Comparison of Pteridine Normalization Methods in Urine for Detection of Bladder Cancer
Abstract
1. Introduction
2. Results and Discussion
2.1. Basic Analyses
2.1.1. Characteristics of Population
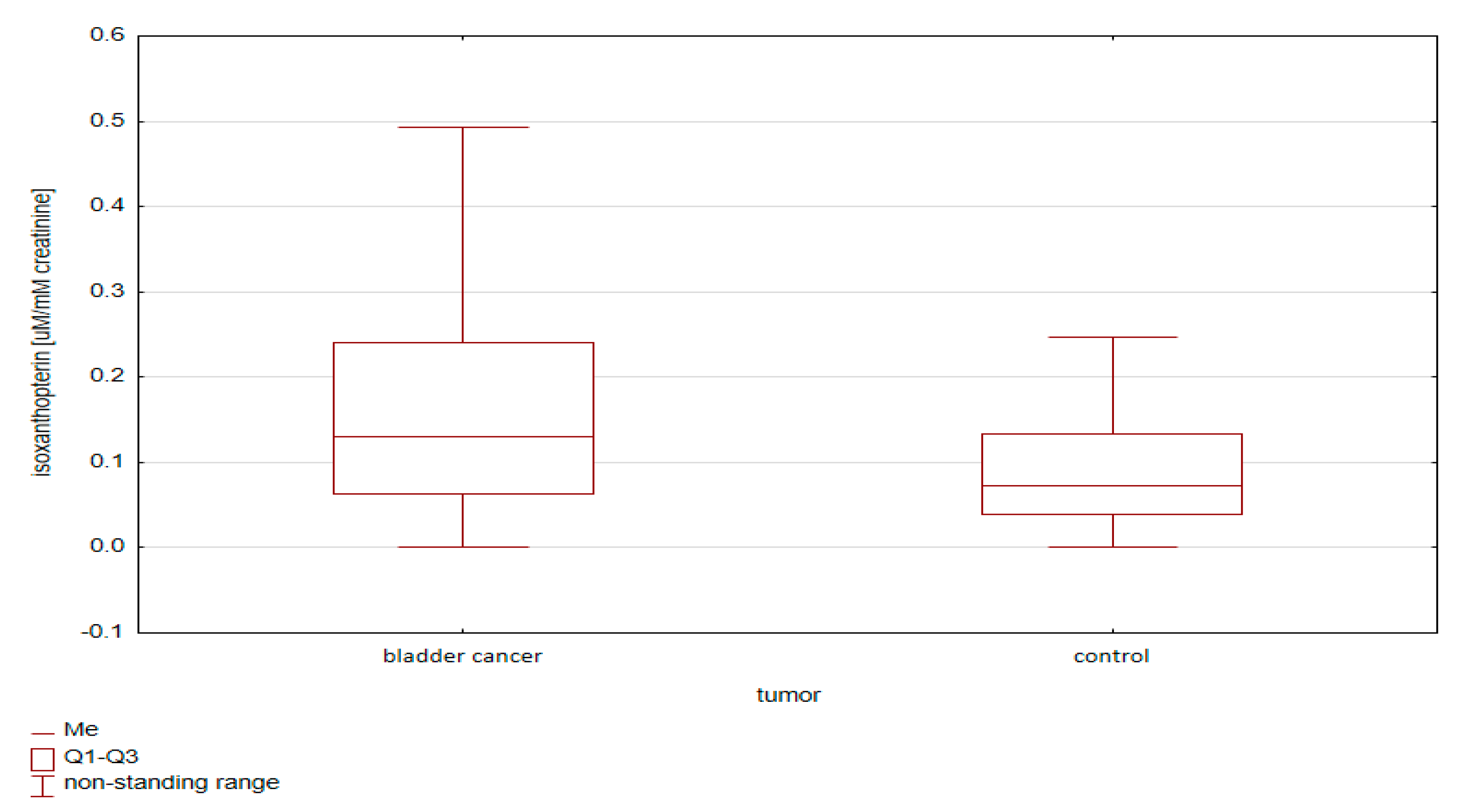
2.1.2. Comparative Analysis of Concentrations of Pterin Compounds in the Studied Groups
- (i)
- Standardization using urine creatinine;
- (ii)
- Standardization using urine specific gravity.
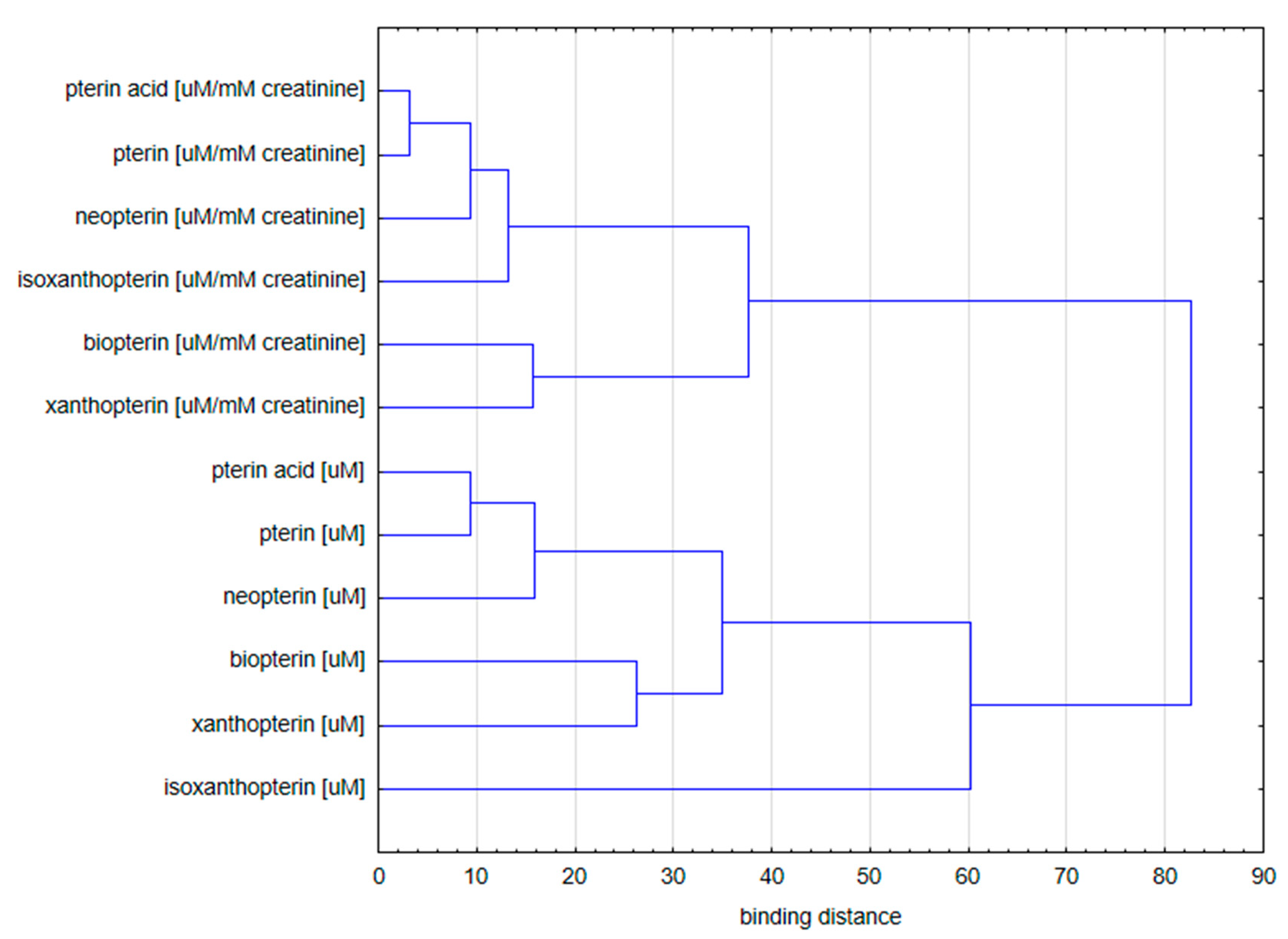
2.1.3. Cluster Analysis
- Concentration of pterin compounds with standardization to creatinine;
- Concentration of pterin compounds with standardization to urine specific gravity.
- (a)
- Isoxanthopterin concentration;
- (b)
- Other pterin compounds standardized to specific urine gravity.
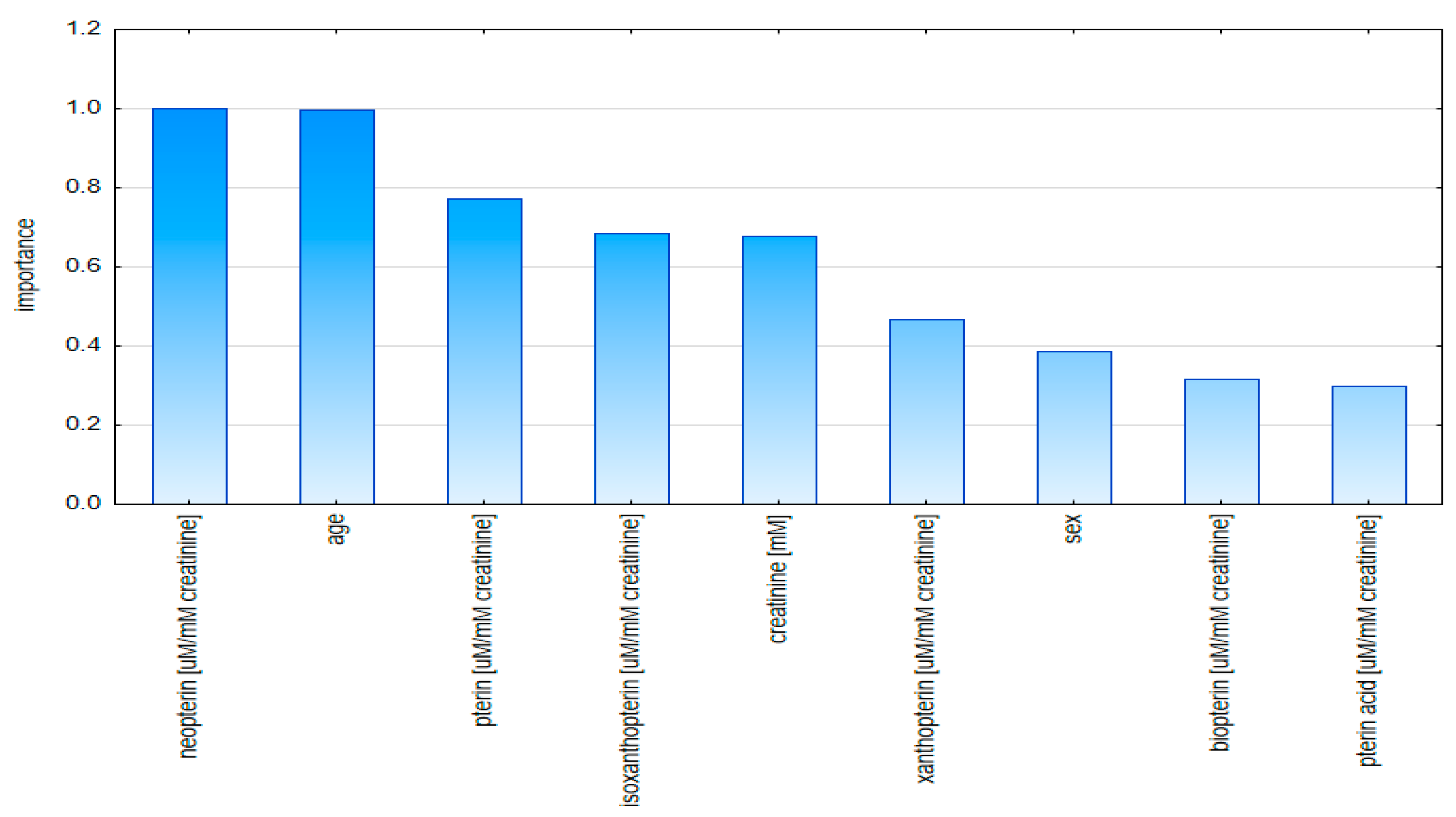
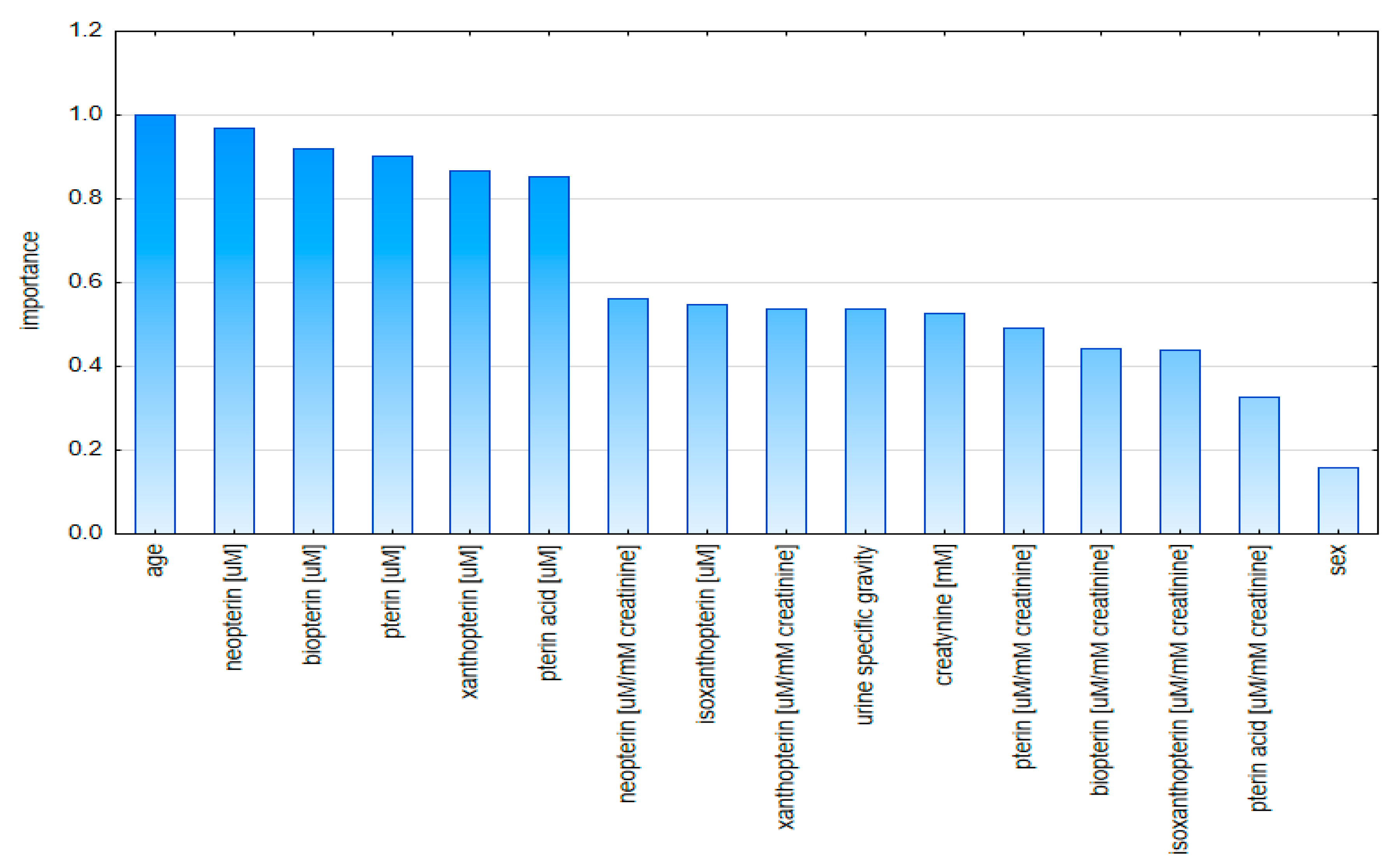
2.2. Multivariate Analysis
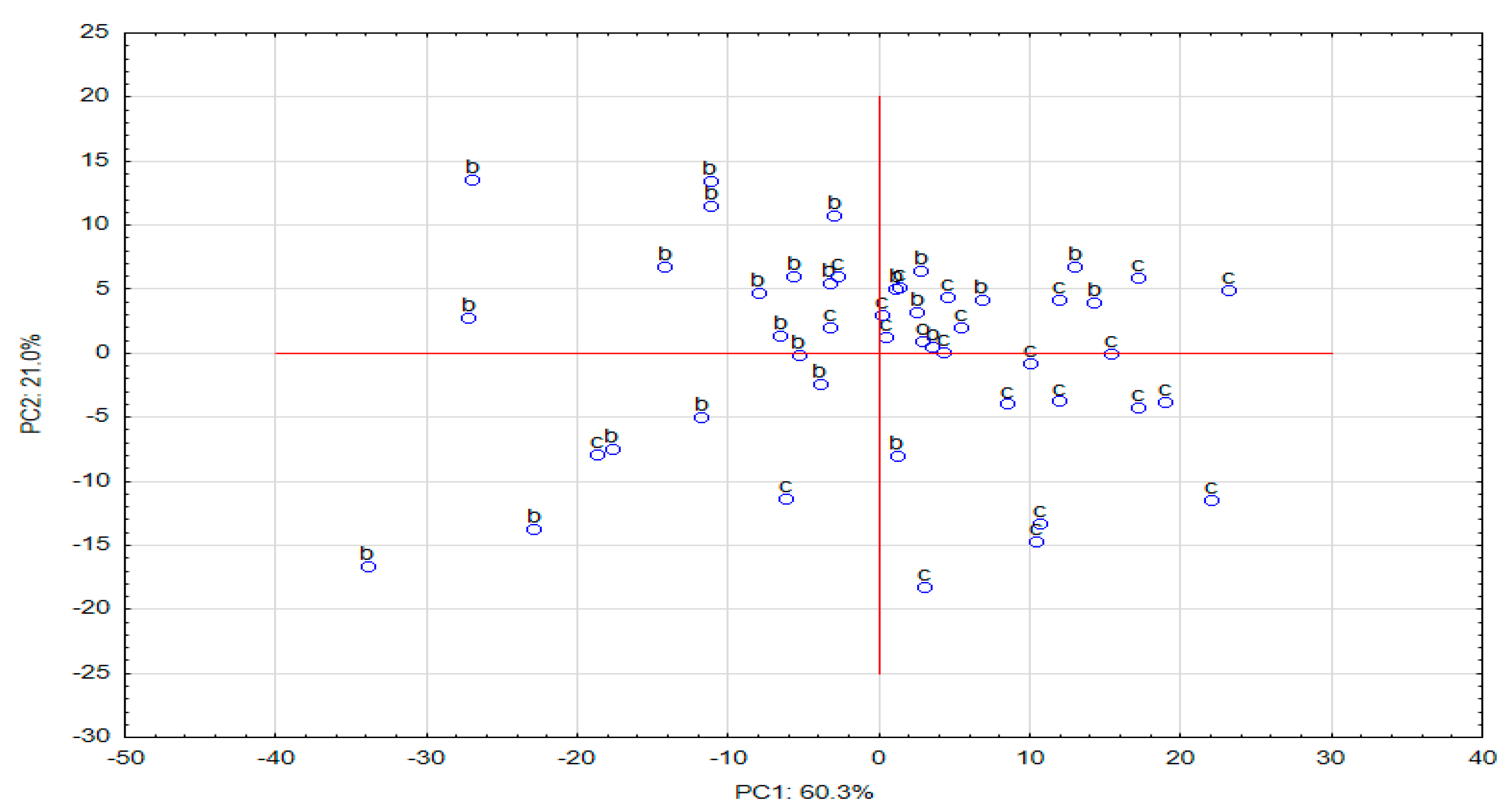
2.2.1. Principal Component Analysis (PCA)
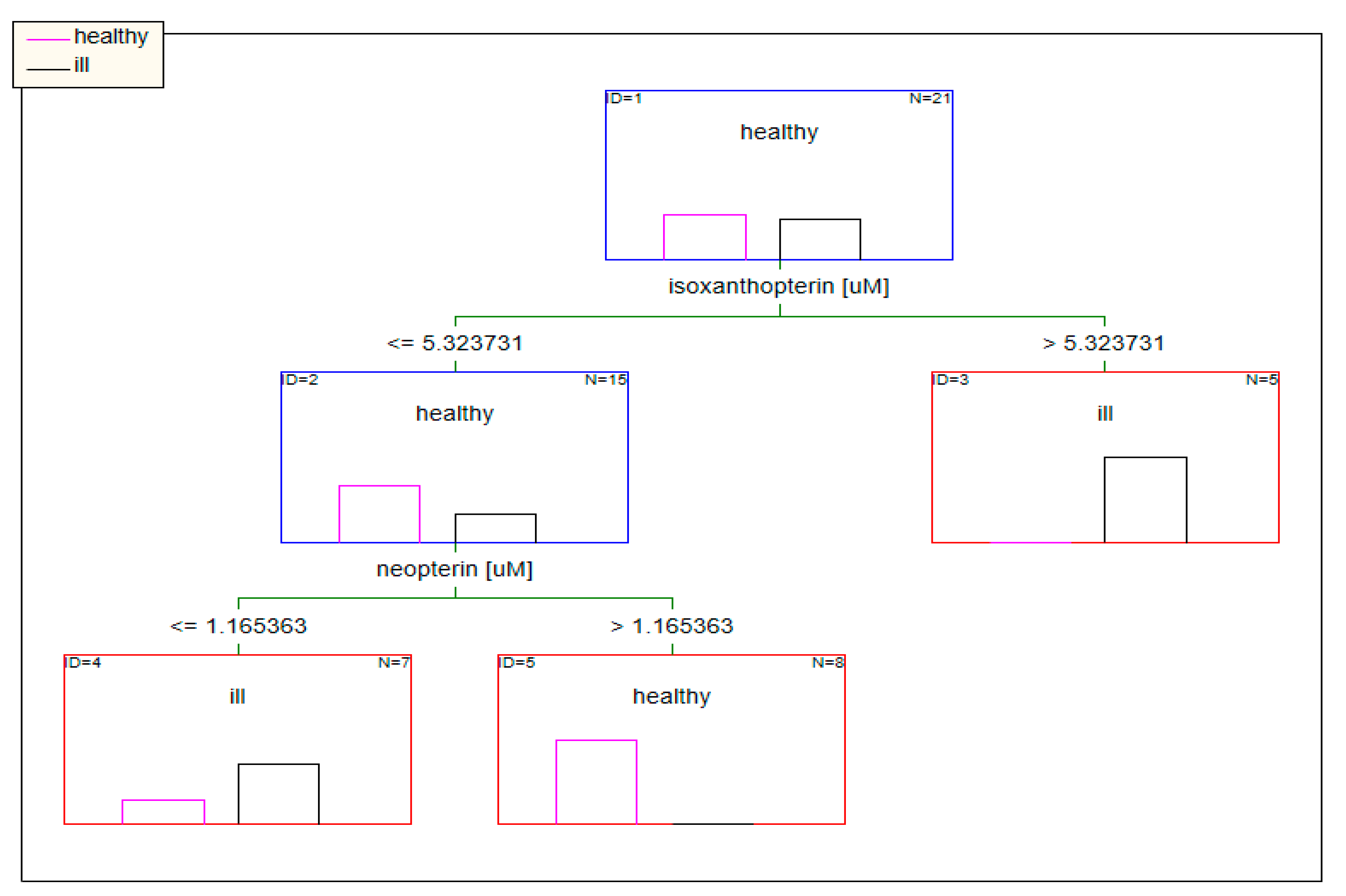
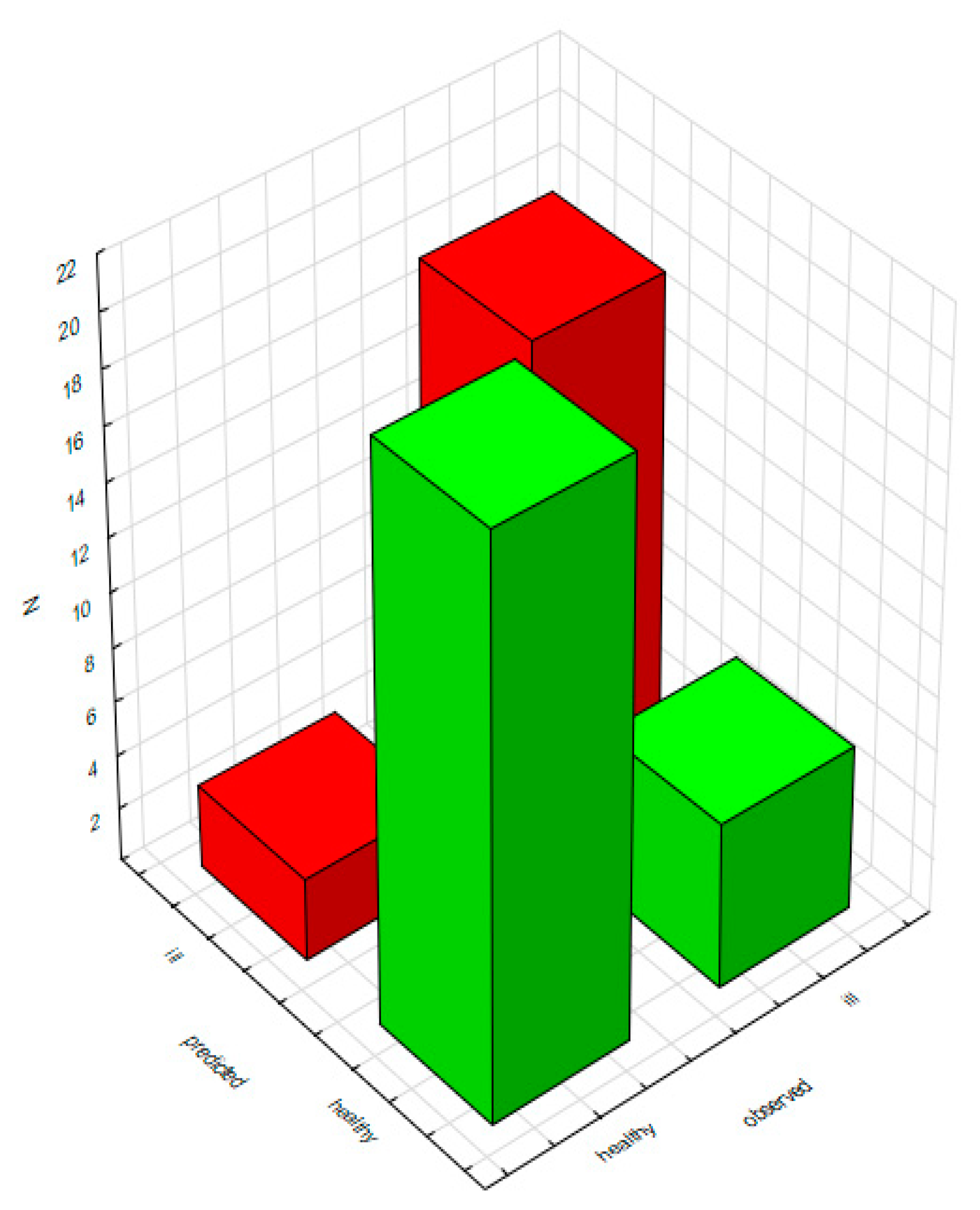
2.2.2. Classification Analysis (Decision Trees)
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Samples and Specimens
3.3. Assays
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Batista, R.; Vinagre, N.; Meireles, S.; Vinagre, J.; Prazeres, H.; Leao, R.; Máximo, V.; Soares, P. Biomarkers for Bladder Cancer Diagnosis and Surveillance: A Comprehensive Review. Diagnostics 2020, 10, 39. [Google Scholar] [CrossRef]
- Wu, L.; Qu, X. Cancer biomarker detection: Recent achievements and challenges. Chem. Soc. Rev. 2015, 44, 2963–2997. [Google Scholar] [CrossRef]
- Vincent, J.L.; Bogossian, E.; Menozzi, M. The Future of Biomarkers. Crit. Care Clin. 2020, 36, 177–180. [Google Scholar] [CrossRef]
- Sarma, A.; Calfee, C.S.; Ware, L.B. Biomarkers and Precision Medicine: State of the Art. Crit. Care Clin. 2019, 36, 155–165. [Google Scholar] [CrossRef]
- Proctor, I.; Stoeber, K.; Williams, G.H. Biomarkers In bladder cancer. Histopatology 2010, 57, 1–13. [Google Scholar] [CrossRef]
- Lokeshwar, V.B.; Habuchi, T.; Grossman, H.B.; Murph, W.M.; Hautmann, S.H.; Hemstree, G.P.; Bono, A.V.; Getzenberg, R.H.; Goebell, P.; Schmitz-Dräger, B.J.; et al. Bladder 95 tumor markers beyond cytology: International Consensus Panel on bladder tumor markers. Urology 2005, 66, 35–63. [Google Scholar] [CrossRef]
- Wiener, H.G.; Mian, C.; Haitel, A.; Pycha, A.; Schatzl, G.; Marberger, M. Can urine bound diagnostic tests replace cystoscopy in the management of bladder cancer? J. Urol. 1998, 159, 1876–1880. [Google Scholar] [CrossRef]
- Habuchi, T.; Marberger, M.; Droller, M.J.; Hemstreet, G.P.; Grossman, H.B.; Schalken, J.A.; Schmitz-Dräger, B.J.; Murphy, W.M.; Bono, A.V.; Goebell, P.; et al. Prognostic markers for bladder cancer: International Consensus Panel on bladder tumor markers. Urology 2005, 66, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Shaw, G.; Paris, A. Is microscopic haematuria a urological emergency? BJU Int. 2002, 90, 355–357. [Google Scholar] [CrossRef] [PubMed]
- Khadra, M.H.; Pickard, R.S.; Charlton, M.; Powell, P.H.; Neal, D.E. A prospective analysis of 1930 patients with haematuria to evaluate current diagnostic practice. J. Urol. 2000, 163, 524–527. [Google Scholar] [CrossRef]
- Sarosdy, M.F.; Hudson, M.A.; Ellis, W.J.; Soloway, M.S.; deVere White, R.; Sheinfeld, J.; Jarowenko, M.V.; Schellhammer, P.F.; Schervish, E.W.; Patel, J.V.; et al. Improved detection of recurrent bladder cancer using the Bard BTA stat test. Urology 1997, 50, 349–353. [Google Scholar] [CrossRef]
- Thomas, L.; Leyh, H.; Marberger, M.; Bombardieri, E.; Bassi, P.; Pagano, F.; Pansadoro, V.; Sternberg, C.N.; Boccon-Gibod, L.; Ravery, V.; et al. Multicenter trial of the quantitative BTA TRAK assay in the detection of bladder cancer. Clin. Chem. 1999, 45, 472–477. [Google Scholar] [PubMed]
- Ellis, W.J.; Blumenstein, B.A.; Ishak, L.M.; Enfield, D.L.I. Clinical evaluation of the BTA TRAK assay and comparison to voided urine cytology and the Bard BTA test in patiens with recurrent bladder tumors. Multi. center study group. Urology 1997, 50, 882–887. [Google Scholar] [CrossRef]
- Sarosdy, M.F.; Schellhammer, P.; Bokinsky, G.; Kahn, P.; Chao, R.; Yore, L.; Zadra, J.; Burzon, D.; Osher, G.; Bridge, J.A.; et al. Clinical evaluation of a multi-target fluorescent in situ hybridization assay for detection of bladder cancer. J. Urol. 2002, 168, 1950–1954. [Google Scholar] [CrossRef]
- Moonen, P.M.; Merkx, G.F.; Peelen, P.; Karthaus, H.F.; Smeets, M.F.; Witjes, J.A. UroVysion Compared with Cytology and Quantitative Cytology in the Surveillance of Non–Muscle-Invasive Bladder Cancer. Eur. Urol. 2007, 51, 1275–1280. [Google Scholar] [CrossRef]
- Replogle-Schwab, T.S.; Pienta, K.J.; Getzenberg, R.H. The Utilization of Nuclear Matrix Proteins for Cancer Diagnosis. Crit. Rev. Eukaryot. Gene Expr. 1996, 6, 103–113. [Google Scholar] [CrossRef]
- Landman, J.; Chang, Y.; Kavaler, E.; Droller, M.J.; Liu, B.C. Sensitivity and specificity of NMP-22, telomerase, and BTA in the detection of human bladder cancer. Urology 1998, 52, 398–402. [Google Scholar] [CrossRef]
- Poulakis, V.; Witzsch, U.; Vries, D.; Altmannsberger, H.M.; Manyak, M.J.; Becht, E. A comparison of urinary nuclear matrix protein-22 and bladder tumor antigen tests with voided urinary cytology in detecting and following bladder cancer: The prognostic value of false-positive results. BJU Int. 2001, 88, 692–701. [Google Scholar] [CrossRef]
- Kośliński, P.; Bujak, R.; Daghir, E.; Markuszewski, M.J. Metabolic profiling of pteridines for determination of potential biomarkers in cancer diseases. Electrophoresis 2011, 32, 2044–2054. [Google Scholar] [CrossRef]
- Basu, P.; Burgmayer, S.J. Pterin chemistry and its relationship to the molybdenum cofactor. Coord. Chem. Rev. 2011, 255, 1016–1038. [Google Scholar] [CrossRef]
- Gamagedara, S.; Gibbons, S.; Ma, Y. Investigation of urinary pteridine levels as potential biomarkers for noninvasive diagnosis of cancer. Clin. Chim. Acta 2011, 412, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Burton, C. Pteridine detection in urine: The future of cancer diagnostics? Biomarkers Med. 2013, 7, 679–681. [Google Scholar] [CrossRef] [PubMed]
- Burton, C.; Shi, H.; Ma, Y. Normalization of urinary pteridines by urine specific gravity for early cancer detection. Clin. Chim. Acta 2014, 435, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Burton, C.; Shi, H.; Ma, Y. Simultaneous Detection of Six Urinary Pteridines and Creatinine by High-Performance Liquid Chromatography-Tandem Mass Spectrometry for Clinical Breast Cancer Detection. Anal. Chem. 2013, 85, 11137–11145. [Google Scholar] [CrossRef]
- Tang, K.W.A.; Toh, Q.C.; Teo, B.W. Normalisation of urinary biomarkers to creatinine for clinical practice and research—When and why. Singap. Med. J. 2015, 56, 7–10. [Google Scholar] [CrossRef]
- Harpole, M.; Davis, J.; Espina, V. Current state of the art for enhancing urine biomarker discovery. Expert Rev. Proteom. 2016, 13, 609–626. [Google Scholar] [CrossRef]
- James, G.D.; Sealey, D.J.E.; Alderman, M.; Ljungman, S.; Mueller, F.B.; Pecker, M.S.; Laragh, J.H. A Longitudinal Study of Urinary Creatinine and Creatinine Clearance in Normal Subjects: Race, Sex, and Age Differences. Am. J. Hypertens. 1988, 1, 124–131. [Google Scholar] [CrossRef]
- Barr, D.B.; Wilder, L.C.; Caudill, S.P.; Gonzalez, A.J.; Needham, L.L.; Pirkle, J.L. Urinary creatinine concentrations in the US population: Implications for urinary biologic monitoring measurements. Environ. Health Perspect. 2005, 113, 192–200. [Google Scholar] [CrossRef]
- Cirillo, M.; Anastasio, P.; De Santo, N.G. Relationship of gender, age, and body mass index to errors in predicted kidney function. Nephrol. Dial. Transplant. 2005, 20, 1791–1798. [Google Scholar] [CrossRef]
- Pimentaa, E.; Jensenb, M.; Jungb, D.; Schaumannc, F.; Boxnickc, S.; Truebela, H. Effect of Diet on Serum Creatinine in Healthy Subjects During a Phase I Study. J. Clin. Med. Res. 2016, 8, 836–839. [Google Scholar] [CrossRef]
- Phipps, W.R.; Duncan, A.M.; Merz, B.E.; Kurzer, M.S. Effect of the Menstrual Cycle on Creatinine Clearance in Normally Cycling Women. Obstet. Gynecol. 1998, 92, 585–588. [Google Scholar] [PubMed]
- Carter, C.E.; Gansevoort, R.T.; Scheven, L. Influence of urine creatinine on the relationship between the albumin-to-creatinine ratio and cardiovascular events. Clin. J. Am. Soc. Nephrol. 2012, 7, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Kleber, M.; Cybulla, M.; Bauchmuller, K.; Ihorst, G.; Koch, B.; Engelhardt, M. Monitoring of renal function in cancer patients: An ongoing challenge for clinical practice. Ann. Oncol. 2007, 18, 950–958. [Google Scholar] [CrossRef]
- Vij, H.S.; Howell, S. Improving the Specific Gravity Adjustment Method for Assessing Urinary Concentrations of Toxic Substances. Am. Ind. Hyg. Assoc. 1998, 59, 375–380. [Google Scholar] [CrossRef]
- Edmands, W.M.B.; Ferrari, P.; Scalbert, A. Normalization to Specific Gravity Prior to Analysis Improves Information Recovery from High Resolution Mass Spectrometry Metabolomic Profiles of Human Urine. Anal. Chem. 2014, 86, 10925–10931. [Google Scholar] [CrossRef]
- Sauvé, J.F.; Levesque, M.; Huard, M.; Truchon, G. Creatinine and Specific Gravity Normalization in Biological Monitoring of Occupational Exposures. J. Occup. Environ. Hyg. 2014, 12, 123–129. [Google Scholar] [CrossRef]
- Singh, G.K.; Balzer, B.W.; Desai, R.; Jimenez, M.; Steinbeck, K.S.; Handelsman, D.J. Requirement for specific gravity and creatinine adjustments for urinary steroids and luteinizing hormone concentrations in adolescents. Ann. Clin. Biochem. Int. J. Lab. Med. 2015, 52, 665–671. [Google Scholar] [CrossRef]
- Bi, H.; Guo, Z.; Jia, X. The key points in the pre-analytical procedures of blood and urine samples in metabolomics studies. Metabolomics 2020, 16, 68. [Google Scholar] [CrossRef]
- Cook, J.D.; Caplan, Y.H.; Lodico, C.P.; Bush, D.M. The characterization of human urine for specimen validity determination in workplace drug testing: A review. J. Anal. Toxicol. 2000, 24, 579–588. [Google Scholar] [CrossRef]
- Miyamoto, R.; Abe, Y.; Takeshita, J. Ward method of hierarchical clustering for non-Euclidean similarity measures. In Proceedings of the 2015 7th International Conference of Soft Computing and Pattern Recognition (SoCPaR), Fukuoka, Japan, 13–15 November 2015; pp. 60–63. [Google Scholar]
- Dunn, W.B.; Bailey, N.J.; Johnson, H.E. Measuring the metabolome: Current analytical technologies. Analyst 2005, 130, 606–625. [Google Scholar] [CrossRef]
- Gaitani, N.; Lehmann, C.; Santamouris, M.; Mihalakakou, G.; Patargias, P. Using principal component and cluster analysis in the heating evaluation of the school building sector. Appl. Energy 2010, 87, 2079–2086. [Google Scholar] [CrossRef]
- Patel, H.H.; Prajapati, P. Study and Analysis of Decision Tree Based Classification Algorithms. Int. J. Comput. Sci. Eng. 2018, 6, 74–78. [Google Scholar] [CrossRef]
- Kośliński, P.; Jarzemski, P.; Markuszewski, M.J.; Kaliszan, R. Determination of pterins in urine by HPLC with UV and fluorescent detection using different types of chromatographic stationary phases (HILIC, RP C8, RP C18). JPBA 2014, 91, 37–45. [Google Scholar] [CrossRef] [PubMed]

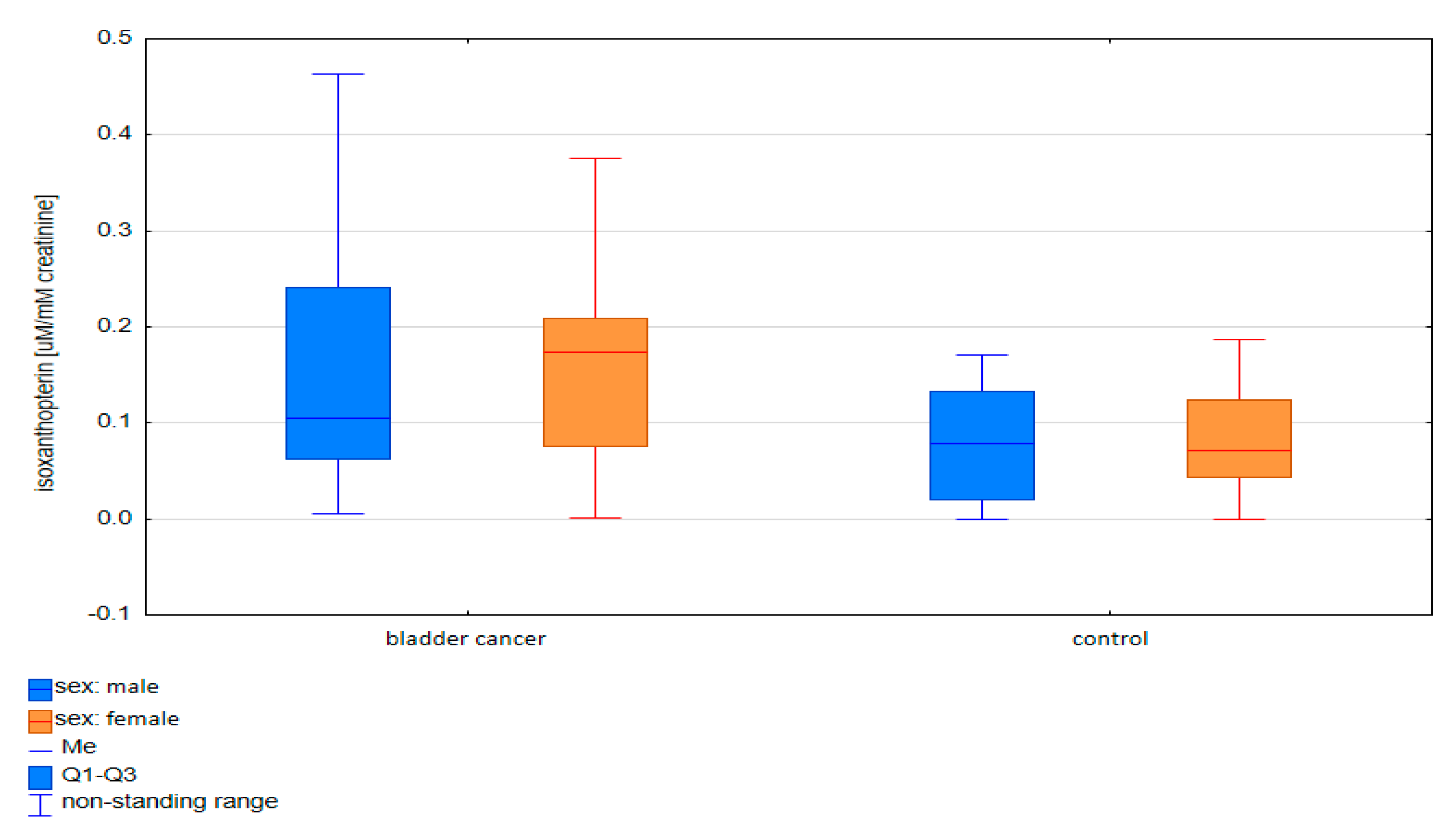



| Compounds | Women p-Value | Men p-Value |
|---|---|---|
| Isoxanthopterin | 0.018 | 0.562 |
| Xanthopterin | 0.066 | 0.003 |
| Neopterin | 0.030 | 0.117 |
| Pterin acid | 0.043 | 0.001 |
| Pterin | 0.038 | 0.030 |
| Standardization for Urine Specific Gravity | Standardization for Creatinine | Aggregated Data | |
|---|---|---|---|
| SEN | 0.806 | 0.833 | 0.750 |
| SPE | 0.808 | 0.480 | 0.875 |
| PPV | 0.833 | 0.606 | 0.857 |
| NPV | 0.778 | 0.750 | 0.778 |
| LRPLUS | 4.194 | 1.603 | 6.000 |
| LRMINUS | 0.240 | 0.347 | 0.286 |
| Acc | 0.807 | 0.653 | 0.813 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kośliński, P.; Pluskota, R.; Mądra-Gackowska, K.; Gackowski, M.; Markuszewski, M.J.; Kędziora-Kornatowska, K.; Koba, M. Comparison of Pteridine Normalization Methods in Urine for Detection of Bladder Cancer. Diagnostics 2020, 10, 612. https://doi.org/10.3390/diagnostics10090612
Kośliński P, Pluskota R, Mądra-Gackowska K, Gackowski M, Markuszewski MJ, Kędziora-Kornatowska K, Koba M. Comparison of Pteridine Normalization Methods in Urine for Detection of Bladder Cancer. Diagnostics. 2020; 10(9):612. https://doi.org/10.3390/diagnostics10090612
Chicago/Turabian StyleKośliński, Piotr, Robert Pluskota, Katarzyna Mądra-Gackowska, Marcin Gackowski, Michał J. Markuszewski, Kornelia Kędziora-Kornatowska, and Marcin Koba. 2020. "Comparison of Pteridine Normalization Methods in Urine for Detection of Bladder Cancer" Diagnostics 10, no. 9: 612. https://doi.org/10.3390/diagnostics10090612
APA StyleKośliński, P., Pluskota, R., Mądra-Gackowska, K., Gackowski, M., Markuszewski, M. J., Kędziora-Kornatowska, K., & Koba, M. (2020). Comparison of Pteridine Normalization Methods in Urine for Detection of Bladder Cancer. Diagnostics, 10(9), 612. https://doi.org/10.3390/diagnostics10090612







