Photodynamic Diagnosis-Assisted Transurethral Resection Using Oral 5-Aminolevulinic Acid Decreases the Risk of Repeated Recurrence in Non-Muscle-Invasive Bladder Cancer: A Cumulative Incidence Analysis by the Person-Time Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Data Collection
2.2. Surgical Procedure and Device
2.3. Follow-Up after Initial TURBT
2.4. Statistical Analysis
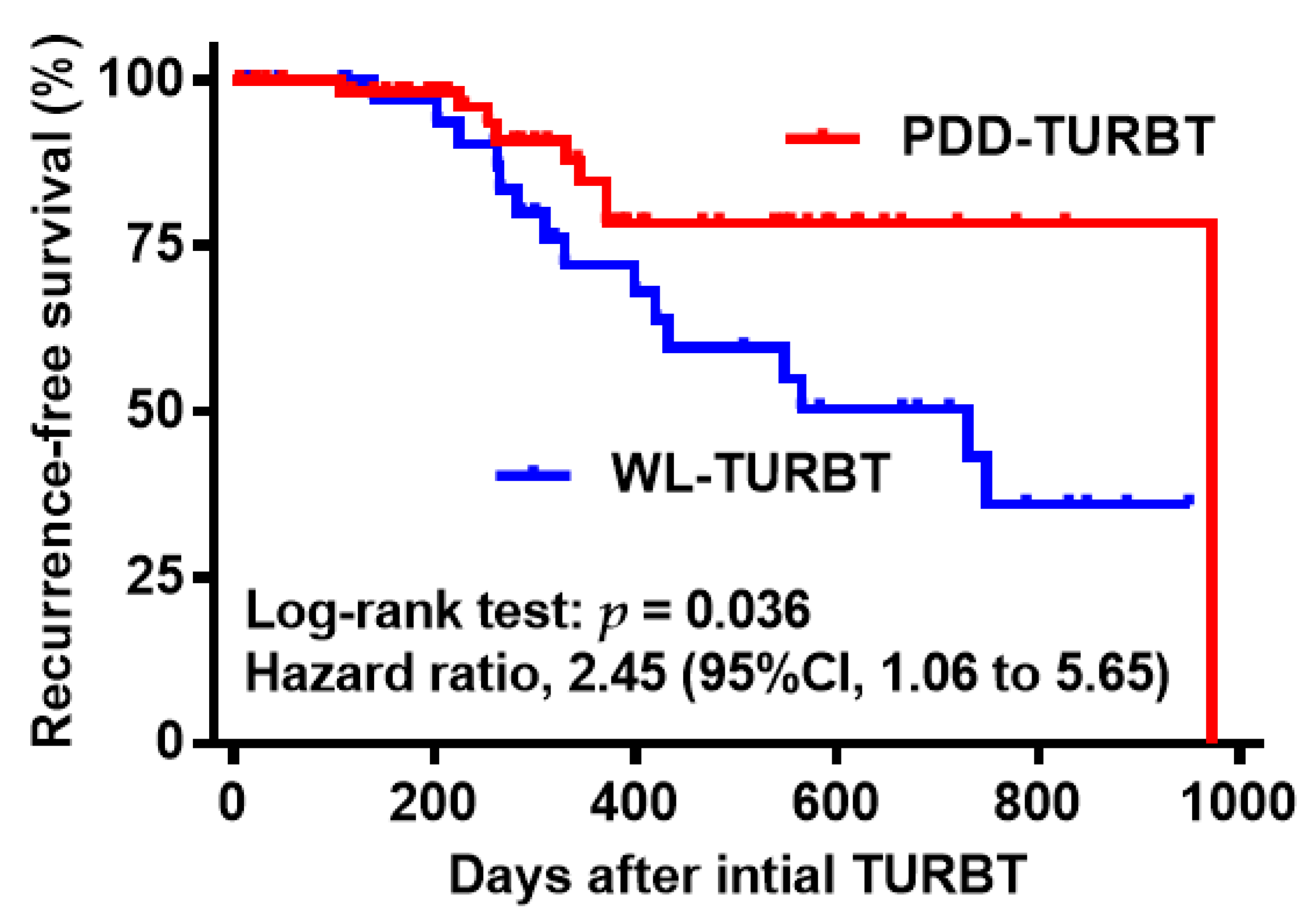
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miyake, M.; Gotoh, D.; Shimada, K.; Tatsumi, Y.; Nakai, Y.; Anai, S.; Torimoto, K.; Aoki, K.; Tanaka, N.; Konishi, N.; et al. Exploration of risk factors predicting outcomes for primary T1 high-grade bladder cancer and validation of the Spanish Urological Club for Oncological Treatment scoring model: Long-term follow-up experience at a single institute. Int. J. Urol. 2015, 22, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Ozono, S.; Hinotsu, S.; Tabata, S.; Takashima, K.; Fujimoto, K.; Okajima, E.; Hirao, Y.; Ohashi, Y.; Akaza, H.; Fukushima, S. Treated natural history of superficial bladder cancer. Jpn. J. Clin. Oncol. 2001, 31, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Hinotsu, S.; Akaza, H.; Ohashi, Y.; Kotake, T. Intravesical chemotherapy for maximum prophylaxis of new early phase superficial bladder carcinoma treated by transurethral resection: A combined analysis of trials by the Japanese Urological Cancer Research Group using smoothed hazard function. Cancer 1999, 86, 1818–1826. [Google Scholar] [CrossRef]
- Kausch, I.; Sommerauer, M.; Montorsi, F.; Stenzl, A.; Jacqmin, D.; Jichlinski, P.; Jocham, D.; Ziegler, A.; Vonthein, R. Photodynamic diagnosis in non-muscle-invasive bladder cancer: A systematic review and cumulative analysis of prospective studies. Eur. Urol. 2010, 57, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Burger, M.; Grossman, H.B.; Droller, M.; Schmidbauer, J.; Hermann, G.; Drăgoescu, O.; Ray, E.; Fradet, Y.; Karl, A.; Burgués, J.P.; et al. Photodynamic Diagnosis of Non-muscle-invasive Bladder Cancer with Hexaminolevulinate Cystoscopy: A Meta-analysis of Detection and Recurrence. Based on Raw Data. Eur. Urol. 2013, 64, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Fukuhara, H.; Shimamoto, T.; Kamada, M.; Iiyama, T.; Miyamura, M.; Kurabayashi, A.; Furihata, M.; Tanimura, M.; Watanabe, H.; et al. Comparison between intravesical and oral administration of 5-aminolevulinic acid in the clinical benefit of photodynamic diagnosis for nonmuscle invasive bladder cancer. Cancer 2012, 118, 1062–1074. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Anai, S.; Fujimoto, K.; Hirao, Y.; Furuse, H.; Kai, F.; Ozono, S.; Hara, T.; Matsuyama, H.; Oyama, M.; et al. Oral 5-aminolevulinic acid mediated photodynamic diagnosis using fluorescence cystoscopy for non-muscle-invasive bladder cancer: A randomized, double-blind, multicentre phase II/III study. Photodiagnosis Photodyn. Ther. 2015, 12, 193–200. [Google Scholar] [CrossRef]
- Nakai, Y.; Inoue, K.; Tsuzuki, T.; Shimamoto, T.; Shuin, T.; Nagao, K.; Matsuyama, H.; Oyama, M.; Furuse, H.; Ozono, S.; et al. Oral 5-aminolevulinic acid-mediated photodynamic diagnosis using fluorescence cystoscopy for non-muscle-invasive bladder cancer: A multicenter phase III study. Int. J. Urol. 2018, 25, 723–729. [Google Scholar] [CrossRef]
- Matsumoto, H.; Shiraishi, K.; Azuma, H.; Inoue, K.; Uemura, H.; Eto, M.; Ohyama, C.; Ogawa, O.; Kikuchi, E.; Kitamura, H.; et al. Clinical Practice Guidelines for Bladder Cancer 2019 edition by the Japanese Urological Association: Revision working position paper. Int. J. Urol. 2020, 27, 362–368. [Google Scholar] [CrossRef]
- Miyake, M.; Nishimura, N.; Fujii, T.; Miyamoto, T.; Iida, K.; Hori, S.; Morizawa, Y.; Gotoh, D.; Nakai, Y.; Anai, S.; et al. Photodynamic Diagnosis-Assisted En Bloc Transurethral Resection of Bladder Tumor for Nonmuscle Invasive Bladder Cancer: Short-Term Oncologic and Functional Outcomes. J. Endourol. 2020. Available online: https://www.liebertpub.com/doi/10.1089/end.2020.0371 (accessed on 3 November 2020).
- Rothman, K.J.; Greenland, S.; Lash, T.L. Modern Epidemiology, 3rd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008. [Google Scholar]
- Chan, I.S.F.; Bohidar, N.R. Exact power and sample size for vaccine efficacy studies. Commun. Statist. Theory Methods 1998, 27, 1305–1322. [Google Scholar] [CrossRef]
- Breslow, N.E.; Day, N.E. Statistical Methods in Cancer Research Volume II: The Design and Analysis of Cohort Studies; IARC Scientific Publication: Lyon, France, 1987. [Google Scholar]
- Dorresteijn, J.A.; Visseren, F.L.; Wassink, A.M.; Gondrie, M.J.; Steyerberg, E.W.; Ridker, P.M.; Cook, N.R.; van der Graaf, Y.; SMART Study Group. Development and validation of a prediction rule for recurrent vascular events based on a cohort study of patients with arterial disease: The SMART risk score. Heart 2013, 99, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Schoenfield, L.J.; Lachin, J.M. Chenodiol (chenodeoxycholic acid) for dissolution of gallstones: The National Cooperative Gallstone Study. A controlled trial of efficacy and safety. Ann. Intern. Med. 1981, 95, 257–282. [Google Scholar] [CrossRef] [PubMed]
- Yahya, N.; Ebert, M.A.; Bulsara, M.; House, M.J.; Kennedy, A.; Joseph, D.J.; Denham, J.W. Urinary symptoms following external beam radiotherapy of the prostate: Dose-symptom correlates with multiple-event and event-count models. Radiother. Oncol. 2015, 117, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Smedinga, H.; Steyerberg, E.W.; Beukers, W.; van Klaveren, D.; Zwarthoff, E.C.; Vergouwe, Y. Prediction of Multiple Recurrent Events: A Comparison of Extended Cox Models in Bladder Cancer. Am. J. Epidemiol. 2017, 186, 612–623. [Google Scholar] [CrossRef]
- Simon, M.; Bosset, P.O.; Rouanne, M.; Benhamou, S.; Radulescu, C.; Molinié, V.; Neuzillet, Y.; Paoletti, X.; Lebret, T. Multiple recurrences and risk of disease progression in patients with primary low-grade (TaG1) non-muscle-invasive bladder cancer and with low and intermediate EORTC-risk score. PLoS ONE 2019, 14, e0211721. [Google Scholar] [CrossRef]
- Inoue, K. 5-Aminolevulinic acid-mediated photodynamic therapy for bladder cancer. Int. J. Urol. 2017, 24, 97–101. [Google Scholar] [CrossRef]
- Lee, J.Y.; Diaz, R.R.; Cho, K.S.; Lim, M.S.; Chung, J.S.; Kim, W.T.; Ham, W.S.; Choi, Y.D. Efficacy and safety of photodynamic therapy for recurrent, high grade nonmuscle invasive bladder cancer refractory or intolerant to bacille Calmette-Guérin immunotherapy. J. Urol. 2013, 190, 1192–1199. [Google Scholar] [CrossRef]
- Berger, A.P.; Steiner, H.; Stenzl, A.; Akkad, T.; Bartsch, G.; Holtl, L. Photodynamic therapy with intravesical instillation of 5-aminolevulinic acid for patients with recurrent superficial bladder cancer, a single-center study. Urology 2003, 61, 338–341. [Google Scholar] [CrossRef]
- Waidelich, R.; Beyer, W.; Knüchel, R.; Stepp, H.; Baumgartner, R.; Schröder, J.; Hofstetter, A.; Kriegmair, M. Whole bladder photodynamic therapy with 5-aminolevulinic acid using a white light source. Urology 2003, 61, 332–337. [Google Scholar] [CrossRef]
- Skyrme, R.J.; French, A.S.; Allman, R.; Mason, M.D.; Matthews, P.N. A phase-1 study of sequential mitomycin C and 5-aminolaevulinic acid-mediated photodynamic therapy in recurrent superficial bladder carcinoma. BJU Int. 2005, 95, 1206–1210. [Google Scholar] [CrossRef]
- Miyake, M.; Matsuyama, H.; Teramukai, S.; Kinoshita, F.; Yokota, I.; Matsumoto, H.; Shimada, K.; Kinjyo, M.; Shimokama, T.; Okumura, K.; et al. A new risk stratification model for intravesical recurrence, disease progression, and cancer-specific death in patients with non-muscle invasive bladder cancer: The J-NICE risk tables. Int. J. Clin. Oncol. 2020, 25, 1364–1376. [Google Scholar] [CrossRef] [PubMed]
- Sylvester, R.J.; van der Meijden, A.P.; Oosterlinck, W.; Witjes, J.A.; Bouffioux, C.; Denis, L.; Newling, D.W.; Kurth, K. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: A combined analysis of 2596 patients from seven EORTC trials. Eur. Urol. 2006, 49, 723–729. [Google Scholar] [CrossRef] [PubMed]



| Variables | Total | WL-TURBT | PDD-TURBT | p Value |
|---|---|---|---|---|
| N | 100 | 40 | 60 | |
| Age, mean ± SD | 75.3 ± 9.0 | 73.9 ± 11.0 | 76.2 ± 7.3 | 0.26 # |
| Sex | 0.79 ## | |||
| Male | 89 | 36 (90%) | 53 (88%) | |
| Female | 11 | 4 (10%) | 7 (12%) | |
| Multiplicity | 0.46 ## | |||
| Single | 48 | 21 (53%) | 27 (45%) | |
| Multiple | 52 | 19 (48%) | 33 (55%) | |
| Tumor size | 0.04 ## | |||
| Less than 1 cm | 17 | 6 (15%) | 11 (18%) | |
| 1–3 cm | 62 | 19 (48%) | 43 (72%) | |
| 3 cm or more | 21 | 15 (37%) | 6 (10%) | |
| T category | 0.066 ## | |||
| Ta | 68 | 23 (58%) | 45 (75%) | |
| T1 | 32 | 17 (42%) | 15 (25%) | |
| Tumor grade (WHO 2004) | 0.13 ## | |||
| Low-grade | 71 | 25 (63%) | 46 (77%) | |
| High-grade | 29 | 15 (37%) | 14 (23%) |
| Variables | Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | ||
| Type of TURBT | WL-TURBT | 1 | 1 | ||||
| PDD-TURBT | 0.35 | 0.15–0.87 | 0.023 | 0.35 | 0.14–0.92 | 0.032 | |
| Age (years) | Less than 70 | 1 | |||||
| 70 or more | 1.03 | 0.91–1.17 | 0.64 | ||||
| Sex | Male | 1 | |||||
| Female | 0.89 | 0.19–4.07 | 0.88 | ||||
| Multiplicity | Single | 1 | |||||
| Multiple | 1.04 | 0.96–1.12 | 0.34 | ||||
| Tumor size | Less than 3 cm | 1 | 1 | ||||
| 3 cm or more | 1.84 | 071–4.80 | 0.21 | 1.01 | 0.98–1.04 | 0.47 | |
| T category | Ta | 1 | |||||
| T1 | 0.82 | 0.34–1.97 | 0.66 | 0.57 | 0.09–3.3 | 0.53 | |
| Tumor grade | Low-grade | 1 | |||||
| High-grade | 0.72 | 0.26–1.96 | 0.66 | 1.14 | 0.14–9.5 | 0.90 | |
| Surgical Method | WL-TURBT | PDD-TURBT |
|---|---|---|
| Patients | 40 | 60 |
| Cumulative incidence (bladder recurrence) | 26 | 9 |
| Person-days at risk | 24,855 | 23,174 |
| Incidence (per 10,000 person-days) | 10.5 | 3.9 |
| Normal approximation confidence interval | 6.44 to 14.5 | 1.35 to 6.42 |
| Incidence rate ratio (Clopper–Pearson confidence interval) | - | 0.37 (0.15 to 0.82) |
| Exact p value | - | 0.011 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyake, M.; Nishimura, N.; Nakai, Y.; Fujii, T.; Owari, T.; Hori, S.; Morizawa, Y.; Gotoh, D.; Anai, S.; Torimoto, K.; et al. Photodynamic Diagnosis-Assisted Transurethral Resection Using Oral 5-Aminolevulinic Acid Decreases the Risk of Repeated Recurrence in Non-Muscle-Invasive Bladder Cancer: A Cumulative Incidence Analysis by the Person-Time Method. Diagnostics 2021, 11, 185. https://doi.org/10.3390/diagnostics11020185
Miyake M, Nishimura N, Nakai Y, Fujii T, Owari T, Hori S, Morizawa Y, Gotoh D, Anai S, Torimoto K, et al. Photodynamic Diagnosis-Assisted Transurethral Resection Using Oral 5-Aminolevulinic Acid Decreases the Risk of Repeated Recurrence in Non-Muscle-Invasive Bladder Cancer: A Cumulative Incidence Analysis by the Person-Time Method. Diagnostics. 2021; 11(2):185. https://doi.org/10.3390/diagnostics11020185
Chicago/Turabian StyleMiyake, Makito, Nobutaka Nishimura, Yasushi Nakai, Tomomi Fujii, Takuya Owari, Shunta Hori, Yosuke Morizawa, Daisuke Gotoh, Satoshi Anai, Kazumasa Torimoto, and et al. 2021. "Photodynamic Diagnosis-Assisted Transurethral Resection Using Oral 5-Aminolevulinic Acid Decreases the Risk of Repeated Recurrence in Non-Muscle-Invasive Bladder Cancer: A Cumulative Incidence Analysis by the Person-Time Method" Diagnostics 11, no. 2: 185. https://doi.org/10.3390/diagnostics11020185
APA StyleMiyake, M., Nishimura, N., Nakai, Y., Fujii, T., Owari, T., Hori, S., Morizawa, Y., Gotoh, D., Anai, S., Torimoto, K., Tanaka, N., Hirao, Y., & Fujimoto, K. (2021). Photodynamic Diagnosis-Assisted Transurethral Resection Using Oral 5-Aminolevulinic Acid Decreases the Risk of Repeated Recurrence in Non-Muscle-Invasive Bladder Cancer: A Cumulative Incidence Analysis by the Person-Time Method. Diagnostics, 11(2), 185. https://doi.org/10.3390/diagnostics11020185







