Simulated Radiation Dose Reduction in Whole-Body CT on a 3rd Generation Dual-Source Scanner: An Intraindividual Comparison
Abstract
:1. Introduction
2. Materials and Methods
2.1. Population
2.2. Radiation Exposure
2.3. Image Acquisition Protocol
2.4. Image Reconstruction Parameters
2.5. Objective Analysis of Image Quality
2.6. Subjective Analysis of Image Quality
2.7. Statistical Analysis
3. Results
3.1. Population and Radiation Dose
3.2. Objective Analysis of Image Quality
3.3. Subjective Analysis of Image Quality
3.3.1. Overall Image Quality
3.3.2. Overall Diagnostic Confidence
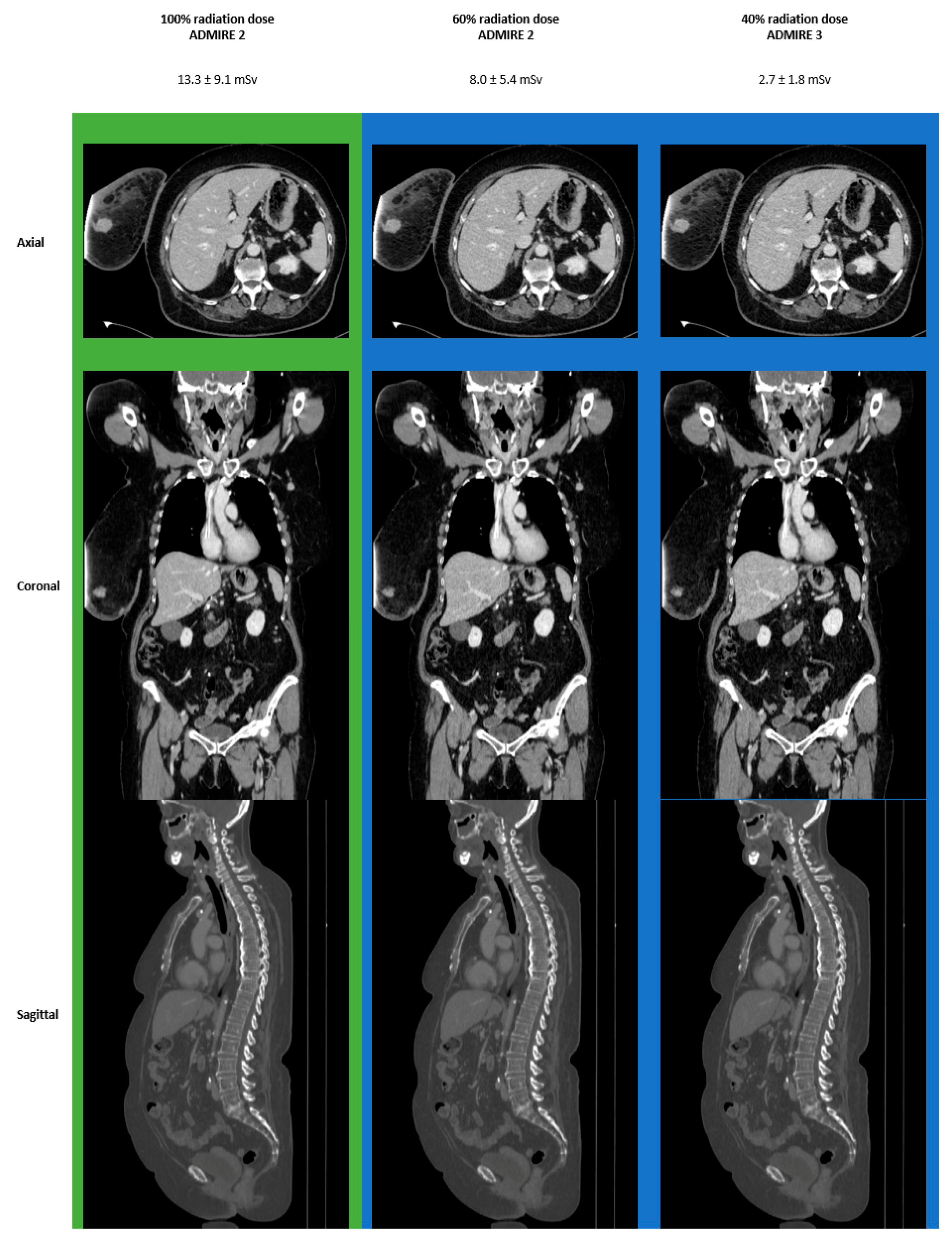
3.4. Images
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zondervan, R.L.; Hahn, P.F.; Sadow, C.A.; Liu, B.; Lee, S.I. Frequent body CT scanning of young adults: Indications, outcomes, and risk for radiation-induced cancer. J. Am. Coll. Radiol. 2011, 8, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Brenner, D.J.; Hall, E.J. Computed Tomography—An Increasing Source of Radiation Exposure. N. Engl. J. Med. 2007, 357, 2277–2284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trugenberger-Schnabel, A.M.D.D.L.-R.; Peter, J.A. Environmental Radioactivity and Radiation Exposure Annual Report 2020; Bundesministerium für Umwelt, Naturschutz und nukleare Sicherheit; N.u.n.S.B., Ed.; Federal Office for the Environment, Nature Conservastion and Nuclear Safety (Bundesamt für Strahlenschutz, BfS): Bonn, Germany, 2018. [Google Scholar]
- de González, A.B.; Mahesh, M.; Kim, K.-P.; Bhargavan, M.; Lewis, R.; Mettler, F.; Land, C. Projected Cancer Risks From Computed Tomographic Scans Performed in the United States in 2007. Arch. Intern. Med. 2009, 169, 2071–2077. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.-H.; Tsai, K.; Kim, S.; Wu, Y.-J.; Demissie, K. Exposure to Tomographic Scans and Cancer Risks. JNCI Cancer Spectr. 2019, 4. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, P.; Sinha, R.; Tumeh, J.W.; Lechowicz, M.J.; Flowers, C. Surveillance Computed Tomography Scans for Patients With Lymphoma: Is the Risk Worth the Benefits? Clin. Lymphoma Myeloma Leuk. 2010, 10, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.G.; Fidler, J.L.; Venkatesh, S.K.; Hough, D.M.; Takahashi, N.; Yu, L.; Johnson, M.; Leng, S.; Holmes, D.R., III; Carter, R. Observer performance with varying radiation dose and reconstruction methods for detection of hepatic metastases. Radiology 2018, 289, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Karpitschka, M.; Augart, D.; Becker, H.; Reiser, M.; Graser, A. Dose reduction in oncological staging multidetector CT: Effect of iterative reconstruction. Br. J. Radiol. 2013, 86, 20120224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mangold, S.; De Cecco, C.N.; Wichmann, J.L.; Canstein, C.; Varga-Szemes, A.; Caruso, D.; Fuller, S.R.; Bamberg, F.; Nikolaou, K.; Schoepf, U.J. Effect of automated tube voltage selection, integrated circuit detector and advanced iterative reconstruction on radiation dose and image quality of 3rd generation dual-source aortic CT angiography: An intra-individual comparison. Eur. J. Radiol. 2016, 85, 972–978. [Google Scholar] [CrossRef]
- Mangold, S.; Wichmann, J.L.; Schoepf, U.J.; Poole, Z.B.; Canstein, C.; Varga-Szemes, A.; Caruso, D.; Bamberg, F.; Nikolaou, K.; De Cecco, C.N. Automated tube voltage selection for radiation dose and contrast medium reduction at coronary CT angiography using 3rd generation dual-source CT. Eur. Radiol. 2016, 26, 3608–3616. [Google Scholar] [CrossRef]
- Afat, S.; Brockmann, C.; Nikoubashman, O.; Muller, M.; Thierfelder, K.M.; Brockmann, M.A.; Nikolaou, K.; Wiesmann, M.; Kim, J.H.; Othman, A.E. Diagnostic Accuracy of Simulated Low-Dose Perfusion CT to Detect Cerebral Perfusion Impairment after Aneurysmal Subarachnoid Hemorrhage: A Retrospective Analysis. Radiology 2018, 287, 643–650. [Google Scholar] [CrossRef] [Green Version]
- Othman, A.E.; Bongers, M.N.; Zinsser, D.; Schabel, C.; Wichmann, J.L.; Arshid, R.; Notohamiprodjo, M.; Nikolaou, K.; Bamberg, F. Evaluation of reduced-dose CT for acute non-traumatic abdominal pain: Evaluation of diagnostic accuracy in comparison to standard-dose CT. Acta Radiol. 2018, 59, 4–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solomon, J.; Mileto, A.; Ramirez-Giraldo, J.C.; Samei, E. Diagnostic Performance of an Advanced Modeled Iterative Reconstruction Algorithm for Low-Contrast Detectability with a Third-Generation Dual-Source Multidetector CT Scanner: Potential for Radiation Dose Reduction in a Multireader Study. Radiology 2015, 275, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Winkelmann, M.T.; Afat, S.; Walter, S.S.; Stock, E.; Schwarze, V.; Brendlin, A.; Kolb, M.; Artzner, C.P.; Othman, A.E. Diagnostic Performance of Different Simulated Low-Dose Levels in Patients with Suspected Cervical Abscess Using a Third-Generation Dual-Source CT Scanner. Diagnostics 2020, 10, 1072. [Google Scholar] [CrossRef] [PubMed]
- Winkelmann, M.T.; Walter, S.S.; Stock, E.; Brendlin, A.; Kolb, M.; Othman, A.E.; Afat, S. Effects of radiation dose reduction on diagnostic performance of 3rd generation Dual Source CT pulmonary angiography. Eur. J. Radiol. 2021, 134, 109426. [Google Scholar] [CrossRef] [PubMed]
- ICRP. The 2007 Recommendations of the International Commission on Radiological Protection. Ann. ICRP 2007, 37, 2–4. [Google Scholar]
- Ulzheimer, S. Stellar Detector Performance in Computed Tomography: The first fully-integrated detector in the CT industry sets a new reference in image quality with HiDynamics, TrueSignal and Ultra Fast Ceramics. Somat. Sess. 2011, 29, 64–66. [Google Scholar]
- Chun, M.; Choi, Y.H.; Kim, J.H. Automated measurement of CT noise in patient images with a novel structure coherence feature. Phys. Med. Biol. 2015, 60, 9107–9122. [Google Scholar] [CrossRef]
- Menzel, H.-G.; Schibilla, H.; Teunen, D. European Guidelines on Quality Criteria for Computed Tomography, EUR 16260. Available online: http://www.drs.dk/guidelines/ct/quality/htmlindex.htm (accessed on 1 May 2020).
- Bobak, C.A.; Barr, P.J.; O’Malley, A.J. Estimation of an inter-rater intra-class correlation coefficient that overcomes common assumption violations in the assessment of health measurement scales. BMC Med. Res. Methodol. 2018, 18, 93. [Google Scholar] [CrossRef]
- Kalra, M.K.; Maher, M.M.; Toth, T.L.; Hamberg, L.M.; Blake, M.A.; Shepard, J.-A.; Saini, S. Strategies for CT radiation dose optimization. Radiology 2004, 230, 619–628. [Google Scholar] [CrossRef]
- Gordic, S.; Desbiolles, L.; Stolzmann, P.; Gantner, L.; Leschka, S.; Husarik, D.; Alkadhi, H. Advanced modelled iterative reconstruction for abdominal CT: Qualitative and quantitative evaluation. Clin. Radiol. 2014, 69, e497–e504. [Google Scholar] [CrossRef]
- Scholtz, J.-E.; Wichmann, J.L.; Hüsers, K.; Albrecht, M.H.; Beeres, M.; Bauer, R.W.; Vogl, T.J.; Bodelle, B. Third-generation dual-source CT of the neck using automated tube voltage adaptation in combination with advanced modeled iterative reconstruction: Evaluation of image quality and radiation dose. Eur. Radiol. 2016, 26, 2623–2631. [Google Scholar] [CrossRef] [PubMed]
- Alshamari, M.; Geijer, M.; Norrman, E.; Liden, M.; Krauss, W.; Jendeberg, J.; Magnuson, A.; Geijer, H. Impact of iterative reconstruction on image quality of low-dose CT of the lumbar spine. Acta Radiol. 2017, 58, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Hara, A.K.; Paden, R.G.; Silva, A.C.; Kujak, J.L.; Lawder, H.J.; Pavlicek, W. Iterative Reconstruction Technique for Reducing Body Radiation Dose at CT: Feasibility Study. Am. J. Roentgenol. 2009, 193, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Ippolito, D.; Casiraghi, A.S.; Franzesi, C.T.; Fior, D.; Meloni, F.; Sironi, S. Low-dose computed tomography with 4(th)-generation iterative reconstruction algorithm in assessment of oncologic patients. World J. Gastrointest. Oncol. 2017, 9, 423–430. [Google Scholar] [CrossRef]
- Moloney, F.; James, K.; Twomey, M.; Ryan, D.; Grey, T.M.; Downes, A.; Kavanagh, R.G.; Moore, N.; Murphy, M.J.; Bye, J.; et al. Low-dose CT imaging of the acute abdomen using model-based iterative reconstruction: A prospective study. Emerg. Radiol. 2019, 26, 169–177. [Google Scholar] [CrossRef]
- Murphy, K.P.; Crush, L.; O’Neill, S.B.; Foody, J.; Breen, M.; Brady, A.; Kelly, P.J.; Power, D.G.; Sweeney, P.; Bye, J.; et al. Feasibility of low-dose CT with model-based iterative image reconstruction in follow-up of patients with testicular cancer. Eur. J. Radiol. Open 2016, 3, 38–45. [Google Scholar] [CrossRef] [Green Version]
- Kataria, B.; Althén, J.N.; Smedby, Ö.; Persson, A.; Sökjer, H.; Sandborg, M. Assessment of image quality in abdominal CT: Potential dose reduction with model-based iterative reconstruction. Eur. Radiol. 2018, 28, 2464–2473. [Google Scholar] [CrossRef] [Green Version]
- Larsson, J.; Båth, M.; Ledenius, K.; Caisander, H.; Thilander-Klang, A. Assessment of clinical image quality in paediatric abdominal CT examinations: Dependency on the level of adaptive statistical iterative reconstruction (ASiR) and the type of convolution kernel. Radiat. Prot. Dosim. 2016, 169, 123–129. [Google Scholar] [CrossRef]
- Miéville, F.A.; Berteloot, L.; Grandjean, A.; Ayestaran, P.; Gudinchet, F.; Schmidt, S.; Brunelle, F.; Bochud, F.O.; Verdun, F.R. Model-based iterative reconstruction in pediatric chest CT: Assessment of image quality in a prospective study of children with cystic fibrosis. Pediatric Radiol. 2013, 43, 558–567. [Google Scholar] [CrossRef] [Green Version]
- Pooler, B.D.; Lubner, M.G.; Kim, D.H.; Chen, O.T.; Li, K.; Chen, G.-H.; Pickhardt, P.J. Prospective evaluation of reduced dose computed tomography for the detection of low-contrast liver lesions: Direct comparison with concurrent standard dose imaging. Eur. Radiol. 2017, 27, 2055–2066. [Google Scholar] [CrossRef] [Green Version]




| Parameter | Male | Female | Total | |
|---|---|---|---|---|
| Patient Population | ||||
| Absolute (n) | 4 | 14 | 18 | |
| Reconstructions (n) | 120 | 420 | 540 | |
| Mean age (years) | 62 ± 25 | 60 ± 14 | 60 ± 15 | |
| Mean BMI (kg/m²) | 26 ± 2 | 30 ± 7 | 30 ± 6 | |
| Diagnosis (n) | ||||
| breast cancer | 11 | 11 | ||
| melanoma | 2 | 1 | 3 | |
| squamos cell carcinoma | 1 | 1 | 2 | |
| oropharyngeal carcinoma | 1 | 1 | ||
| lymphoma | 1 | 1 | ||
| Image Acquisistion parameters | ||||
| kV | 100.00 ± 00.0 | 115.71 ± 22.08 | 112.22 ± 20.52 | |
| mAs | 183.25 ± 16.01 | 218.43 ± 50.51 | 210.61 ± 47.19 | |
| Mean CTDIvol (mGy) | 7.37 ± 0.65 | 15.03 ± 10.52 | 13.33 ± 9.8 | |
| Mean DLP (mGy*cm) | 570.85 ± 59.85 | 1229.8 ± 905.42 | 1083.37 ± 840.93 | |
| Mean Estimated Effective Radiation Dose (mSv) | ||||
| 100% | 7.9 ± 1.4 | 14.9 ± 9.8 | 13.3 ± 9.1 | |
| 80% | 6.3 ± 1.1 | 11.9 ± 7.8 | 10.7 ± 7.2 | |
| 60% | 4.7 ± 0.8 | 8.9 ± 5.9 | 8.0 ± 5.4 | |
| 40% | 3.2 ± 0.6 | 6.0 ± 3.9 | 5.3 ± 3.6 | |
| 20% | 1.6 ± 0.3 | 3.0 ± 2.0 | 2.7 ± 1.8 | |
| Radiation Dose (%) | ED (mSv) | Reconstruction | Noise (SD of HU) | p | r |
|---|---|---|---|---|---|
| vs. 100% ADMIRE 2 | |||||
| 100 | 13.3 ± 9.1 | FBP | 10.51 ± 1.30 | 1.000 | 0.44 |
| ADMIRE 1 | 9.22 ± 1.12 | 1.000 | <0.1 | ||
| ADMIRE 2 | 8.98 ± 2.62 | ||||
| ADMIRE 3 | 7.06 ± 0.85 | 1.000 | 0.38 | ||
| ADMIRE 4 | 6.14 ± 1.25 | 1.000 | 0.58 | ||
| ADMIRE 5 | 4.62 ± 0.58 | 0.215 | >0.7 | ||
| 80 | 10.7 ± 7.2 | FBP | 11.92 ± 1.53 | 1.000 | >0.7 |
| ADMIRE 1 | 10.46 ± 1.33 | 1.000 | 0.42 | ||
| ADMIRE 2 | 9.18 ± 1.31 | 1.000 | <0.1 | ||
| ADMIRE 3 | 7.95 ± 1.02 | 1.000 | 0.18 | ||
| ADMIRE 4 | 6.88 ± 1.38 | 1.000 | 0.46 | ||
| ADMIRE 5 | 5.14 ± 0.66 | 0.728 | >0.7 | ||
| 60 | 8.0 ± 4.5 | FBP | 13.74 ± 1.86 | 0.007 | >0.7 |
| ADMIRE 1 | 12.08 ± 1.63 | 0.491 | >0.7 | ||
| ADMIRE 2 | 10.55 ± 1.61 | 1.000 | 0.46 | ||
| ADMIRE 3 | 9.18 ± 1.24 | 1.000 | <0.1 | ||
| ADMIRE 4 | 7.92 ± 1.65 | 1.000 | 0.21 | ||
| ADMIRE 5 | 5.86 ± 0.80 | 1.000 | 0.62 | ||
| 40 | 5.3 ± 3.6 | FBP | 16.78 ± 2.37 | <0.001 | >0.7 |
| ADMIRE 1 | 14.65 ± 2.18 | 0.002 | >0.7 | ||
| ADMIRE 2 | 12.95 ± 2.09 | 0.090 | >0.7 | ||
| ADMIRE 3 | 11.27 ± 1.73 | 1.000 | 0.60 | ||
| ADMIRE 4 | 9.58 ± 2.02 | 1.000 | 0.19 | ||
| ADMIRE 5 | 7.00 ± 1.09 | 1.000 | 0.40 | ||
| 20 | 2.7 ± 1.8 | FBP | 23.85 ± 3.61 | <0.001 | >0.7 |
| ADMIRE 1 | 20.97 ± 3.14 | <0.001 | >0.7 | ||
| ADMIRE 2 | 18.33 ± 3.39 | <0.001 | > 0.7 | ||
| ADMIRE 3 | 15.85 ± 2.45 | <0.001 | >0.7 | ||
| ADMIRE 4 | 13.55 ± 2.85 | 0.033 | >0.7 | ||
| ADMIRE 5 | 9.98 ± 1.53 | 1.000 | 0.29 | ||
| Radiation Dose (%) | ED (mSv) | Reconstruction | Rating | ICC | ICC: 95% CI | p | r | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Median | IQR | Average Measure | Lower Bound | Upper Bound | vs. 100% ADMIRE 2 | |||||
| 100 | 13.3 ± 9.1 | FBP | 5 | 4–5 | 0.942 | 0.874 | – | 0.976 | 1.000 | <0.1 |
| ADMIRE 1 | 5 | 4–5 | 0.896 | 0.775 | – | 0.958 | 1.000 | <0.1 | ||
| ADMIRE 2 | 5 | 4–5 | 1.000 | |||||||
| ADMIRE 3 | 5 | 4–5 | 1.000 | 1.000 | <0.1 | |||||
| ADMIRE 4 | 5 | 4–5 | 0.896 | 0.775 | – | 0.958 | 1.000 | <0.1 | ||
| ADMIRE 5 | 5 | 4–5 | 0.750 | 0.457 | – | 0.899 | 1.000 | <0.1 | ||
| 80 | 10.7 ± 7.2 | FBP | 4 | 3–5 | 0.983 | 0.962 | – | 0.993 | 0.971 | >0.7 |
| ADMIRE 1 | 4 | 3–5 | 0.980 | 0.957 | – | 0.992 | 1.000 | 0.68 | ||
| ADMIRE 2 | 4 | 4–5 | 0.968 | 0.930 | – | 0.987 | 1.000 | 0.48 | ||
| ADMIRE 3 | 4 | 4–5 | 0.970 | 0.935 | – | 0.988 | 1.000 | 0.46 | ||
| ADMIRE 4 | 4 | 3–5 | 0.950 | 0.891 | – | 0.980 | 1.000 | 0.60 | ||
| ADMIRE 5 | 4 | 3–5 | 0.956 | 0.904 | – | 0.982 | 1.000 | 0.68 | ||
| 60 | 8.0 ± 4.5 | FBP | 3 | 3–4 | 0.968 | 0.930 | – | 0.987 | 0.003 | >0.7 |
| ADMIRE 1 | 4 | 3–4 | 0.961 | 0.916 | – | 0.984 | 0.542 | >0.7 | ||
| ADMIRE 2 | 4 | 4–5 | 0.949 | 0.889 | – | 0.979 | 1.000 | 0.56 | ||
| ADMIRE 3 | 4 | 4–5 | 0.958 | 0.909 | – | 0.983 | 1.000 | 0.54 | ||
| ADMIRE 4 | 4 | 3–4 | 0.885 | 0.748 | – | 0.953 | 1.000 | 0.68 | ||
| ADMIRE 5 | 4 | 3–4 | 0.907 | 0.798 | – | 0.962 | 0.542 | >0.7 | ||
| 40 | 5.3 ± 3.6 | FBP | 2 | 1–2 | 0.968 | 0.930 | – | 0.987 | <0.001 | >0.7 |
| ADMIRE 1 | 3 | 2–3 | 0.961 | 0.916 | – | 0.984 | <0.001 | >0.7 | ||
| ADMIRE 2 | 3 | 3–4 | 0.949 | 0.889 | – | 0.979 | <0.001 | >0.7 | ||
| ADMIRE 3 | 4 | 4–5 | 0.958 | 0.909 | – | 0.983 | 1.000 | 0.46 | ||
| ADMIRE 4 | 3 | 2–3 | 0.885 | 0.748 | – | 0.953 | <0.001 | >0.7 | ||
| ADMIRE 5 | 3 | 2–3 | 0.907 | 0.798 | – | 0.962 | <0.001 | >0.7 | ||
| 20 | 2.7 ± 1.8 | FBP | 2 | 1–2 | 0.974 | 0.943 | – | 0.989 | <0.001 | >0.7 |
| ADMIRE 1 | 2 | 1–2 | 0.971 | 0.938 | – | 0.988 | <0.001 | >0.7 | ||
| ADMIRE 2 | 2 | 1–2 | 0.968 | 0.930 | – | 0.987 | <0.001 | >0.7 | ||
| ADMIRE 3 | 2 | 1–3 | 0.968 | 0.930 | – | 0.987 | <0.001 | >0.7 | ||
| ADMIRE 4 | 3 | 2–3 | 0.933 | 0.853 | – | 0.973 | <0.001 | >0.7 | ||
| ADMIRE 5 | 3 | 2–3 | 0.936 | 0.861 | – | 0.974 | <0.001 | >0.7 | ||
| Radiation Dose (%) | ED (mSv) | Reconstruction | Rating | ICC | ICC: 95% CI | p | r | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Median | IQR | Average Measure | Lower Bound | Upper Bound | vs. 100% ADMIRE 2 | |||||
| 100 | 13.3 ± 9.1 | FBP | 5 | 4–5 | 0.958 | 0.909 | – | 0.983 | 1.000 | <0.1 |
| ADMIRE 1 | 5 | 4–5 | 0.942 | 0.874 | – | 0.976 | 1.000 | <0.1 | ||
| ADMIRE 2 | 5 | 4–5 | 1.000 | |||||||
| ADMIRE 3 | 5 | 4–5 | 1.000 | 1.000 | <0.1 | |||||
| ADMIRE 4 | 5 | 4–5 | 0.942 | 0.874 | – | 0.976 | 1.000 | <0.1 | ||
| ADMIRE 5 | 5 | 4–5 | 0.919 | 0.823 | – | 0.967 | 1.000 | <0.1 | ||
| 80 | 10.7 ± 7.2 | FBP | 4 | 3–5 | 0.985 | 0.969 | – | 0.994 | 0.777 | >0.7 |
| ADMIRE 1 | 4 | 3–5 | 0.984 | 0.966 | – | 0.994 | 1.000 | 0.70 | ||
| ADMIRE 2 | 4 | 4–5 | 0.974 | 0.943 | – | 0.989 | 1.000 | 0.37 | ||
| ADMIRE 3 | 4 | 4–5 | 0.973 | 0.941 | – | 0.989 | 1.000 | 0.40 | ||
| ADMIRE 4 | 4 | 3–5 | 0.959 | 0.910 | – | 0.983 | 1.000 | 0.59 | ||
| ADMIRE 5 | 4 | 3–5 | 0.962 | 0.918 | – | 0.985 | 1.000 | 0.67 | ||
| 60 | 8.0 ± 4.5 | FBP | 3 | 3–4 | 0.974 | 0.943 | – | 0.989 | 0.002 | >0.7 |
| ADMIRE 1 | 4 | 3–4 | 0.971 | 0.823 | – | 0.967 | 0.429 | >0.7 | ||
| ADMIRE 2 | 4 | 4–5 | 0.968 | 0.930 | – | 0.987 | 1.000 | 0.45 | ||
| ADMIRE 3 | 4 | 4–5 | 0.966 | 0.926 | – | 0.986 | 1.000 | 0.48 | ||
| ADMIRE 4 | 4 | 3–4 | 0.915 | 0.814 | – | 0.966 | 1.000 | 0.67 | ||
| ADMIRE 5 | 4 | 3–4 | 0.926 | 0.839 | – | 0.970 | 0.639 | >0.7 | ||
| 40 | 5.3 ± 3.6 | FBP | 2 | 1–2 | 0.974 | 0.943 | – | 0.989 | <0.001 | >0.7 |
| ADMIRE 1 | 3 | 2–3 | 0.971 | 0.938 | – | 0.988 | <0.001 | >0.7 | ||
| ADMIRE 2 | 3 | 3–4 | 0.968 | 0.930 | – | 0.987 | 0.002 | >0.7 | ||
| ADMIRE 3 | 4 | 3–4 | 0.966 | 0.926 | – | 0.986 | 1.000 | 0.40 | ||
| ADMIRE 4 | 3 | 2–3 | 0.915 | 0.814 | – | 0.966 | <0.001 | >0.7 | ||
| ADMIRE 5 | 3 | 2–3 | 0.926 | 0.839 | – | 0.970 | <0.001 | >0.7 | ||
| 20 | 2.7 ± 1.8 | FBP | 2 | 1–2 | 0.975 | 0.947 | – | 0.990 | <0.001 | >0.7 |
| ADMIRE 1 | 2 | 1–2 | 0.975 | 0.945 | – | 0.990 | <0.001 | >0.7 | ||
| ADMIRE 2 | 2 | 1–2 | 0.974 | 0.943 | – | 0.989 | <0.001 | >0.7 | ||
| ADMIRE 3 | 2 | 1–3 | 0.974 | 0.943 | – | 0.989 | <0.001 | >0.7 | ||
| ADMIRE 4 | 3 | 2–3 | 0.945 | 0.879 | – | 0.978 | <0.001 | >0.7 | ||
| ADMIRE 5 | 3 | 2–3 | 0.946 | 0.882 | – | 0.978 | <0.001 | >0.7 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brendlin, A.S.; Winkelmann, M.T.; Do, P.L.; Schwarze, V.; Peisen, F.; Almansour, H.; Bongers, M.N.; Artzner, C.P.; Weiss, J.; Kim, J.H.; et al. Simulated Radiation Dose Reduction in Whole-Body CT on a 3rd Generation Dual-Source Scanner: An Intraindividual Comparison. Diagnostics 2021, 11, 118. https://doi.org/10.3390/diagnostics11010118
Brendlin AS, Winkelmann MT, Do PL, Schwarze V, Peisen F, Almansour H, Bongers MN, Artzner CP, Weiss J, Kim JH, et al. Simulated Radiation Dose Reduction in Whole-Body CT on a 3rd Generation Dual-Source Scanner: An Intraindividual Comparison. Diagnostics. 2021; 11(1):118. https://doi.org/10.3390/diagnostics11010118
Chicago/Turabian StyleBrendlin, Andreas S., Moritz T. Winkelmann, Phuong Linh Do, Vincent Schwarze, Felix Peisen, Haidara Almansour, Malte N. Bongers, Christoph P. Artzner, Jakob Weiss, Jong Hyo Kim, and et al. 2021. "Simulated Radiation Dose Reduction in Whole-Body CT on a 3rd Generation Dual-Source Scanner: An Intraindividual Comparison" Diagnostics 11, no. 1: 118. https://doi.org/10.3390/diagnostics11010118
APA StyleBrendlin, A. S., Winkelmann, M. T., Do, P. L., Schwarze, V., Peisen, F., Almansour, H., Bongers, M. N., Artzner, C. P., Weiss, J., Kim, J. H., Othman, A. E., & Afat, S. (2021). Simulated Radiation Dose Reduction in Whole-Body CT on a 3rd Generation Dual-Source Scanner: An Intraindividual Comparison. Diagnostics, 11(1), 118. https://doi.org/10.3390/diagnostics11010118






