Aging and Allostasis: Using Bayesian Network Analytics to Explore and Evaluate Allostatic Markers in the Context of Aging
Abstract
1. Introduction
2. Materials and Methods
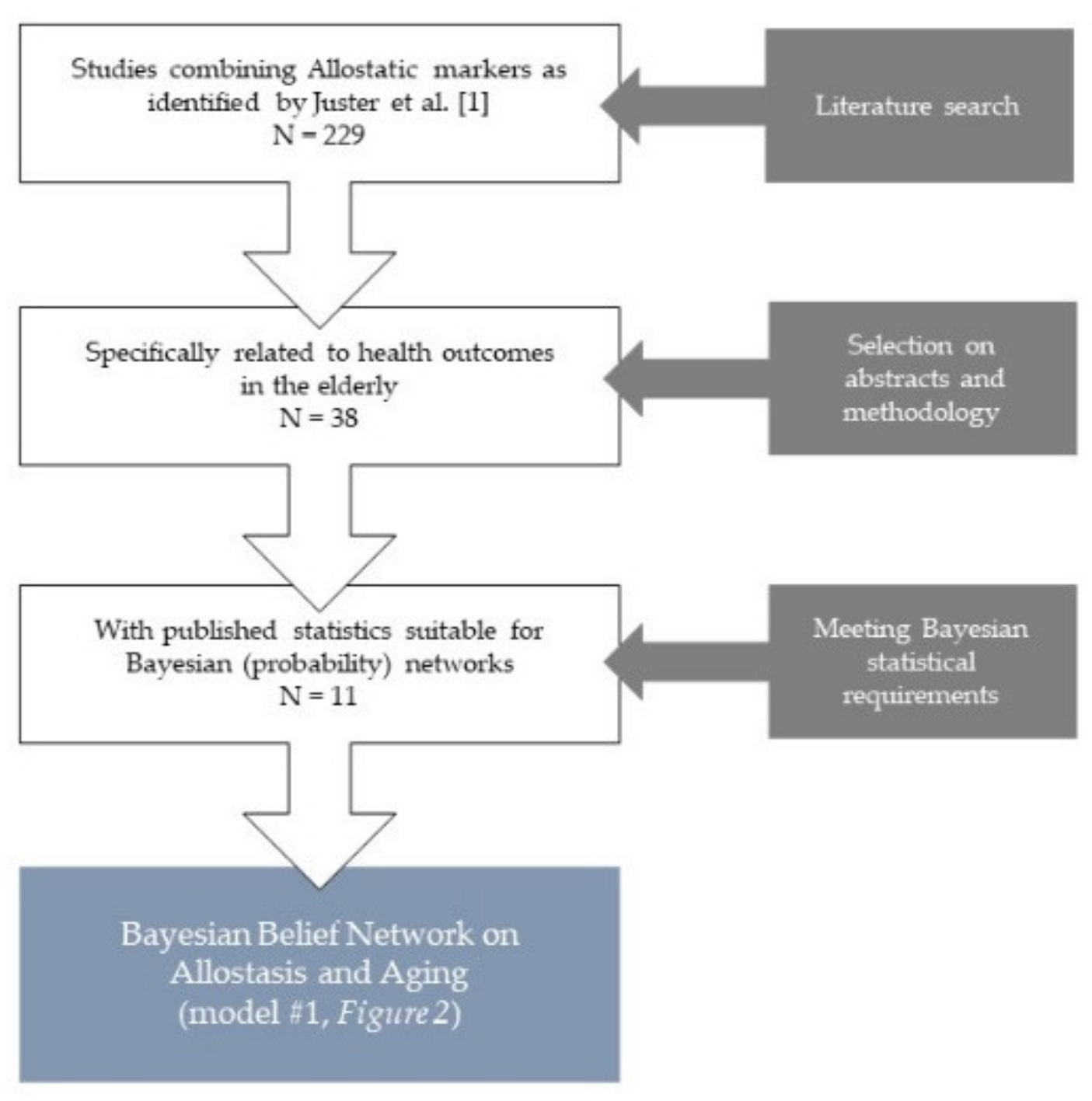
2.1. Search Strategy
2.2. Study Selection
2.3. Bayesian Belief Networks
2.4. Modeling
3. Results
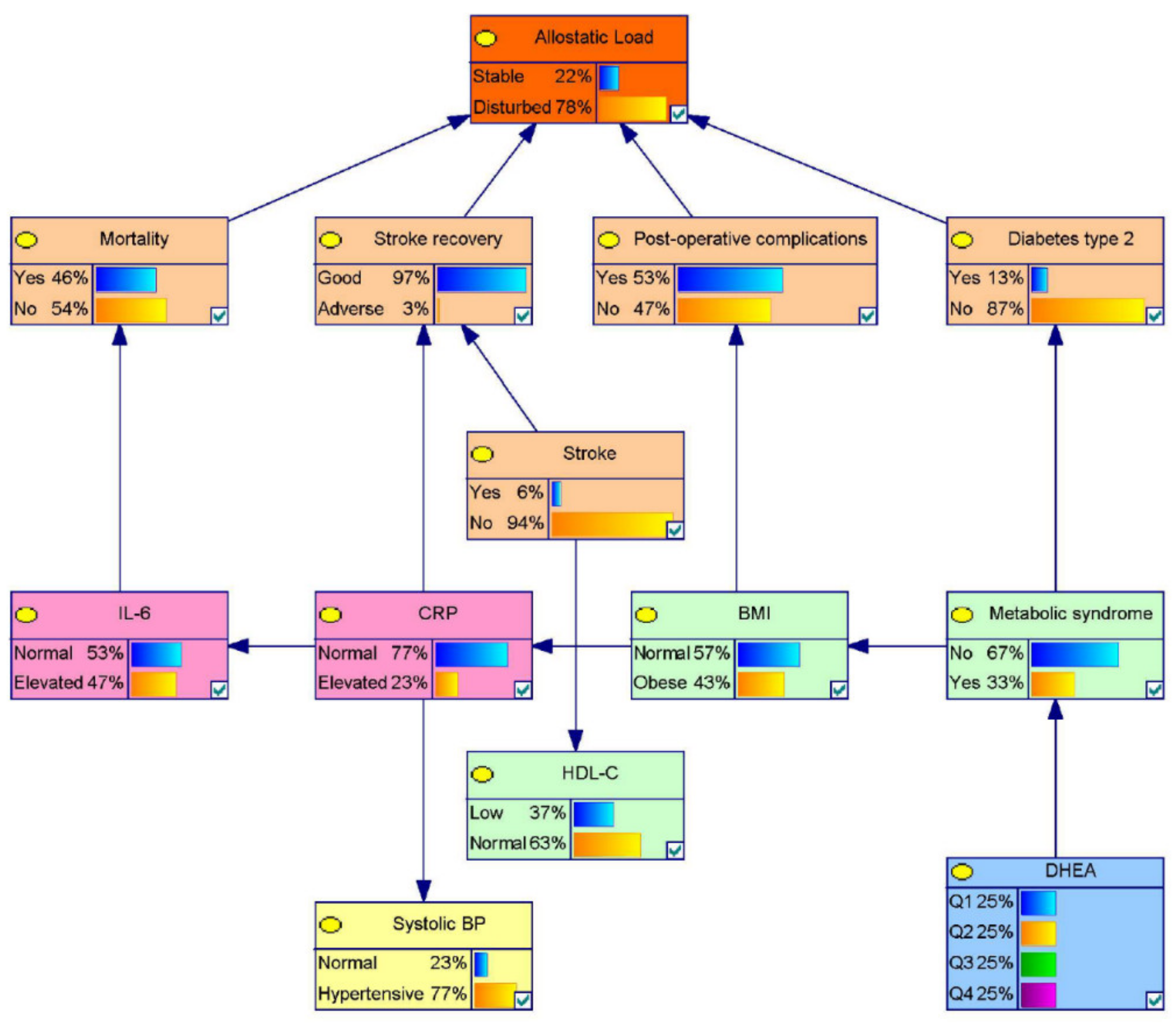
3.1. Model 1: A Probabilistic Allostatic Model in Elderly
Sensitivity Analysis for the Allostasis Model for Elderly
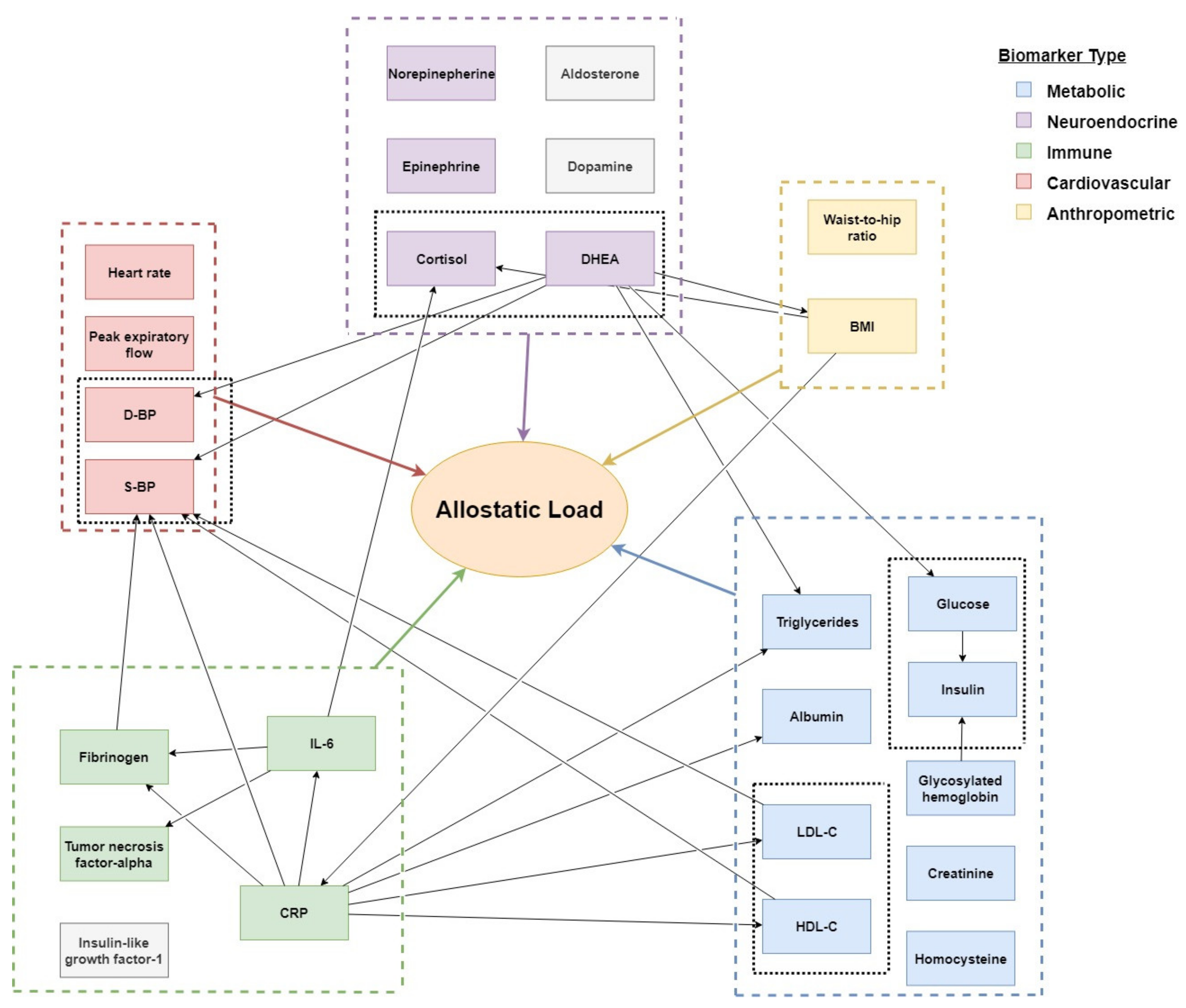
3.2. Model 2: An Extended Framework Covering Allostasis in Elderly
4. Discussion
4.1. The Concept of Allostasis in Aging Populations
4.2. Limitations
4.3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Juster, R.P.; McEwen, B.S.; Lupien, S.J. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci. Biobehav. Rev. 2010, 35, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Huber, M.; André Knottnerus, J.; Green, L.; Van Der Horst, H.; Jadad, A.R.; Kromhout, D.; Leonard, B.; Lorig, K.; Loureiro, M.I.; Van Der Meer, J.W.M.; et al. How should we define health? BMJ 2011, 343, d4163. [Google Scholar] [CrossRef] [PubMed]
- Piazza, J.R.; Stawski, R.S.; Sheffler, J.L. Age, Daily Stress Processes, and Allostatic Load: A Longitudinal Study. J. Aging Health 2019. [Google Scholar] [CrossRef] [PubMed]
- Sterling, P.; Eyer, J. Allostasis: A new paradigm to explain arousal pathology. In Handbook of Life Stress, Cognition and Health; John Wiley & Sons: Oxford, UK, 1988; pp. 629–649. ISBN 0-471-91269-7. [Google Scholar]
- Seeman, T.; Gruenewald, T.; Karlamangla, A.; Sidney, S.; Liu, K.; Mcewen, B.; Schwartz, J. Modeling multisystem biological risk in young adults: The Coronary Artery Risk Development in Young Adults Study. Am. J. Hum. Biol. 2010, 22, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Kallen, V.L.; Marck, J.W.; Stam, J.V.; Issa, A.; Johnson, B.; Van Meeteren, N.L.U. Psychophysiological Models to Identify and Monitor Elderly with a Cardiovascular Condition: The Added Value of Psychosocial Parameters to Routinely Applied Physiological Assessments. Sensors 2020, 20, 3240. [Google Scholar] [CrossRef] [PubMed]
- Mocayar Marón, F.J.; Ferder, L.; Saraví, F.D.; Manucha, W. Hypertension linked to allostatic load: From psychosocial stress to inflammation and mitochondrial dysfunction. Stress 2019, 22, 169–181. [Google Scholar] [CrossRef]
- Juster, R.P.; Sindi, S.; Marin, M.F.; Perna, A.; Hashemi, A.; Pruessner, J.C.; Lupien, S.J. A clinical allostatic load index is associated with burnout symptoms and hypocortisolemic profiles in healthy workers. Psychoneuroendocrinology 2011, 36, 797–805. [Google Scholar] [CrossRef]
- Whittaker, A.C.; Delledonne, M.; Finni, T.; Garagnani, P.; Greig, C.; Kallen, V.; Kokko, K.; Lord, J.; Maier, A.B.; Meskers, C.G.M.; et al. Physical Activity and Nutrition INfluences In ageing (PANINI): Consortium mission statement. Aging Clin. Exp. Res. 2018, 30, 685–692. [Google Scholar] [CrossRef]
- Taylor, J.; McFarland, M.J.; Carr, D.C. Age, Perceptions of Mattering, and Allostatic Load. J. Aging Health 2019. [Google Scholar] [CrossRef]
- Lipsitz, L.A. Physiological Complexity, Aging, and the Path to Frailty. Sci. Aging Knowl. Environ. 2004, 2004, pe16. [Google Scholar] [CrossRef]
- Seeman, T.E.; Singer, B.H.; Rowe, J.W.; Horwitz, R.I.; McEwen, B.S. Price of Adaptation—Allostatic Load and Its Health Consequences: MacArthur Studies of Successful Aging. Arch. Intern. Med. 1997, 157, 2259–2268. [Google Scholar] [CrossRef] [PubMed]
- Tabbarah, M.; Crimmins, E.M.; Seeman, T.E. The relationship between cognitive and physical performance: MacArthur studies of successful aging. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2002, 57, M228–M235. [Google Scholar] [CrossRef]
- Karlamangla, A.S.; Singer, B.H.; McEwen, B.S.; Rowe, J.W.; Seeman, T.E. Allostatic load as a predictor of functional decline: MacArthur studies of successful aging. J. Clin. Epidemiol. 2002, 55, 696–710. [Google Scholar] [CrossRef]
- McEwen, B.S.; Seeman, T. Protective and Damaging Effects of Mediators of Stress: Elaborating and Testing the Concepts of Allostasis and Allostatic Load. Ann. N. Y. Acad. Sci. 1999, 896, 30–47. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Biomarkers for assessing population and individual health and disease related to stress and adaptation. Metabolism 2015, 64, S2–S10. [Google Scholar] [CrossRef]
- Scheffer, M.; Carpenter, S.R.; Lenton, T.M.; Bascompte, J.; Brock, W.; Dakos, V.; van de Koppel, J.; van de Leemput, I.A.; Levin, S.A.; van Nes, E.H.; et al. Anticipating Critical Transitions. Science 2012, 338, 344–348. [Google Scholar] [CrossRef]
- Ghimire, S.; Hill, C.V.; Sy, F.S.; Rodriguez, R. Decline in telomere length by age and effect modification by gender, allostatic load and comorbidities in National Health and Nutrition Examination Survey (1999–2002). PLoS ONE 2019, 14, e0221690. [Google Scholar] [CrossRef]
- Shiels, P.G.; Stenvinkel, P.; Kooman, J.P.; McGuinness, D. Circulating markers of ageing and allostatic load: A slow train coming. Pract. Lab. Med. 2017, 7, 49–54. [Google Scholar] [CrossRef]
- McCrory, C.; Fiorito, G.; McLoughlin, S.; Polidoro, S.; Cheallaigh, C.N.; Bourke, N.; Karisola, P.; Alenius, H.; Vineis, P.; Layte, R.; et al. Epigenetic clocks and allostatic load reveal potential sex-specific drivers of biological aging. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2020. [Google Scholar] [CrossRef]
- Bäckman, L.; Nyberg, L.; Lindenberger, U.; Li, S.C.; Farde, L. The correlative triad among aging, dopamine, and cognition: Current status and future prospects. Neurosci. Biobehav. Rev. 2006, 30, 791–807. [Google Scholar] [CrossRef]
- Bauer, J.H. Age-Related Changes in the Renin-Aldosterone System. Drugs Aging 1993, 3, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Van den Beld, A.W.; Kaufman, J.M.; Zillikens, M.C.; Lamberts, S.W.J.; Egan, J.M.; van der Lely, A.J. The physiology of endocrine systems with ageing. Lancet Diabetes Endocrinol. 2018, 6, 647–658. [Google Scholar] [CrossRef]
- Shiels, P.G.; Buchanan, S.; Selman, C.; Stenvinkel, P. Allostatic load and ageing: Linking the microbiome and nutrition with age-related health. Biochem. Soc. Trans. 2019, 47, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Do Nascimento Falcão Freire, A.; de Souza Barbosa, J.F.; Pereira, D.S.; Dos Santos Gomes, C.; Guerra, R.O. Allostatic load and stress biomarkers in a sample of community-dwelling older adults. Arch. Gerontol. Geriatr. 2020, 87, 104006. [Google Scholar] [CrossRef]
- Seeman, T.E.; McEwen, B.S.; Rowe, J.W.; Singer, B.H. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proc. Natl. Acad. Sci. USA 2001, 98, 4770–4775. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.; Tahir, M.R.; Bongers, B.C.; Kallen, V.L.; Slooter, G.D.; van Meeteren, N.L. Prehabilitation before major intra-abdominal cancer surgery: A systematic review of randomised controlled trials. Eur. J. Anaesthesiol. 2019, 36, 933–945. [Google Scholar] [CrossRef]
- Lipsitz, L.A.; Goldberger, A.L. Loss of ‘Complexity’ and Aging: Potential Applications of Fractals and Chaos Theory to Senescence. JAMA J. Am. Med. Assoc. 1992, 267, 1806–1809. [Google Scholar] [CrossRef]
- Bishop, C.M. Pattern Recognition and Machine Learning; Springer: New York, NY, USA, 2006; ISBN 0387310738. [Google Scholar]
- Nielsen, T.D.; Jensen, F.V. Bayesian Networks and Decision Graphs; Springer Science & Business Media: Basel, Switzerland, 2009. [Google Scholar]
- Ai, A.L.; Kabbaj, M.; Kathy, L.L. Body affects mind? Preoperative behavioral and biological predictors for postoperative symptoms in mental health. J. Behav. Med. 2014, 37, 289–299. [Google Scholar] [CrossRef]
- Jackson, S.E.; Kirschbaum, C.; Steptoe, A. Hair cortisol and adiposity in a population-based sample of 2,527 men and women aged 54 to 87 years. Obesity 2017, 25, 539–544. [Google Scholar] [CrossRef]
- Heaney, J.L.J.; Carroll, D.; Phillips, A.C. Physical activity, life events stress, cortisol, and DHEA: Preliminary findings that physical activity may buffer against the negative effects of stress. J. Aging Phys. Act. 2014, 22, 465–473. [Google Scholar] [CrossRef]
- Peeters, G.M.E.E.; Van Schoor, N.M.; Van Rossum, E.F.C.; Visser, M.; Lips, P. The relationship between cortisol, muscle mass and muscle strength in older persons and the role of genetic variations in the glucocorticoid receptor. Clin. Endocrinol. 2008, 69, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Samaras, N.; Samaras, D.; Frangos, E.; Forster, A.; Philippe, J. A review of age-related dehydroepiandrosterone decline and its association with well-known geriatric syndromes: Is treatment beneficial? Rejuvenation Res. 2013, 16, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Baulieu, E.E.; Thomas, G.; Legrain, S.; Lahlou, N.; Roger, M.; Debuire, B.; Faucounau, V.; Girard, L.; Hervy, M.P.; Latour, F.; et al. Dehydroepiandrosterone (DHEA), DHEA sulfate, and aging: Contribution of the DHEAge Study to a sociobiomedical issue. Proc. Natl. Acad. Sci. USA 2000, 97, 4279–4284. [Google Scholar] [CrossRef] [PubMed]
- Pascualya, M.; Petrie, E.C.; Brodkin, K.; Peskind, E.R.; Veith, R.C.; Raskind, M.A. Effects of advanced aging on plasma catecholamine responses to the cold pressor test. Neurobiol. Aging 1999, 20, 637–642. [Google Scholar] [CrossRef]
- Reeves, S.; Bench, C.; Howard, R. Ageing and the nigrostriatal dopaminergic system. Int. J. Geriatr. Psychiatry 2002, 17, 359–370. [Google Scholar] [CrossRef]
- Li, H.; Liu, W.; Xie, J. Circulating interleukin-6 levels and cardiovascular and all-cause mortality in the elderly population: A meta-analysis. Arch. Gerontol. Geriatr. 2017, 73, 257–262. [Google Scholar] [CrossRef]
- Bruunsgaard, H.; Skinhøj, P.; Pedersen, A.N.; Schroll, M.; Pedersen, B.K. Ageing, tumour necrosis factor-alpha (TNF-α) and atherosclerosis. Clin. Exp. Immunol. 2000, 121, 255–260. [Google Scholar] [CrossRef]
- Tang, Y.; Fung, E.; Xu, A.; Lan, H.Y. C-reactive protein and ageing. Clin. Exp. Pharmacol. Physiol. 2017, 44, 9–14. [Google Scholar] [CrossRef]
- Seck, T.; Scheppach, B.; Scharla, S.; Diel, I.; Blum, W.F.; Bismar, H.; Schmid, G.; Krempien, B.; Ziegler, R.; Pfeilschifter, J. Concentration of Insulin-Like Growth Factor (IGF)-I and -II in Iliac Crest Bone Matrix from Pre- and Postmenopausal Women: Relationship to Age, Menopause, Bone Turnover, Bone Volume, and Circulating IGFs1. J. Clin. Endocrinol. Metab. 1998, 83, 2331–2337. [Google Scholar] [CrossRef]
- Garnero, P.; Sornay-Rendu, E.; Delmas, P.D. Low serum IGF-1 and occurrence of osteoporotic fractures in postmenopausal women. Lancet 2000, 355, 898–899. [Google Scholar] [CrossRef]
- Hager, K.; Felicetti, M.; Seefried, G.; Platt, D. Fibrinogen and aging. Aging Clin. Exp. Res. 1994, 6, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Holzer, M.; Trieb, M.; Konya, V.; Wadsack, C.; Heinemann, A.; Marsche, G. Aging affects high-density lipoprotein composition and function. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2013, 1831, 1442–1448. [Google Scholar] [CrossRef]
- Dubowitz, N.; Xue, W.; Long, Q.; Ownby, J.G.; Olson, D.E.; Barb, D.; Rhee, M.K.; Mohan, A.V.; Watson-Williams, P.I.; Jackson, S.L.; et al. Aging is associated with increased HbA1c levels, independently of glucose levels and insulin resistance, and also with decreased HbA1c diagnostic specificity. Diabet. Med. 2014, 31, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.; Kritchevsky, S.B.; Newman, A.B.; Goodpaster, B.H.; Tylavsky, F.A.; Nevitt, M.C.; Harris, T.B. Lower serum albumin concentration and change in muscle mass: The Health, Aging and Body Composition Study. Am. J. Clin. Nutr. 2005, 82, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Friedlander, J.I.; Moreira, D.M.; Hartman, C.; Elsamra, S.E.; Smith, A.D.; Okeke, Z. Age-related changes in 24-hour urine composition must be considered in the medical management of nephrolithiasis. J. Endourol. 2014, 28, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Pařízková, M.; Andel, R.; Lerch, O.; Marková, H.; Gažová, I.; Vyhnálek, M.; Hort, J.; Laczó, J. Homocysteine and Real-Space Navigation Performance among Non-Demented Older Adults. J. Alzheimer’s Dis. 2017, 55, 951–964. [Google Scholar] [CrossRef]
- Matsui, T.; Arai, H.; Yuzuriha, T.; Yao, H.; Miura, M.; Hashimoto, S.; Higuchi, S.; Matsushita, S.; Morikawa, M.; Kato, A.; et al. Elevated plasma homocysteine levels and risk of silent brain infarction in elderly people. Stroke 2001, 32, 1116–1119. [Google Scholar] [CrossRef]
- Rigaud, A.S.; Forette, B. Hypertension in Older Adults. J. Gerontol. Ser. A 2001, 56, M217–M225. [Google Scholar] [CrossRef]
- Janssens, J.P.; Pache, J.C.; Nicod, L.P. Physiological changes in respiratory function associated with ageing. Eur. Respir. J. 1999, 13, 197–205. [Google Scholar] [CrossRef]
- Stevens, J.; Katz, E.G.; Huxley, R.R. Associations between gender, age and waist circumference. Eur. J. Clin. Nutr. 2010, 64, 6–15. [Google Scholar] [CrossRef]
- Huxley, R.; Mendis, S.; Zheleznyakov, E.; Reddy, S.; Chan, J. Body mass index, waist circumference and waist:hip ratio as predictors of cardiovascular riska review of the literature. Eur. J. Clin. Nutr. 2010, 64, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.; Ho, S.C.; Yu, A.L.M.; Sham, A. Is waist circumference a useful measure in predicting health outcomes in the elderly? Int. J. Obes. 2002, 26, 1349–1355. [Google Scholar] [CrossRef] [PubMed]
- Prospective Studies Collaboration Body-mass index and cause-specific mortality in 900 000 adults: Collaborative analyses of 57 prospective studies. Lancet 2009, 373, 1083–1096. [CrossRef]
- Song, Y.M.; Sung, J.; Smith, G.D.; Ebrahim, S. Body Mass Index and Ischemic and Hemorrhagic Stroke: A Prospective Study in Korean Men. Stroke 2004, 35, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Visscher, T.L.S.; Seidell, J.C.; Molarius, A.; van der Kuip, D.; Hofman, A.; Witteman, J.C.M. A comparison of body mass index, waist–hip ratio and waist circumference as predictors of all-cause mortality among the elderly: The Rotterdam study. Int. J. Obes. 2001, 25, 1730–1735. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chang, H.H.; Wen, C.J.; Lin, W.Y.; Chen, C.Y.; Hong, B.S.; Huang, K.C. Elevated serum dehydroepiandrosterone sulphate level correlates with increased risk for metabolic syndrome in the elderly men. Eur. J. Clin. Investig. 2010, 40, 220–225. [Google Scholar] [CrossRef]
- Lee, J.K.; Bettencourt, R.; Brenner, D.; Le, T.A.; Barrett-Connor, E.; Loomba, R. Association between serum interleukin-6 concentrations and mortality in older adults: The rancho bernardo study. PLoS ONE 2012, 7. [Google Scholar] [CrossRef]
- Barone, M.; Viggiani, M.T.; Losurdo, G.; Principi, M.; Leandro, G.; Di Leo, A. Systematic review with meta-analysis: Post-operative complications and mortality risk in liver transplant candidates with obesity. Aliment. Pharmacol. Ther. 2017, 46, 236–245. [Google Scholar] [CrossRef]
- Sacco, R.L.; Benson, R.T.; Kargman, D.E.; Boden-Albala, B.; Tuck, C.; Lin, I.F.; Cheng, J.F.; Paik, M.C.; Shea, S.; Berglund, L. High-Density Lipoprotein Cholesterol and Ischemic Stroke in the ElderlyThe Northern Manhattan Stroke Study. JAMA 2001, 285, 2729–2735. [Google Scholar] [CrossRef]
- Bruckert, E. Epidemiology of low HDL-cholesterol: Results of studies and surveys. Eur. Heart J. Suppl. 2006, 8, 17–22. [Google Scholar] [CrossRef]
- Boix, R.; del Barrio, J.L.; Saz, P.; Reñe, R.; Manubens, J.M.; Lobo, A.; Gascón, J.; de Arce, A.; Díaz-Guzmán, J.; Bergareche, A.; et al. Stroke prevalence among the Spanish elderly: An analysis based on screening surveys. BMC Neurol. 2006, 6. [Google Scholar] [CrossRef] [PubMed]
- Sattar, N.; McConnachie, A.; Shaper, A.G.; Blauw, G.J.; Buckley, B.M.; de Craen, A.J.; Ford, I.; Forouhi, N.G.; Freeman, D.J.; Jukema, J.W.; et al. Can metabolic syndrome usefully predict cardiovascular disease and diabetes? Outcome data from two prospective studies. Lancet 2008, 371, 1927–1935. [Google Scholar] [CrossRef]
- Butcher, S.K.; Killampalli, V.; Lascelles, D.; Wang, K.; Alpar, K.E.; Lord, J.M. Raised cortisol: DHEAS ratios in the elderly after injury: Potential impact upon neutrophil function and immunity. Aging Cell 2005, 4, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Phillips, A.C.; Burns, V.E.; Lord, J.M. Stress and exercise: Getting the balance right for aging immunity. Exerc. Sport Sci. Rev. 2007, 35, 35–39. [Google Scholar] [CrossRef]
- Porges, S.W. Orienting in a defensive world: Mammalian modifications of our evolutionary heritage. A Polyvagal Theory. Psychophysiology 1995. [Google Scholar] [CrossRef]
- Gupta, D.; Morley, J.E. Hypothalamic-Pituitary-Adrenal (HPA) Axis and Aging. Compr. Physiol. 2014, 4, 1495–1510. [Google Scholar]
- Buford, T.W.; Willoughby, D.S. Impact of DHEA(S) and cortisol on immune function in aging: A brief review. Appl. Physiol. Nutr. Metab. 2008, 33, 429–433. [Google Scholar] [CrossRef]
- Belanger, A. Changes in serum concentrations of conjugated and unconjugated steroids in 40- to 80-year-old men. J. Clin. Endocrinol. Metab. 1994, 79, 1086–1090. [Google Scholar] [CrossRef]
- Goldwater, D.; Karlamangla, A.; Merkin, S.S.; Seeman, T. Compared to non-drinkers, individuals who drink alcohol have a more favorable multisystem physiologic risk score as measured by allostatic load. PLoS ONE 2019, 14, e0223168. [Google Scholar] [CrossRef]
- Galen Buckwalter, J.; Castellani, B.; Mcewen, B.; Karlamangla, A.S.; Rizzo, A.A.; John, B.; O’donnell, K.; Seeman, T. Allostatic load as a complex clinical construct: A case-based computational modeling approach. Complexity 2016, 21, 291–306. [Google Scholar] [CrossRef]
- Stephan, K.E.; Manjaly, Z.M.; Mathys, C.D.; Weber, L.A.E.; Paliwal, S.; Gard, T.; Tittgemeyer, M.; Fleming, S.M.; Haker, H.; Seth, A.K.; et al. Allostatic self-efficacy: A metacognitive theory of dyshomeostasis-induced fatigue and depression. Front. Hum. Neurosci. 2016, 10. [Google Scholar] [CrossRef] [PubMed]
- Al Saedi, A.; Feehan, J.; Phu, S.; Duque, G. Current and emerging biomarkers of frailty in the elderly. Clin. Interv. Aging 2019, 14, 389–398. [Google Scholar] [CrossRef] [PubMed]

| No | Allostatic Biomarker | Included in Models 1 and 2 | Included in Model 2 (√), or Excluded (X) | Effects of Aging | Associated Health Outcomes | References |
|---|---|---|---|---|---|---|
| Neuroendocrine | ||||||
| 1 | Cortisol | √ | Stable or increasing; remains highly responsive | Depressed mood; anxiety; hostility; and adiposity. | Ai et al., 2014 [31] Jackson et al., 2017 [32] Heaney et al., 2014 [33] Peeters et al., 2008 [34] | |
| 2 | Dehydroepiandrosterone (DHEA) | √ | Significant decline with aging; age 70–80 years ~20% compared to those aged 20–30 years | Musculoskeletal disorders; Cognitive disorders; Mood disorders; Cardiovascular disease; Sexual functioning; Menopause symptoms. | Heaney et al., 2014 [33] Samaras et al., 2013 [35] Baulieu et al., 2000 [36] | |
| 3 | Epinephrine (EPI) | √ | Higher basal plasma concentrations and higher responses to acute stressor. | Pascualya et al., 1999 [37] | ||
| 4 | Norepinephrine (NE) | √ | Higher basal plasma concentrations and higher responses to acute stressor. | Pascualya et al., 1999 [37] | ||
| 5 | Dopamine | X | Averaged 6–10% loss per decade, resulting in 40–50% loss between 18 and 88 years. | Parkinson; dementia. | Bäckman et al., 2006 [21] Reeves et al., 2002 [38] | |
| 6 | Aldosterone | X | Reduced (plasma) concentrations up to 50% at age 70 years. | Associated with progress of coronary artery disease. | Bauer, 1993 [22] | |
| Immune | ||||||
| 7 | Interleukin-6 | √ | Age-related pro-inflammatory state due to intrinsic dysregulation of the immune system. | Inflammation; morbidity; cardiovascular disease; diabetes mellitus, sarcopenia, dementia; depressed mood; anxiety; and hostility. | Ai et al., 2014 [31] Li et al., 2017 [39] | |
| 8 | Tumor necrosis factor-alpha (TNF-α) | √ | Increasing circulating levels of TNF-α. | Atherosclerosis. | Bruunsgaard et al., 2000 [40] | |
| 9 | C-reactive protein or high sensitivity C-reactive protein | √ | General risk factor associated with aging-related diseases. | Cardiovascular disease; hypertension; diabetes mellitus; kidney disease; atherosclerosis; depressed mood; anxiety; and hostility. | Ai et al., 2014 [31] Bruunsgaard et al., 2000 [40] Tang et al., 2017 [41] | |
| 10 | Insulin like growth factor-1 (IGF-1) | X | Declining (up to 60%). | Bone loss; and osteoporotic fractures. | Seck et al., 1998 [42] Garnero et al., 2000 [43] | |
| 11 | Fibrinogen | √ | Increase in 25 mq/dl per decade. | Cardio- and cerebrovascular disease. | Hager et al., 1994 [44] | |
| Metabolic | ||||||
| 12 | HDL-cholesterol | √ | Seems stable. | Cardiovascular diseases. | Holzer et al., 2013 [45] | |
| 13 | LDL-cholesterol | √ | Increases with aging. | Cardiovascular diseases. | Holzer et al., 2013 [45] | |
| 14 | Triglycerides | X | Increases with aging | Cardiovascular diseases. | Holzer et al., 2013 [45] | |
| 15 | Glycosylated hemoglobin (HbA1c) | √ | Increases with aging. | Cardiovascular & ischemic heart diseases; and diabetes. | Dubowitz et al., 2014 [46] | |
| 16 | Glucose | √ | Glucose hemostasis gets disturbed with aging. | Primarily, though not exclusively, diabetes. | Van den Beld et al., 2018 [23] | |
| 17 | Insulin | √ | Decreases with aging. | Diabetes and associated health problems. | Van den Beld et al., 2018 [23] | |
| 18 | Albumin | X | Decreases with aging. | Loss of appendicular muscle mass; sarcopenia. | Visser et al., 2005 [47] | |
| 19 | Creatinine | √ | Decreases with aging. | Renal function. | Friedlander et al., 2014 [48] | |
| 20 | Homocysteine | X | Increases with aging. | Alzheimer’s disease; lower cognitive performance; stroke. | Pařízková et al., 2017 [49] Matsui et al., 2001 [50] | |
| Cardiovascular & Respiratory | ||||||
| 21 | Systolic blood pressure * | √ | High incidence of hypertension (>140 mm Hg) in elderly. | Hypertension; cardiovascular problems; cerebrovascular morbidity; mortality. | Rigaud et al., 2001 [51] | |
| 22 | Diastolic blood pressure * | √ | High incidence of hypertension (>90 mm Hg) in elderly. | Hypertension; cardiovascular problems; cerebrovascular morbidity; mortality. | Rigaud et al., (2001) [51] | |
| 23 | Peak expiratory flow | X | Decreases with aging. | Asthma; COPD; decreased lung function. | Janssens et al., 1999 [52] | |
| 24 | Heart rate * | √ | Cardiovascular morbidity and mortality. | |||
| Anthropometric | ||||||
| 25 | Waist-to-hip ratio | √ | Increases with aging. | Cardiovascular disease; diabetes mellitus. | Stevens et al., 2010 [53] Huxley et al., 2010 [54] Woo et al., 2002 ** [55] | |
| 26 | Body mass index (BMI) | √ | Many health-related issues, e.g., ischemic heart disease; stroke; and overall mortality. | Prospective Studies Collaboration, 2009 [56] Song et al., 2004 [57] Visscher et al., 2001 [58] |
| Biomarkers or Outcomes | Reported Health Outcome(s) or Interactions | Cutoff Criteria | Reported or Calculated Risk Ratio | References |
|---|---|---|---|---|
| Dehydroepiandrosterone | Metabolic syndrome | 1.44 µmol/L | 1 | Chen et al., 2010 [59] |
| 2.31 µmol/L | 1.66 | |||
| 3.4 µmol/L | 1 | |||
| 13.5 µmol/L | 2.68 | |||
| C-reactive protein | Stroke recovery | ≤8 mg/L | 1.36 | Rigaud et al., 2001 [51] |
| Interleukin-6 | 1.97 | |||
| Systolic blood pressure | 1.18 | |||
| Interleukin-6 | Mortality | ≤1.8 pg/mL | 1.49 | Li et al., 2017 [39] Lee et al., 2012 [60] |
| Body mass index | Postoperative complications | 18.5–24.9 kg/m2 | 1.6 | Barone et al., 2017 [61] Lee et al., 2012 [60] |
| C-reactive protein | 1.4 | |||
| Stroke | HDL-C | Boolean | 1.24 | Sacco et al., 2001 [62] Bruckert et al., 2006 [63] Boix et al., 2006 [64] |
| Metabolic syndrome | Type II diabetes | Boolean | 4.42 | Sattar et al., 2008 [65] |
| Body mass index | Boolean | 6.76 | Lee et al., 2012 [60] |
| Allostatic Load | |||
|---|---|---|---|
| * Stable | * Disturbed | ||
| Mortality within 8 years | Yes | 0% | 59% |
| No | 100% | 41% | |
| Stroke | Yes | 4% | 7% |
| No | 96% | 93% | |
| Stroke recovery | Positive | 100% | 96% |
| Adverse | 0% | 4% | |
| Postoperative complications | Yes | 0% | 68% |
| No | 100 | 32% | |
| Type II diabetes | Yes | 0% | 16% |
| No | 100% | 84% | |
| Interleukin-6 | Elevated | 38% | 50% |
| Normal | 62% | 50% | |
| C-reactive protein | Elevated | 20% | 24% |
| Normal | 80% | 76% | |
| Body mass index | Obese | 26% | 48% |
| Normal | 74% | 52% | |
| Metabolic syndrome | Yes | 19% | 37% |
| No | 81% | 63% | |
| DHEA | Q1 | 27% | 25% |
| Q2 | 25% | 25% | |
| Q3 | 25% | 25% | |
| Q4 | 23% | 25% | |
| Parameter | Short Comment(s) | References | Reported Age Ranges |
|---|---|---|---|
| Cortisol | Highly responsive up until high age typically use in cortico-DHEA ratio in aging studies | Heaney et al., 2014 [33] | 65–88 |
| Peeters et al., 2008 [34] | 65+ | ||
| Epinephrine | Significant response to an acute stressor: helps a person to cope with physical and emotional stress | Pascualya et al., 1999 [37] | 24–26, 69–71, 83–85 |
| Norepinephrine | Insignificant response to acute stressor; however, in elderly this increase is due to increase concentration of plasma concentration and decrease in clearance | Pascualya et al., 1999 [37] | 24–26, 69–71, 83–85 |
| Interleukin-6 | Due to any dysregulation in the immune systems, circulating interleukin-6 levels are independently associated with greater risk of cardiovascular and all-cause mortality in the general elderly population | Li et al., 2017 [39] | 60+ |
| C-reactive protein | Important risk factor in elderly | Ai et al., 2014 [31] | 35+ |
| Bruunsgaard et al., 2000 [40] | 19–31, All 81 (n = 130) | ||
| Tang et al., 2017 [41] | Review: 80+ | ||
| Fibrinogen | Increases by 25 mg/dL/decade: good indicator for variance in an older population | Hager et al., 1994 [44] | 23–96 |
| High-density lipoprotein- cholesterol | Good indicator for variance, does not change significantly with aging | Holzer et al., 2013 [45] | 25–28, 65–69 |
| Creatinine | 24-hr urine creatinine decreases with aging | Friedlander et al., 2014 [48] | <45–65+ |
| Systolic and diastolic blood pressure | Indicator of hypertension; however, variance component is not that much | Rigaud et al. 2001 [68] | 60–84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kallen, V.; Tahir, M.; Bedard, A.; Bongers, B.; van Riel, N.; van Meeteren, N. Aging and Allostasis: Using Bayesian Network Analytics to Explore and Evaluate Allostatic Markers in the Context of Aging. Diagnostics 2021, 11, 157. https://doi.org/10.3390/diagnostics11020157
Kallen V, Tahir M, Bedard A, Bongers B, van Riel N, van Meeteren N. Aging and Allostasis: Using Bayesian Network Analytics to Explore and Evaluate Allostatic Markers in the Context of Aging. Diagnostics. 2021; 11(2):157. https://doi.org/10.3390/diagnostics11020157
Chicago/Turabian StyleKallen, Victor, Muhammad Tahir, Andrew Bedard, Bart Bongers, Natal van Riel, and Nico van Meeteren. 2021. "Aging and Allostasis: Using Bayesian Network Analytics to Explore and Evaluate Allostatic Markers in the Context of Aging" Diagnostics 11, no. 2: 157. https://doi.org/10.3390/diagnostics11020157
APA StyleKallen, V., Tahir, M., Bedard, A., Bongers, B., van Riel, N., & van Meeteren, N. (2021). Aging and Allostasis: Using Bayesian Network Analytics to Explore and Evaluate Allostatic Markers in the Context of Aging. Diagnostics, 11(2), 157. https://doi.org/10.3390/diagnostics11020157









