef] [PubMed]
- Terry, R.L.; Schnider, C.M.; Holden, B.A.; Cornish, R.; Grant, T.; Sweeney, D.; La Hood, D.; Back, A. CCLRU standards for success of daily and extended wear contact lenses. Optom. Vis. Sci. Off. Publ. Am. Acad. Optom. 1993, 70, 234–243. [Google Scholar] [CrossRef]
- Armstrong, R.A.; Davies, L.N.; Dunne, M.C.; Gilmartin, B. Statistical guidelines for clinical studies of human vision. Ophthalmic Physiol. Opt. 2011, 31, 123–136. [Google Scholar] [CrossRef]
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II Epidemiology Report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef]
- Bron, A.J.; Yokoi, N.; Gafney, E.; Tiffany, J.M. Predicted phenotypes of dry eye: Proposed consequences of its natural history. Ocul. Surf. 2009, 7, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.; Yang, M.; Rootman, D. Natural history of inflammatory and non-inflammatory dry eye in thyroid eye disease. Orbit 2021, 40, 389–393. [Google Scholar] [CrossRef]
- Lienert, J.P.; Tarko, L.; Uchino, M.; Christen, W.G.; Schaumberg, D.A. Long-term Natural History of Dry Eye Disease from the Patient’s Perspective. Ophthalmology 2016, 123, 425–433. [Google Scholar] [CrossRef]
- Pflugfelder, S.C.; Stern, M.E. Biological functions of tear film. Exp. Eye Res. 2020, 197, 108115. [Google Scholar] [CrossRef]
- Miller, W.L.; Doughty, M.J.; Narayanan, S.; Leach, N.E.; Tran, A.; Gaume, A.L.; Bergmanson, J.P. A comparison of tear volume (by tear meniscus height and phenol red thread test) and tear fluid osmolality measures in non-lens wearers and in contact lens wearers. Eye Contact Lens 2004, 30, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Bilkhu, P.; Wolffsohn, J.; Purslow, C. Provocation of the ocular surface to investigate the evaporative pathophysiology of dry eye disease. Contact Lens Anterior Eye 2021, 44, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Du, C.; Zhu, D.; Shen, M.; Cui, L.; Wang, J. Daytime variations of tear osmolarity and tear meniscus volume. Eye Contact Lens 2012, 38, 282–287. [Google Scholar] [CrossRef]
- Pult, H. Relationships Between Meibomian Gland Loss and Age, Sex, and Dry Eye. Eye Contact Lens 2018, 44 (Suppl. S2), S318–S324. [Google Scholar] [CrossRef]
- Markoulli, M.; Duong, T.B.; Lin, M.; Papas, E. Imaging the Tear Film: A Comparison Between the Subjective Keeler Tearscope-Plus and the Objective Oculus(R) Keratograph 5M and LipiView(R) Interferometer. Curr. Eye Res. 2018, 43, 155–162. [Google Scholar] [CrossRef]
- Singh, S.; Srivastav, S.; Modiwala, Z.; Ali, M.H.; Basu, S. Repeatability, reproducibility and agreement between three different diagnostic imaging platforms for tear film evaluation of normal and dry eye disease. Eye 2023, 37, 2042–2047. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.B.; Liu, L.; Anchouche, S.; Yung, A.; Mittal, S.K.; Blanco, T.; Dohlman, T.H.; Yin, J.; Dana, R. Ocular redness—I: Etiology, pathogenesis, and assessment of conjunctival hyperemia. Ocul. Surf. 2021, 21, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Queiruga, J.; Pena-Verdeal, H.; Sabucedo-Villamarin, B.; Garcia-Resua, C.; Giraldez, M.J.; Yebra-Pimentel, E. Analysis of the Differences in Ocular Surface Damage and Inflammatory Signs between Healthy and Evaporative Dry Eye Participants. Ocul. Immunol. Inflamm. 2023, 31, 970–977. [Google Scholar] [CrossRef] [PubMed]
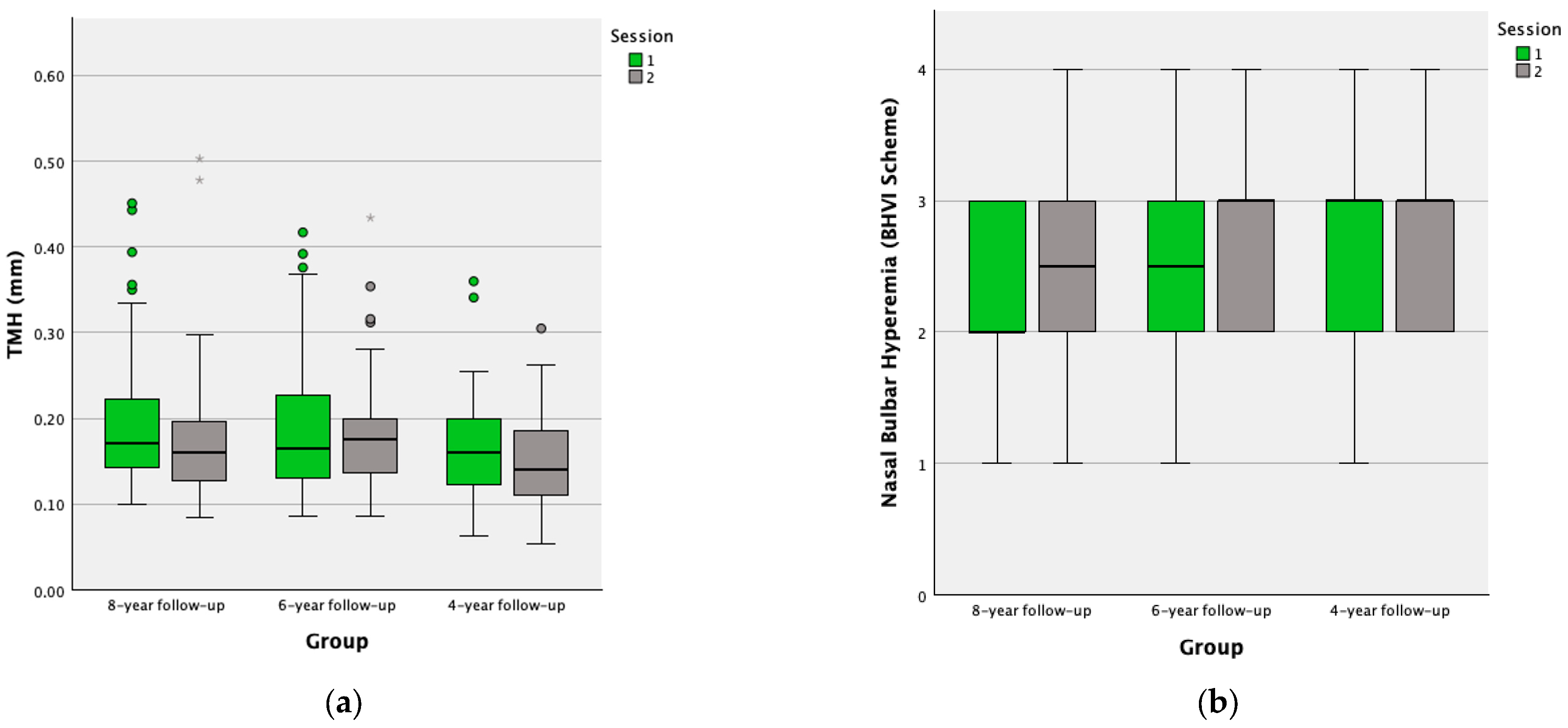

| Ocular Parameter | N | Session | Mean ± SD | Median (IQR) | p-Value | |
|---|---|---|---|---|---|---|
| TMH (mm) | 61 | 1 | 0.20 ± 0.79 | 0.18 (0.15–0.23) | 0.006 * | |
| 2 | 0.18 ± 0.79 | 0.17 (0.13–0.20) | ||||
| Bulbar hyperemia (BHVI Scheme) | Nasal | 34 | 1 | 2.18 ± 0.67 | 2 (2–3) | 0.010 * |
| 2 | 2.59 ± 0.60 | 3 (2–3) | ||||
| Temporal | 34 | 1 | 2.21 ± 0.81 | 2 (2–3) | 0.027 * | |
| 2 | 2.53 ± 0.71 | 2 (2–3) | ||||
| Mean | 34 | 1 | 2.19 ± 0.65 | 2 (1.88–2.50) | 0.003 * | |
| 2 | 2.56 ± 0.56 | 2.50 (2–3) | ||||
| LLP (Guillon’s Scheme) | 38 | 1 | 3.13 ± 1.32 | 3 (2–4) | 0.016 * | |
| 2 | 2.50 ± 1.20 | 2 (2–3) | ||||
| Ocular Parameter | N | Session | Mean ± SD | Median (IQR) | p-Value | |
|---|---|---|---|---|---|---|
| TMH (mm) | 30 | 1 | 0.20 ± 0.09 | 0.18 (0.13–0.26) | 0.165 | |
| 2 | 0.19 ± 0.08 | 0.18 (0.14–0.20) | ||||
| Bulbar hyperemia (BHVI Scheme) | Nasal | 30 | 1 | 2.47 ± 0.82 | 3 (2–3) | 0.022 * |
| 2 | 2.83 ± 0.65 | 3 (2–3) | ||||
| Temporal | 30 | 1 | 2.37 ± 0.67 | 2 (2–3) | 0.007 * | |
| 2 | 2.80 ± 0.71 | 3 (2–3) | ||||
| Mean | 30 | 1 | 2.42 ± 0.66 | 2.50 (2–3) | 0.002 * | |
| 2 | 2.82 ± 0.61 | 3 (2.38–3.13) | ||||
| LLP (Guillon’s Scheme) | 30 | 1 | 2.93 ± 1.34 | 3 (2–4) | 0.009 * | |
| 2 | 2.17 ± 1.09 | 2 (1–2.25) | ||||
| Ocular Parameter | N | Session | Mean ± SD | Median (IQR) | p-Value | |
|---|---|---|---|---|---|---|
| TMH (mm) | 51 | 1 | 0.16 ± 0.61 | 0.16 (0.11–0.20) | 0.189 | |
| 2 | 0.15 ± 0.05 | 0.14 (0.11–0.18) | ||||
| Bulbar Hyperemia (BHVI Scheme) | Nasal | 55 | 1 | 2.56 ± 0.66 | 3 (2–3) | 0.382 |
| 2 | 2.66 ± 0.67 | 3 (2–3) | ||||
| Temporal | 55 | 1 | 2.35 ± 0.70 | 2 (2–3) | 0.714 | |
| 2 | 2.38 ± 0.71 | 2 (2–3) | ||||
| Mean | 55 | 1 | 2.45 ± 0.59 | 2.50 (2–3) | 0.601 | |
| 2 | 2.52 ± 0.62 | 2.50 (2–3) | ||||
| LLP (Guillon’s Scheme) | 54 | 1 | 3.04 ± 1.30 | 2 (2–4) | <0.001 * | |
| 2 | 2.26 ± 1.17 | 2 (1.75–3) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Queiruga, J.; Pena-Verdeal, H.; Sabucedo-Villamarin, B.; Garcia-Resua, C.; Giraldez, M.J.; Yebra-Pimentel, E. Temporal Progression of Entry Factors into the Vicious Circle of Dry Eye in Untreated Sufferers. Life 2024, 14, 806. https://doi.org/10.3390/life14070806
Garcia-Queiruga J, Pena-Verdeal H, Sabucedo-Villamarin B, Garcia-Resua C, Giraldez MJ, Yebra-Pimentel E. Temporal Progression of Entry Factors into the Vicious Circle of Dry Eye in Untreated Sufferers. Life. 2024; 14(7):806. https://doi.org/10.3390/life14070806
Chicago/Turabian StyleGarcia-Queiruga, Jacobo, Hugo Pena-Verdeal, Belen Sabucedo-Villamarin, Carlos Garcia-Resua, Maria J. Giraldez, and Eva Yebra-Pimentel. 2024. "Temporal Progression of Entry Factors into the Vicious Circle of Dry Eye in Untreated Sufferers" Life 14, no. 7: 806. https://doi.org/10.3390/life14070806








