Physiological Adaptation of Chromochloris zofingiensis in Three-Phased Cultivation Performed in a Pilot-Scale Photobioreactor
Abstract
:1. Introduction
2. Materials and Methods
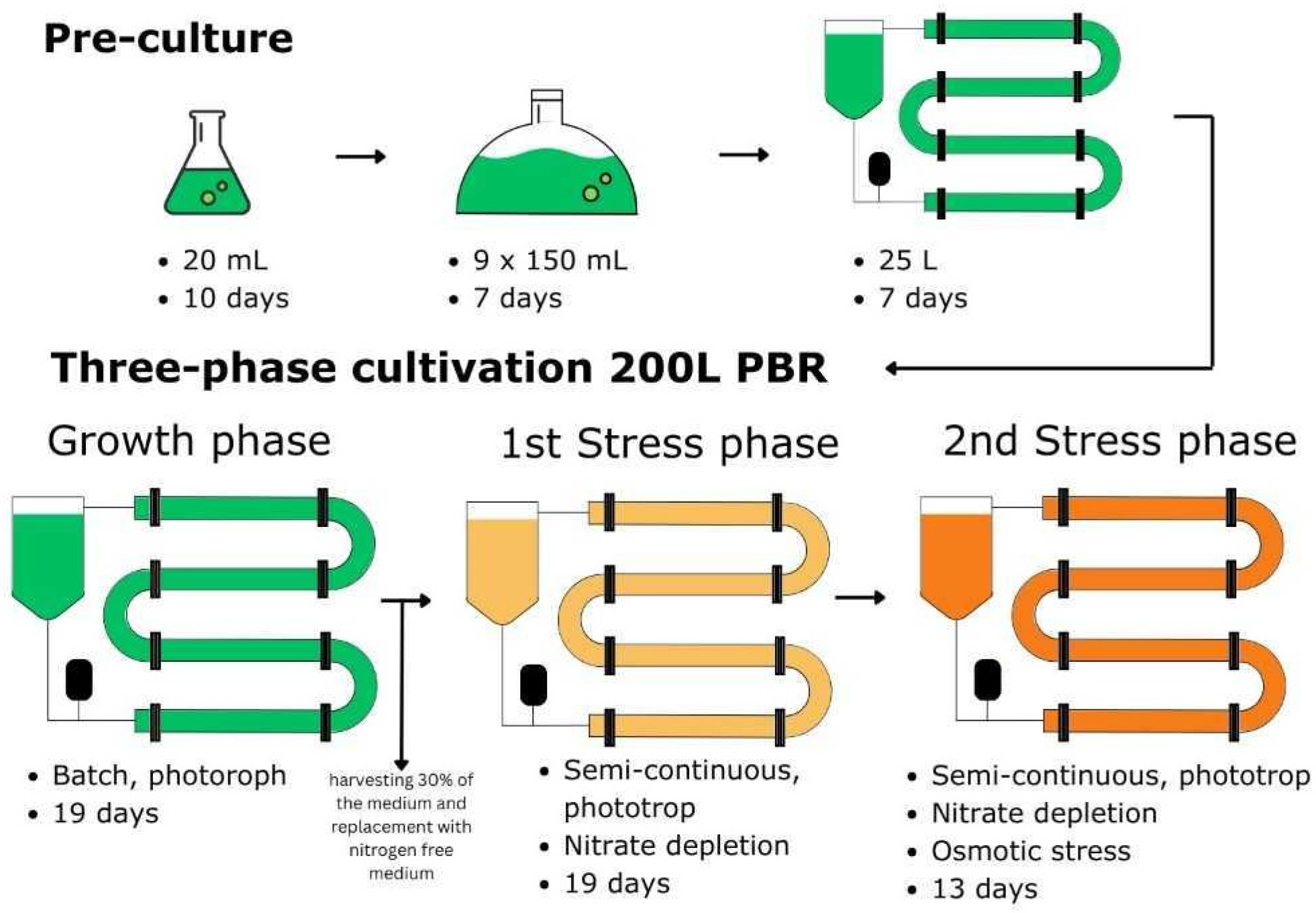
2.1. Microalgae Strain and Cultivation Conditions
2.2. Analytics
2.2.1. Biomass Dry Weight (DW) Concentration
2.2.2. Quantification of Nitrate
2.2.3. Pigments Extraction and Quantification
2.2.4. Water and Ash Content
2.2.5. Elemental Analysis and Protein Content
2.2.6. Total Lipid Content
3. Results and Discussion
3.1. State-of-the-Art of Chromochloris Zofingiensis Performance
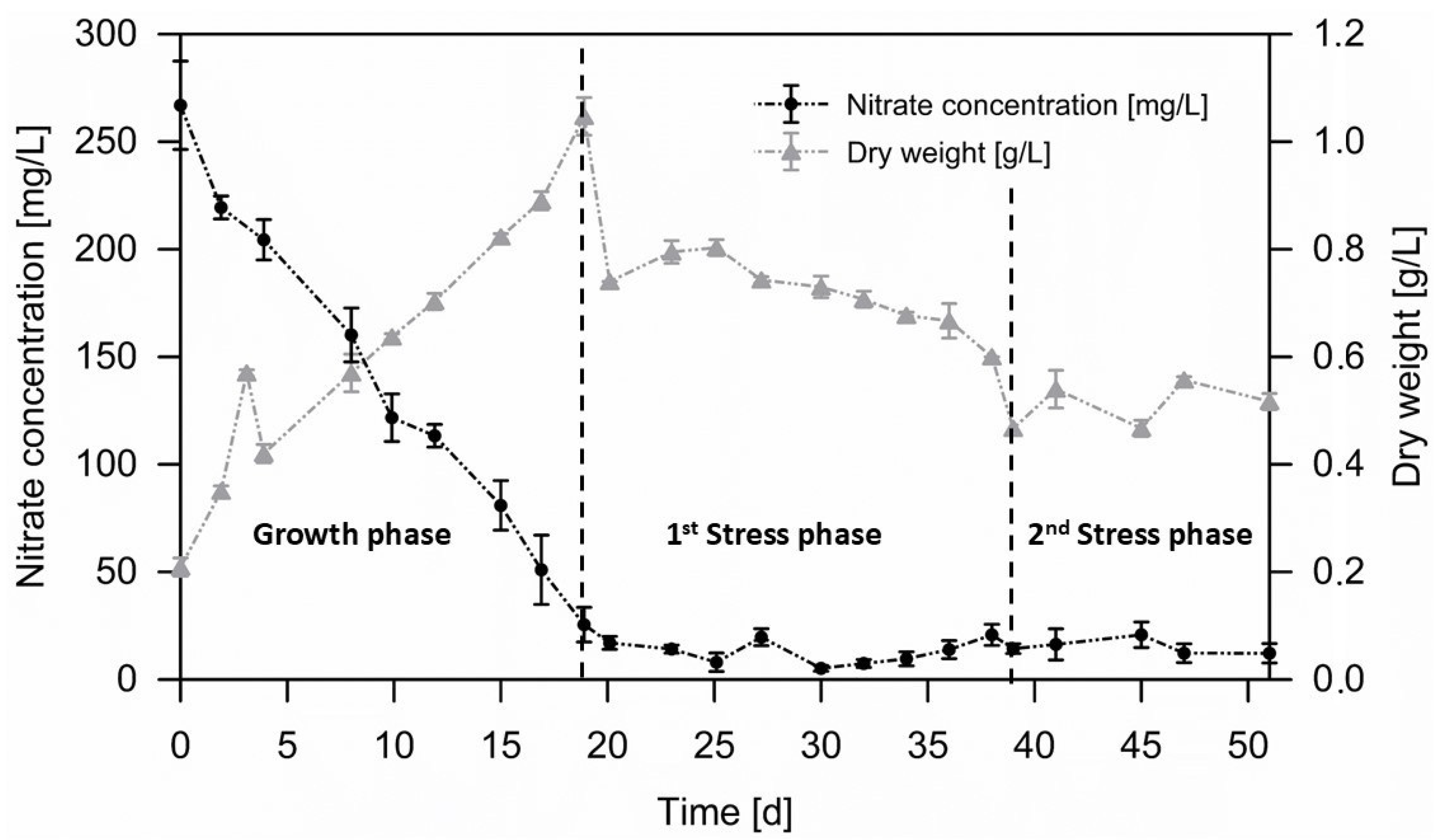
3.2. Biomass Accumulation Phase
3.3. Pigments
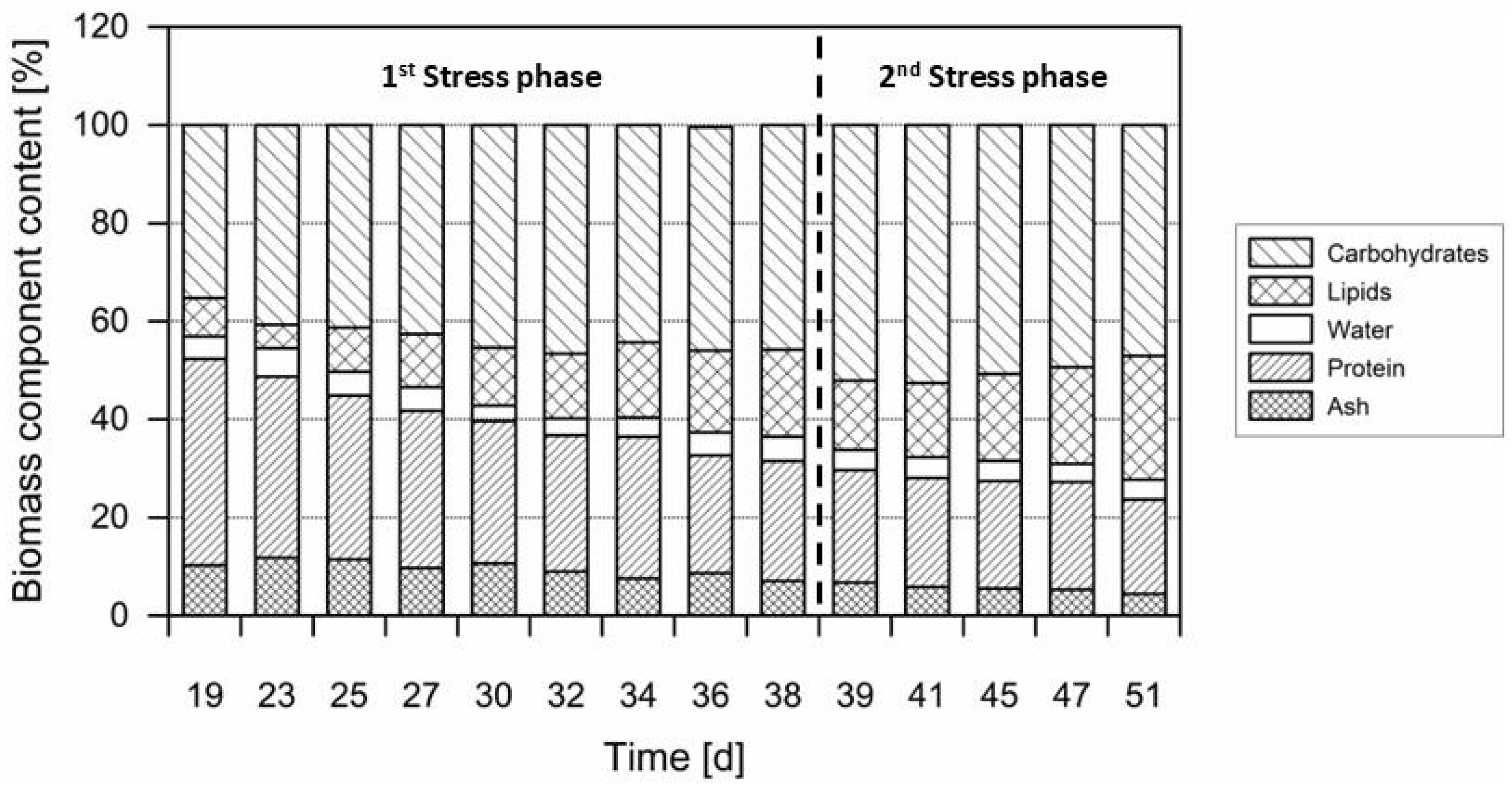
3.4. Macroelements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments

Conflicts of Interest
References
- Mendes, M.C.; Navalho, S.; Ferreira, A.; Paulino, C.; Figueiredo, D.; Silva, D.; Gao, F.; Gama, F.; Bombo, G.; Jacinto, R.; et al. Algae as Food in Europe: An Overview of Species Diversity and Their Application. Foods 2022, 11, 1871. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.J.; Janssen, M.; Südfeld, C.; D’Adamo, S.; Wijffels, R.H. Hypes, Hopes, and the Way Forward for Microalgal Biotechnology. Trends Biotechnol. 2023, 41, 452–471. [Google Scholar] [CrossRef]
- Fatima, V.C.; Rita, D.S.F.D.A.; Javier, S.L.; Jordi, G.G. Algae Industry in Europe; European Commission: Ispra, Italy, 2022. [Google Scholar]
- Chen, C.; Tang, T.; Shi, Q.; Zhou, Z.; Fan, J. The Potential and Challenge of Microalgae as Promising Future Food Sources. Trends Food Sci. Technol. 2022, 126, 99–112. [Google Scholar] [CrossRef]
- Zhou, L.; Li, K.; Duan, X.; Hill, D.; Barrow, C.; Dunshea, F.; Martin, G.; Suleria, H. Bioactive Compounds in Microalgae and Their Potential Health Benefits. Food Biosci. 2022, 49, 101932. [Google Scholar] [CrossRef]
- Mendes, A.; Lopes da Silva, T.; Reis, A. DHA Concentration and Purification from the Marine Heterotrophic Microalga Crypthecodinium Cohnii CCMP 316 by Winterization and Urea Complexation. Food Technol. Biotechnol. 2006, 45, 38–44. [Google Scholar]
- Kumar, R.; Hegde, A.S.; Sharma, K.; Parmar, P.; Srivatsan, V. Microalgae as a Sustainable Source of Edible Proteins and Bioactive Peptides—Current Trends and Future Prospects. Food Res. Int. 2022, 157, 111338. [Google Scholar] [CrossRef]
- Becker, E.W. Micro-Algae as a Source of Protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tibbetts, S.M.; McGinn, P.J. Microalgae as Sources of High-Quality Protein for Human Food and Protein Supplements. Foods 2021, 10, 3002. [Google Scholar] [CrossRef]
- Liu, Y.; Ren, X.; Fan, C.; Wu, W.; Zhang, W.; Wang, Y. Health Benefits, Food Applications, and Sustainability of Microalgae-Derived N-3 PUFA. Foods 2022, 11, 1883. [Google Scholar] [CrossRef]
- Buono, S.; Langellotti, A.L.; Martello, A.; Rinna, F.; Fogliano, V. Functional Ingredients from Microalgae. Food Funct. 2014, 5, 1669–1685. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, M.; Fang, Z. Recent Development in Efficient Processing Technology for Edible Algae: A Review. Trends Food Sci. Technol. 2019, 88, 251–259. [Google Scholar] [CrossRef]
- Huo, S.; Wang, Z.; Zhu, S.; Shu, Q.; Zhu, L.; Qin, L.; Zhou, W.; Feng, P.; Zhu, F.; Yuan, Z.; et al. Biomass Accumulation of Chlorella Zofingiensis G1 Cultures Grown Outdoors in Photobioreactors. Front. Energy Res. 2018, 6, 49. [Google Scholar] [CrossRef]
- Wood, E.E.; Ross, M.E.; Jubeau, S.; Montalescot, V.; MacKechnie, K.; Marchington, R.E.; Davey, M.P.; McNeill, S.; Hamilton, C.; Stanley, M.S. From Green to Orange: The Change in Biochemical Composition of Phototrophic-Mixotrophic Chromochloris Zofingiensis in Pilot-Scale Photobioreactors. Algal Res. 2023, 75, 103238. [Google Scholar] [CrossRef]
- Patel, A.K.; Albarico, F.P.J.B.; Perumal, P.K.; Vadrale, A.P.; Nian, C.T.; Chau, H.T.B.; Anwar, C.; Wani, H.M.U.D.; Pal, A.; Saini, R.; et al. Algae as an Emerging Source of Bioactive Pigments. Bioresour. Technol. 2022, 351, 126910. [Google Scholar] [CrossRef] [PubMed]
- Jung, F.; Braune, S.; Jung, C.H.G.; Krüger-Genge, A.; Waldeck, P.; Petrick, I.; Küpper, J.-H. Lipophilic and Hydrophilic Compounds from Arthrospira Platensis and Its Effects on Tissue and Blood Cells—An Overview. Life 2022, 12, 1497. [Google Scholar] [CrossRef]
- Mimouni, V.; Ulmann, L.; Pasquet, V.; Mathieu, M.; Picot, L.; Bougaran, G.; Cadoret, J.-P.; Morant-Manceau, A.; Schoefs, B. The Potential of Microalgae for the Production of Bioactive Molecules of Pharmaceutical Interest. Curr. Pharm. Biotechnol. 2012, 13, 2733–2750. [Google Scholar] [CrossRef]
- Sun, D.; Wu, S.; Li, X.; Ge, B.; Zhou, C.; Yan, X.; Ruan, R.; Cheng, P. The Structure, Functions and Potential Medicinal Effects of Chlorophylls Derived from Microalgae. Mar. Drugs 2024, 22, 65. [Google Scholar] [CrossRef]
- Tounsi, L.; Ben Hlima, H.; Hentati, F.; Hentati, O.; Derbel, H.; Michaud, P.; Abdelkafi, S. Microalgae: A Promising Source of Bioactive Phycobiliproteins. Mar. Drugs 2023, 21, 440. [Google Scholar] [CrossRef]
- Huss, V.A.R.; Frank, C.; Hartmann, E.C.; Hirmer, M.; Kloboucek, A.; Seidel, B.M.; Wenzeler, P.; Kessler, E. Biochemical Taxonomy And Molecular Phylogeny Of The Genus Chlorella Sensu Lato (Chlorophyta). J. Phycol. 1999, 35, 587–598. [Google Scholar] [CrossRef]
- Koren, I.; Boussiba, S.; Khozin-Goldberg, I.; Zarka, A. Chromochloris Zofingiensis (Chlorophyceae) Divides by Consecutive Multiple Fission Cell-Cycle under Batch and Continuous Cultivation. Biology 2021, 10, 157. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, Y.; Bai, F.; Liu, J. The Oleaginous Astaxanthin-Producing Alga Chromochloris Zofingiensis: Potential from Production to an Emerging Model for Studying Lipid Metabolism and Carotenogenesis. Biotechnol. Biofuels 2021, 14, 119. [Google Scholar] [CrossRef] [PubMed]
- Wood, E.E.; Ross, M.E.; Jubeau, S.; Montalescot, V.; Stanley, M.S. Progress towards a Targeted Biorefinery of Chromochloris Zofingiensis: A Review. Biomass Conv. Bioref. 2024, 14, 8127–8152. [Google Scholar] [CrossRef]
- Imamoglu, E.; Vardar-Sukan, F.; Dalay, M.C. Effect of Different Culture Media and Light Intensities on Growth of Haematococcus Pluvialis. Int. J. Nat. Eng. Sci. 2007, 1, 5–9. [Google Scholar]
- Vitali, L.; Lolli, V.; Sansone, F.; Kumar, A.; Concas, A.; Lutzu, G.A. Lipid Content and Fatty Acid Methyl Ester Profile by Chromochloris Zofingiensis under Chemical and Metabolic Stress. Biomass Conv. Bioref. 2023, 15, 4941–4954. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, Y.; Hu, Q.; Han, D. Metabolomic Analysis Reveals Astaxanthin Biosynthesis in Heterotrophic Microalga Chromochloris Zofingiensis. Bioresour. Technol. 2023, 374, 128811. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, J.; Yang, S.; Liang, Q.; Gu, Z.; Wang, Y.; Mou, H.; Sun, H. Selecting a Preculture Strategy for Improving Biomass and Astaxanthin Productivity of Chromochloris Zofingiensis. Appl. Microbiol. Biotechnol. 2024, 108, 117. [Google Scholar] [CrossRef]
- Ibañez, M.V.; Leonardi, R.J.; Heinrich, J.M.; Steingroewer, J.; Walther, T.; Felix, K. A Rapid Assessment of the Radiative Properties from a Suspension of Chromochloris Zofingiensis. J. Photochem. Photobiol. 2020, 3–4, 100007. [Google Scholar] [CrossRef]
- Bleisch, R.; Mühlstädt, G.; Hilpmann, G.; Seibel, L.; Steingröwer, J.; Zahn, S.; Wagemans, A.M.; Krujatz, F. A Robust, Non-Invasive and Fast Routine for the Quantification of the Nutritional Composition of Microalgae Biomass Slurries Based on near-Infrared Spectroscopy. Algal Res. 2025, 85, 103882. [Google Scholar] [CrossRef]
- Witthohn, M.; Schmidt, A.-K.; Strieth, D.; Ulber, R.; Muffler, K. A Modified Method for a Fast and Economic Determination of Nitrate Concentrations in Microalgal Cultures. Algal Res. 2023, 69, 102957. [Google Scholar] [CrossRef]
- Franke, S.; Steingröwer, J.; Walther, T.; Krujatz, F. The Oxygen Paradigm—Quantitative Impact of High Concentrations of Dissolved Oxygen on Kinetics and Large-Scale Production of Arthrospira Platensis. ChemEngineering 2022, 6, 14. [Google Scholar] [CrossRef]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as Well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Li, Y.; Miao, F.; Geng, Y.; Lu, D.; Zhang, C.; Zeng, M. Accurate Quantification of Astaxanthin from Haematococcus Crude Extract Spectrophotometrically. Chin. J. Ocean. Limnol. 2012, 30, 627–637. [Google Scholar] [CrossRef]
- Casella, P.; Iovine, A.; Mehariya, S.; Marino, T.; Musmarra, D.; Molino, A. Smart Method for Carotenoids Characterization in Haematococcus Pluvialis Red Phase and Evaluation of Astaxanthin Thermal Stability. Antioxidants 2020, 9, 422. [Google Scholar] [CrossRef] [PubMed]
- Templeton, D.W.; Laurens, L.M. Nitrogen-to-Protein Conversion Factors Revisited for Applications of Microalgal Biomass Conversion to Food, Feed and Fuel. Algal Res. 2015, 11, 359–367. [Google Scholar] [CrossRef]
- Sägesser, C.; Kallfelz, J.M.; Boulos, S.; Hammer, L.; Böcker, L.; Portmann, R.; Nyström, L.; Mathys, A. A Novel Approach for the Protein Determination in Food-Relevant Microalgae. Bioresour. Technol. 2023, 390, 129849. [Google Scholar] [CrossRef]
- Safi, C.; Charton, M.; Pignolet, O.; Silvestre, F.; Vaca-Garcia, C.; Pontalier, P.-Y. Influence of Microalgae Cell Wall Characteristics on Protein Extractability and Determination of Nitrogen-to-Protein Conversion Factors. J. Appl. Phycol. 2013, 25, 523–529. [Google Scholar] [CrossRef]
- Jensen, W.B. The Origin of the Soxhlet Extractor. J. Chem. Educ. 2007, 84, 1913. [Google Scholar] [CrossRef]
- Yang, F.; Cheng, C.; Long, L.; Hu, Q.; Jia, Q.; Wu, H.; Xiang, W. Extracting Lipids from Several Species of Wet Microalgae Using Ethanol at Room Temperature. Energy Fuels 2015, 29, 2380–2386. [Google Scholar] [CrossRef]
- Zemke, P.E.; Sommerfeld, M.R.; Hu, Q. Assessment of Key Biological and Engineering Design Parameters for Production of Chlorella Zofingiensis (Chlorophyceae) in Outdoor Photobioreactors. Appl. Microbiol. Biotechnol. 2013, 97, 5645–5655. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, Y.; Xu, Q.; Jin, H.; Hu, Q.; Han, D. Effective Two-Stage Heterotrophic Cultivation of the Unicellular Green Microalga Chromochloris Zofingiensis Enabled Ultrahigh Biomass and Astaxanthin Production. Front. Bioeng. Biotechnol. 2022, 10, 834230. [Google Scholar] [CrossRef]
- Huo, S.; Wang, Z.; Zhu, S.; Zhou, W.; Dong, R.; Yuan, Z. Cultivation of Chlorella Zofingiensis in Bench-Scale Outdoor Ponds by Regulation of pH Using Dairy Wastewater in Winter, South China. Bioresour. Technol. 2012, 121, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Deng, Z.; Hu, Z.; Fan, L. Lipid Accumulation and Growth of Chlorella Zofingiensis in Flat Plate Photobioreactors Outdoors. Bioresour. Technol. 2011, 102, 10577–10584. [Google Scholar] [CrossRef] [PubMed]
- Ajala, S.O.; Alexander, M.L. Assessment of Chlorella Vulgaris, Scenedesmus Obliquus, and Oocystis Minuta for Removal of Sulfate, Nitrate, and Phosphate in Wastewater. Int. J. Energy Environ. Eng. 2020, 11, 311–326. [Google Scholar] [CrossRef]
- Minyuk, G.; Sidorov, R.; Solovchenko, A. Effect of Nitrogen Source on the Growth, Lipid, and Valuable Carotenoid Production in the Green Microalga Chromochloris Zofingiensis. J. Appl. Phycol. 2020, 32, 923–935. [Google Scholar] [CrossRef]
- Ihadjadene, Y.; Ascoli, L.; Syed, T.; Urbas, L.; Walther, T.; Mühlstädt, G.; Streif, S.; Krujatz, F. Experimental and Model-Based Parameterization of the Fundamental Process Kinetics of Chromochloris Zofingiensis. Algal Res. 2025, 88, 104012. [Google Scholar] [CrossRef]
- Sedjati, S.; Santosa, G.; Yudiati, E.; Supriyantini, E.; Ridlo, A.; Kimberly, F. Chlorophyll and Carotenoid Content of Dunaliella Salina at Various Salinity Stress and Harvesting Time. IOP Conf. Ser. Earth Environ. Sci. 2019, 246, 012025. [Google Scholar] [CrossRef]
- Yuan, J.; Peng, J.; Yin, K.; Wang, J. Potential Health-promoting Effects of Astaxanthin: A High-value Carotenoid Mostly from Microalgae. Mol. Nutr. Food Res. 2011, 55, 150–165. [Google Scholar] [CrossRef]
- Del Campo, J.A.; Rodríguez, H.; Moreno, J.; Vargas, M.Á.; Rivas, J.; Guerrero, M.G. Accumulation of Astaxanthin and Lutein in Chlorella Zofingiensis (Chlorophyta). Appl. Microbiol. Biotechnol. 2004, 64, 848–854. [Google Scholar] [CrossRef]
- Li, Q.; You, J.; Qiao, T.; Zhong, D.-B.; Yu, X. Sodium Chloride Stimulates the Biomass and Astaxanthin Production by Haematococcus Pluvialis via a Two-Stage Cultivation Strategy. Bioresour. Technol. 2022, 344, 126214. [Google Scholar] [CrossRef]
- Ariyadasa, T.U.; Thevarajah, B.; Anthonio, R.A.D.P.; Nimarshana, P.H.V.; Wasath, W.A.J. From Present to Prosperity: Assessing the Current Status and Envisioning Opportunities in the Industrial-Scale Cultivation of Haematococcus Pluvialis for Astaxanthin Production. Phytochem. Rev. 2024, 23, 749–779. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, D.; Zhang, Y.; Chen, F. Glucose Triggers Cell Structure Changes and Regulates Astaxanthin Biosynthesis in Chromochloris Zofingiensis. Algal Res. 2019, 39, 101455. [Google Scholar] [CrossRef]
- Mulders, K.J.M.; Janssen, J.H.; Martens, D.E.; Wijffels, R.H.; Lamers, P.P. Effect of Biomass Concentration on Secondary Carotenoids and Triacylglycerol (TAG) Accumulation in Nitrogen-Depleted Chlorella Zofingiensis. Algal Res. 2014, 6, 8–16. [Google Scholar] [CrossRef]
- Sun, N.; Wang, Y.; Li, Y.-T.; Huang, J.-C.; Chen, F. Sugar-Based Growth, Astaxanthin Accumulation and Carotenogenic Transcription of Heterotrophic Chlorella Zofingiensis (Chlorophyta). Process Biochem. 2008, 43, 1288–1292. [Google Scholar] [CrossRef]
- Chen, J.-H.; Wei, D.; Lim, P.-E. Enhanced Coproduction of Astaxanthin and Lipids by the Green Microalga Chromochloris Zofingiensis: Selected Phytohormones as Positive Stimulators. Bioresour. Technol. 2020, 295, 122242. [Google Scholar] [CrossRef]
- Zhu, S.; Huang, W.; Xu, J.; Wang, Z.; Xu, J.; Yuan, Z. Metabolic Changes of Starch and Lipid Triggered by Nitrogen Starvation in the Microalga Chlorella Zofingiensis. Bioresour. Technol. 2014, 152, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Chen, T.; Liu, J.; Liu, J.; Guo, B.; Guo, B.; Ma, X.; Ma, X.; Sun, P.; Sun, P.; et al. Light Attenuates Lipid Accumulation While Enhancing Cell Proliferation and Starch Synthesis in the Glucose-Fed Oleaginous Microalga Chlorella Zofingiensis. Sci. Rep. 2015, 5, 14936. [Google Scholar] [CrossRef]
- Davey, M.P.; Horst, I.; Duong, G.-H.; Tomsett, E.V.; Litvinenko, A.C.P.; Howe, C.J.; Smith, A.G. Triacylglyceride Production and Autophagous Responses in Chlamydomonas Reinhardtii Depend on Resource Allocation and Carbon Source. Eukaryot. Cell 2014, 13, 392–400. [Google Scholar] [CrossRef]
- Laurens, L.M.L.; Wolfrum, E.J. High-Throughput Quantitative Biochemical Characterization of Algal Biomass by NIR Spectroscopy; Multiple Linear Regression and Multivariate Linear Regression Analysis. J. Agric. Food Chem. 2013, 61, 12307–12314. [Google Scholar] [CrossRef]


| Culture Conditions | DW [g/L] | Astax [%] of DW | CAR /Chloro | Total Macro Components [%] of DW | Source | |||
|---|---|---|---|---|---|---|---|---|
| Lipids | Carbohydrates | Ash | Proteins | |||||
| Batch, Tub, po, 200 L, 19 d | 1.05 | 0.10 | 0.66 | 10.2 | 35.2 | 7.8 | 42.1 | this study |
| repB, Tub, po, Nx, oss, 19 d + 31 d | 0.52 | 0.49 | 2.40 | 25.1 | 47.0 | 4.4 | 19.2 | |
| Batch, SR, 500 L ht, 9 d | 182.3 | 0.07 | - | 42.0 | - | - | - | [41] |
| FBatch, SR, 7.5 L, ht, Nx, oss, 15 d | 235.4 | 0.14 | - | - | - | - | - | |
| Batch, Tub, po, 65 L, 15 d | - | - | 0.23 | 11.7 | 25.0 | 9.9 | 34.8 | [14] |
| Batch, Tub, mix, 65 L, 15 + 8 d | 5.13 | 0.29 | 6.71 | 60.0 | 33.9 | 8.1 | 6.1 | |
| Batch, FP, po, 240 L, 14 d | 2.60 | - | - | 15.0 | 30.0 | - | - | [40] |
| Batch, FP, po, 55 L, 14 d + 14 d, Nx | 2.93 | - | - | 35.0 | 40.0 | - | 15.0 | |
| Batch, Tub, 12 L, po, 20 d | - | - | - | 20.0 | - | - | - | [25] |
| Batch, Tub, 12 L, po, oss 20 d + 5 d | 1.22 | - | - | 30.3 | - | - | - | |
| Batch, SR, 20 L mix, 15 d + 5 d | 121.5 | 0.56 | - | - | - | - | - | [27] |
| Batch, OP, 100 L, po, 1 d | 0.34 | - | - | 31.8 | - | - | 20.0 | [42] |
| Batch, OP, 60 L, po, 9 d | 0.9 | - | 54.5 | - | - | - | [43] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bleisch, R.; Ihadjadene, Y.; Torrisi, A.; Walther, T.; Mühlstädt, G.; Steingröwer, J.; Streif, S.; Krujatz, F. Physiological Adaptation of Chromochloris zofingiensis in Three-Phased Cultivation Performed in a Pilot-Scale Photobioreactor. Life 2025, 15, 648. https://doi.org/10.3390/life15040648
Bleisch R, Ihadjadene Y, Torrisi A, Walther T, Mühlstädt G, Steingröwer J, Streif S, Krujatz F. Physiological Adaptation of Chromochloris zofingiensis in Three-Phased Cultivation Performed in a Pilot-Scale Photobioreactor. Life. 2025; 15(4):648. https://doi.org/10.3390/life15040648
Chicago/Turabian StyleBleisch, Richard, Yob Ihadjadene, Agnese Torrisi, Thomas Walther, Gunnar Mühlstädt, Juliane Steingröwer, Stefan Streif, and Felix Krujatz. 2025. "Physiological Adaptation of Chromochloris zofingiensis in Three-Phased Cultivation Performed in a Pilot-Scale Photobioreactor" Life 15, no. 4: 648. https://doi.org/10.3390/life15040648
APA StyleBleisch, R., Ihadjadene, Y., Torrisi, A., Walther, T., Mühlstädt, G., Steingröwer, J., Streif, S., & Krujatz, F. (2025). Physiological Adaptation of Chromochloris zofingiensis in Three-Phased Cultivation Performed in a Pilot-Scale Photobioreactor. Life, 15(4), 648. https://doi.org/10.3390/life15040648








