Analyses of the Relation between BPPV and Thyroid Diseases: A Nested Case-Control Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Definition of Benign Paroxysmal Vertigo (Dependent Variable)
2.3. Definition of Thyroid Diseases and Levothyroxine Use (Independent Variable)
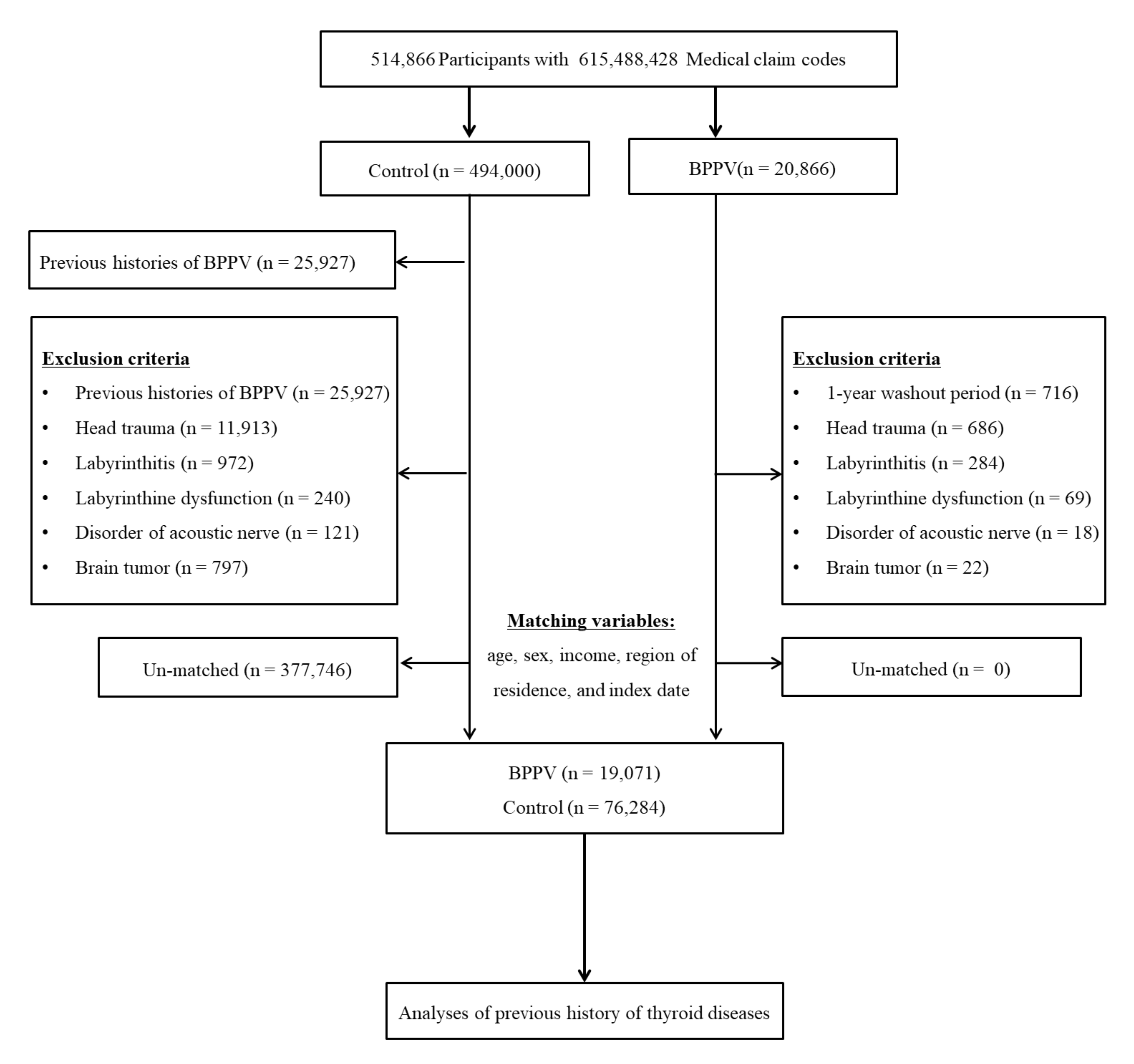
2.4. Participant Selection
2.5. Covariates
2.6. Statistical Analyses
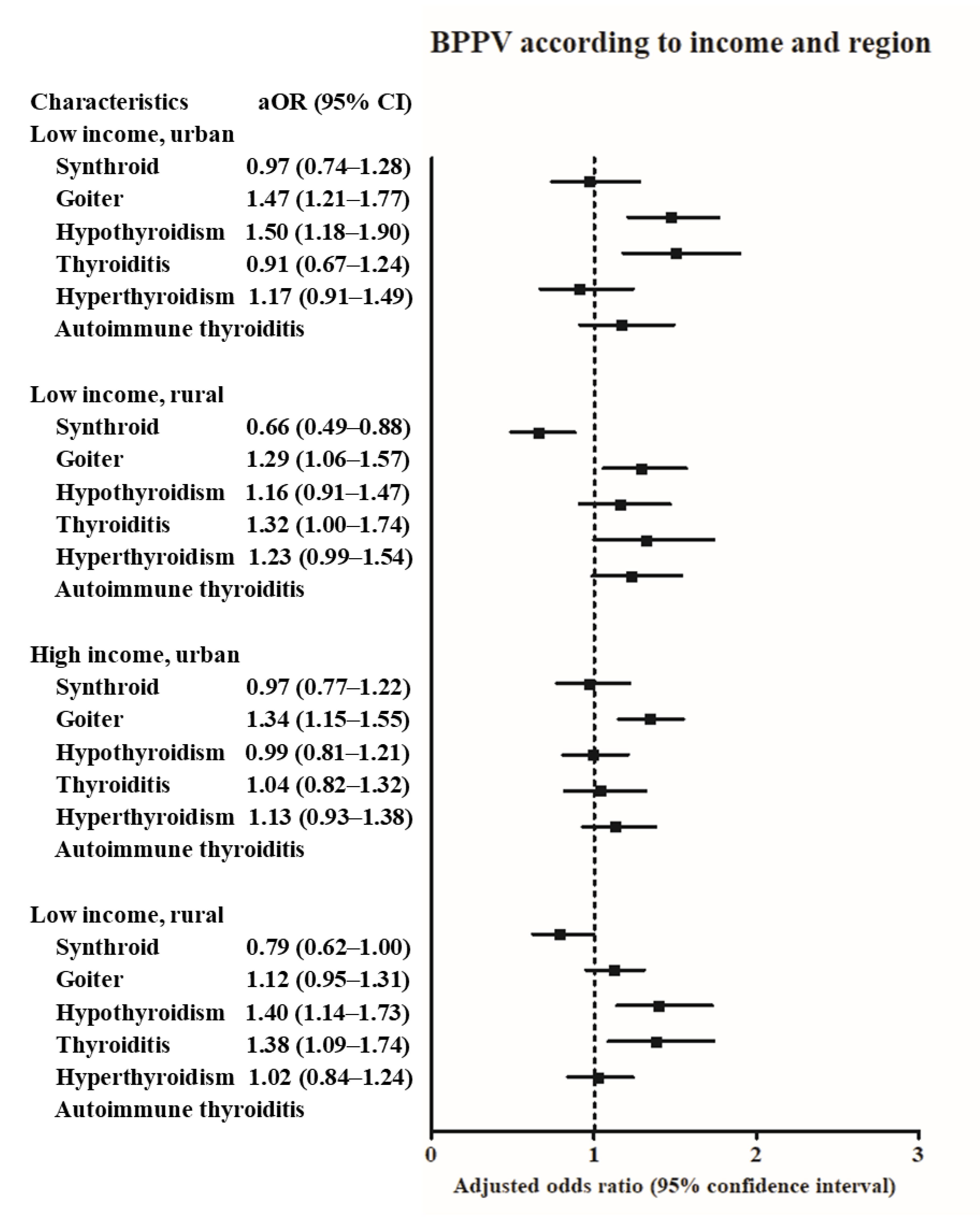
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brent, G.A. Mechanisms of thyroid hormone action. J. Clin. Investig. 2012, 122, 3035–3043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giorda, C.B.; Carna, P.; Romeo, F.; Costa, G.; Tartaglino, B.; Gnavi, R. Prevalence, incidence and associated comorbidities of treated hypothyroidism: An update from a European population. Eur. J. Endocrinol. 2017, 176, 533–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanderpump, M.P.; Tunbridge, W.M. Epidemiology and prevention of clinical and subclinical hypothyroidism. Thyroid 2002, 12, 839–847. [Google Scholar] [CrossRef]
- Trovato, M. A historical excursus of diagnostic methods for Hashimoto thyroiditis and Graves’ disease. Gazz. Med. Ital. Arch. Sci. Med. 2020, 179, 479–485. [Google Scholar] [CrossRef]
- Nyström, H.F.; Jansson, S.; Berg, G.E.B. Incidence rate and clinical features of hyperthyroidism in a long-term iodine sufficient area of Sweden (Gothenburg) 2003–2005. Clin. Endocrinol. 2013, 78, 768–776. [Google Scholar] [CrossRef]
- Taylor, P.N.; Albrecht, D.; Scholz, A.; Gutierrez-Buey, G.; Lazarus, J.H.; Dayan, C.M.; Okosieme, O.E. Global epidemiology of hyperthyroidism and hypothyroidism. Nat. Rev. Endocrinol. 2018, 14, 301–316. [Google Scholar] [CrossRef]
- Biondi, B.; Kahaly, G.J.; Robertson, R.P. Thyroid Dysfunction and Diabetes Mellitus: Two Closely Associated Disorders. Endocr. Rev. 2019, 40, 789–824. [Google Scholar] [CrossRef] [Green Version]
- Wolffenbuttel, B.H.R.; Wouters, H.J.C.M.; Slagter, S.N.; Van Waateringe, R.P.; Kobold, A.C.M.; Van Vliet-Ostaptchouk, J.V.; Links, T.P.; Van Der Klauw, M.M. Thyroid function and metabolic syndrome in the population-based LifeLines cohort study. BMC Endocr. Disord. 2017, 17, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jabbar, A.; Pingitore, A.; Pearce, S.H.S.; Zaman, A.J.A.; Iervasi, A.P.G.; Razvi, A.J.S.H.S.P.A.Z.S. Thyroid hormones and cardiovascular disease. Nat. Rev. Cardiol. 2017, 14, 39–55. [Google Scholar] [CrossRef]
- Andrade, C.L.O.; Machado, G.C.; Fernandes, L.D.C.; Albuquerque, J.M.; Casais-E-Silva, L.L.; Ramos, H.E.; Alves, C.A.D. Mechanisms involved in hearing disorders of thyroid ontogeny: A literature review. Arch. Endocrinol. Metab. 2017, 61, 501–505. [Google Scholar] [CrossRef] [Green Version]
- Chiarella, G.G.; Russo, D.; Monzani, F.F.; Petrolo, C.C.; Fattori, B.B.; Pasqualetti, G.; Cassandro, E.E.; Costante, G. Hashimoto Thyroiditis and Vestibular Dysfunction. Endocr. Pract. 2017, 23, 863–868. [Google Scholar] [CrossRef]
- Chiarella, G.; Tognini, S.; Nacci, A.; Sieli, R.; Costante, G.; Petrolo, C.; Mancini, V.; Guzzi, P.H.; Pasqualetti, G.; Cassandro, E.; et al. Vestibular disorders in euthyroid patients with Hashimoto’s thyroiditis: Role of thyroid autoimmunity. Clin. Endocrinol. 2014, 81, 600–605. [Google Scholar] [CrossRef]
- Von Brevern, M.; Radtke, A.; Lezius, F.; Feldmann, M.; Ziese, T.; Lempert, T.; Neuhauser, H. Epidemiology of benign paroxysmal positional vertigo: A population based study. J. Neurol. Neurosurg. Psychiatry 2007, 78, 710–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Brevern, M.; Bertholon, P.; Brandt, T.; Fife, T.; Imai, T.; Nuti, D.; Newman-Toker, D. Benign paroxysmal positional vertigo: Diagnostic criteria. J. Vestib. Res. 2015, 25, 105–117. [Google Scholar] [CrossRef] [Green Version]
- Brandt, T.; Steddin, S. Current view of the mechanism of benign paroxysmal positioning vertigo: Cupulolithiasis or canalolithiasis? J. Vestib. Res. 1993, 3, 373–382. [Google Scholar]
- Luryi, A.L.; Lawrence, J.; Larouere, M.; Babu, S.; Bojrab, D.I.; Zappia, J.; Sargent, E.W.; Schutt, C.A. Treatment of Patients with Benign Paroxysmal Positional Vertigo and Severe Immobility Using the Particle Repositioning Chair: A Retrospective Cohort Study. Ann. Otol. Rhinol. Laryngol. 2018, 127, 390–394. [Google Scholar] [CrossRef]
- Mumford, C.J. Post-traumatic benign paroxysmal positional vertigo. Pract. Neurol. 2019, 19, 354–355. [Google Scholar] [CrossRef]
- Riga, M.; Bibas, A.G.; Xenellis, J.; Korres, S. Inner ear disease and benign paroxysmal positional vertigo: A critical review of incidence, clinical characteristics, and management. Int. J. Otolaryngol. 2011, 2011, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Papi, G.; Guidetti, G.; Corsello, S.M.; Di Donato, C.; Pontecorvi, A. The association between benign paroxysmal positional vertigo and autoimmune chronic thyroiditis is not related to thyroid status. Thyroid 2010, 20, 237–238. [Google Scholar] [CrossRef]
- Papi, G.; Corsello, S.M.; Milite, M.T.; Zanni, M.; Ciardullo, A.V.; Donato, C.D.; Pontecorvi, A. Association between benign paroxysmal positional vertigo and autoimmune chronic thyroiditis. Clin. Endocrinol. 2009, 70, 169–170. [Google Scholar] [CrossRef] [PubMed]
- Sari, K.; Yildirim, T.; Börekçi, H.; Akin, I.; Aydin, R.; Ozkiris, M. The relationship between benign paroxysmal positional vertigo and thyroid autoimmunity. Acta Otolaryngol. 2015, 135, 754–757. [Google Scholar] [CrossRef]
- Santos, M.D.; Bittar, R.S.M. Vertigo and metabolic disorders. Int. Tinnitus J. 2012, 17, 16–20. [Google Scholar]
- Rybak, L.P. Metabolic disorders of the vestibular system. Otolaryngol. Head Neck Surg. 1995, 112, 128–132. [Google Scholar] [CrossRef]
- Hsu, C.L.; Tsai, S.J.; Shen, C.C.; Lu, T.; Hung, Y.M.; Hu, L.Y. Risk of benign paroxysmal positional vertigo in patients with depressive disorders: A nationwide population-based cohort study. BMJ Open 2019, 9, e026936. [Google Scholar] [CrossRef] [Green Version]
- Miskiewicz-Orczyk, K.A.; Lisowska, G.; Kajdaniuk, D.; Wojtulek, M. Can Hashimoto’s thyroiditis cause vertigo? [Czy choroba Hashimoto moze byc przyczyna zawrotow glowy?]. Endokrynol. Pol. 2020, 70, 76–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.Y.; Min, C.; Oh, D.J.; Choi, H.G. Tobacco Smoking and Alcohol Consumption Are Related to Benign Parotid Tumor: A Nested Case-Control Study Using a National Health Screening Cohort. Clin. Exp. Otorhinolaryngol. 2019, 12, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.G.; Lee, J.K.; Kong, I.G.; Lim, H.; Kim, S.Y. Osteoporosis increases the risk of benign paroxysmal positional vertigo: A nested case-control study using a national sample cohort. Eur. Arch. Oto Rhino Laryngol. 2019, 276, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Song, Y.S.; Wee, J.H.; Min, C.; Yoo, D.M.; Choi, H.G. Association between Meniere’s disease and thyroid diseases: A nested case-control study. Sci. Rep. 2020, 10, 18224. [Google Scholar] [CrossRef]
- Kim, S.Y.; Min, C.; Oh, D.J.; Choi, H.G. Bidirectional Association Between GERD and Asthma: Two Longitudinal Follow-Up Studies Using a National Sample Cohort. J. Allergy Clin. Immunol. Pract. 2020, 8, 1005–1013.e9. [Google Scholar] [CrossRef]
- World Health Organization. Regional Office for the Western Pacific. The Asia-Pacific Perespective: Redefining Obesity and its Treatment; Health Communications Australia: Sydney, Australia, 2000. [Google Scholar]
- Quan, H.; Li, B.; Couris, C.M.; Fushimi, K.; Graham, P.; Hider, P.; Januel, J.M.; Sundararajan, V. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am. J. Epidemiol. 2011, 173, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Quan, H.; Sundararajan, V.; Halfon, P.; Fong, A.; Burnand, B.; Luthi, J.C.; Saunders, L.D.; Beck, C.A.; Feasby, T.E.; Ghali, W.A. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care 2005, 43, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Modugno, G.C.; Pirodda, A.; Ferri, G.G.; Montana, T.; Rasciti, L.; Ceroni, A.R. A relationship between autoimmune thyroiditis and benign paroxysmal positional vertigo? Med. Hypotheses 2000, 54, 614–615. [Google Scholar] [CrossRef] [PubMed]
- Picciotti, P.M.; Di Cesare, T.; Tricarico, L.; De Corso, E.; Galli, J.; Paludetti, G. Is drug consumption correlated with benign paroxysmal positional vertigo (BPPV) recurrence? Eur. Arch. Oto Rhino Laryngol. 2020, 277, 1609–1616. [Google Scholar] [CrossRef]
- Tomer, Y. Mechanisms of autoimmune thyroid diseases: From genetics to epigenetics. Annu. Rev. Pathol. 2014, 9, 147–156. [Google Scholar] [CrossRef] [Green Version]
- Diez, J.J. Hypothyroidism in patients older than 55 years: An analysis of the etiology and assessment of the effectiveness of therapy. J. Gerontol. A Biol. Sci. Med. Sci. 2002, 57, M315–M320. [Google Scholar] [CrossRef] [Green Version]
- Keim, R.J.; Sachs, G.B. Positional nystagmus in association with macroglobulinemia. Ann. Otol. Rhinol. Laryngol. 1975, 84, 223–227. [Google Scholar] [CrossRef]
- Girasoli, L.; Cazzador, D.; Padoan, R.; Nardello, E.; Felicetti, M.; Zanoletti, E.; Schiavon, F.; Bovo, R. Update on Vertigo in Autoimmune Disorders, from Diagnosis to Treatment. J. Immunol. Res. 2018, 2018, 1–16. [Google Scholar] [CrossRef]
- Manzari, L. Enlarged vestibular aqueduct (EVA) related with recurrent benign paroxysmal positional vertigo (BPPV). Med. Hypotheses 2008, 70, 61–65. [Google Scholar] [CrossRef]
- Wemeau, J.L.; Kopp, P. Pendred syndrome. Best Pract. Res. Clin. Endocrinol. Metab. 2017, 31, 213–224. [Google Scholar] [CrossRef]
- Sugiura, M.; Sato, E.; Nakashima, T.; Sugiura, J.; Furuhashi, A.; Yoshino, T.; Nakayama, A.; Mori, N.; Murakami, H.; Naganawa, S. Long-term follow-up in patients with Pendred syndrome: Vestibular, auditory and other phenotypes. Eur. Arch. Oto Rhino Laryngol. 2005, 262, 737–743. [Google Scholar] [CrossRef]
- Song, J.-J.; Hong, S.K.; Kim, J.S.; Koo, J.-W. Enlarged vestibular aqueduct may precipitate benign paroxysmal positional vertigo in children. Acta Otolaryngol. 2012, 132, S109–S117. [Google Scholar] [CrossRef] [PubMed]
- Belguith-Maalej, S.; Rebuffat, S.A.; Charfeddine, I.; Mnif, M.; Nadir, R.F.; Abid, M.; Ghorbel, A.; Peraldi-Roux, S.; Ayadi, H.; Hadj-Kacem, H. SLC26A4 expression among autoimmune thyroid tissues. Immunobiology 2011, 216, 571–578. [Google Scholar] [CrossRef]
- Mian, C.; Lacroix, L.; Alzieu, L.; Nocera, M.; Talbot, M.; Bidart, J.M.; Schlumberger, M.; Caillou, B. Sodium iodide symporter and pendrin expression in human thyroid tissues. Thyroid 2001, 11, 825–830. [Google Scholar] [CrossRef]
- He, J.W.; Gong, Q.; Wang, X.F.; Xiao, Z. High stimulus rate brainstem auditory evoked potential in benign paroxysmal positional vertigo. Eur. Arch. Oto Rhino Laryngol. 2015, 272, 2095–2100. [Google Scholar] [CrossRef] [PubMed]
- Amor-Dorado, J.C.; Llorca, J.; Costa-Ribas, C.; Garcia-Porrua, C.; Gonzalez-Gay, M.A. Giant cell arteritis: A new association with benign paroxysmal positional vertigo. Laryngoscope 2004, 114, 1420–1425. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Shamanna, K. Management of Benign Paroxysmal Positional Vertigo: A Comparative Study between Epleys Manouvre and Betahistine. Int. Tinnitus J. 2017, 21, 30–34. [Google Scholar] [CrossRef] [Green Version]
- Jalali, M.M.; Gerami, H.; Saberi, A.; Razaghi, S. The Impact of Betahistine versus Dimenhydrinate in the Resolution of Residual Dizziness in Patients with Benign Paroxysmal Positional Vertigo: A Randomized Clinical Trial. Ann. Otol Rhinol Laryngol. 2020, 129, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Murdin, L.; Hussain, K.; Schilder, A.G. Betahistine for symptoms of vertigo. Cochrane Database Syst. Rev. 2016, CD010696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tani, N.; Ishikawa, M.; Watanabe, M.; Ikeda, T.; Ishikawa, T. Thyroid-related hormones as potential markers of hypoxia/ischemia. Hum. Cell 2020, 33, 545–558. [Google Scholar] [CrossRef] [Green Version]
- Razvi, S. Novel uses of thyroid hormones in cardiovascular conditions. Endocrine 2019, 66, 115–123. [Google Scholar] [CrossRef] [Green Version]

| Characteristics | Total Participants | ||
|---|---|---|---|
| BPPV (n, %) | Control (n, %) | p-Value | |
| Age (years old) | 1.000 | ||
| 40–44 | 250 (1.3) | 1000 (1.3) | |
| 45–49 | 1359 (7.1) | 5436 (7.1) | |
| 50–54 | 2939 (15.4) | 11,756 (15.4) | |
| 55–59 | 3338 (17.5) | 13,352 (17.5) | |
| 60–64 | 3302 (17.3) | 13,208 (17.3) | |
| 65–69 | 3083 (16.2) | 12,332 (16.2) | |
| 70–74 | 2524 (13.2) | 10,096 (13.2) | |
| 75–79 | 1551 (8.1) | 6204 (8.1) | |
| 80–84 | 579 (3.0) | 2316 (3.0) | |
| 85+ | |||
| Sex | 1.000 | ||
| Male | 6878 (36.1) | 27,512 (36.1) | |
| Female | 12,193 (63.9) | 48,772 (63.9) | |
| Income | 1.000 | ||
| 1 (lowest) | 2961 (15.5) | 11,844 (15.5) | |
| 2 | 2295 (12.0) | 9180 (12.0) | |
| 3 | 2875 (15.1) | 11,500 (15.1) | |
| 4 | 4081 (21.4) | 16,324 (21.4) | |
| 5 (highest) | 6859 (36.0) | 27,436 (36.0) | |
| Region of residence | 1.000 | ||
| Urban | 8689 (45.6) | 34,756 (45.6) | |
| Rural | 10,382 (54.4) | 41,528 (54.4) | |
| Obesity † | <0.001 * | ||
| Underweight | 385 (2.0) | 1935 (2.5) | |
| Normal | 6392 (33.5) | 27,089 (35.5) | |
| Overweight | 5428 (28.5) | 20,652 (27.1) | |
| Obese I | 6263 (32.8) | 24,098 (31.6) | |
| Obese II | 603 (3.2) | 2510 (3.3) | |
| Smoking status | <0.001 * | ||
| Nonsmoker | 15,397 (80.7) | 60,147 (78.9) | |
| Past smoker | 2003 (10.5) | 7047 (9.2) | |
| Current smoker | 1671 (8.8) | 9090 (11.9) | |
| Alcohol consumption | <0.001 * | ||
| <1 time a week | 14,398 (75.5) | 55,586 (72.9) | |
| ≥1 time a week | 4673 (24.5) | 20,698 (27.1) | |
| CCI score | |||
| 0 | 12,245 (64.2) | 52,632 (69.0) | <0.001 * |
| 1 | 3376 (17.7) | 10,586 (13.9) | |
| 2 | 1731 (9.1) | 6030 (7.9) | |
| 3 | 804 (4.2) | 3040 (4.0) | |
| ≥4 | 915 (4.8) | 3996 (5.2) | |
| Thyroid cancer | 367 (1.9) | 298 (1.7) | 0.036 * |
| Osteoporosis | 6091 (31.9) | 20,328 (26.7) | <0.001 * |
| Period of taking levothyroxine | <0.001 * | ||
| <3month | 18,313 (96.0) | 73,728 (96.7) | |
| ≥3month | 758 (4.0) | 2556 (3.4) | |
| Goiter | 1051 (5.5) | 3132 (4.1) | <0.001 * |
| Hypothyroidism | 898 (4.7) | 2809 (3.7) | <0.001 * |
| Thyroiditis | 400 (2.1) | 1241 (1.6) | <0.001 * |
| Hyperthyroidism | 583 (3.1) | 1870 (2.5) | <0.001 * |
| Autoimmune thyroiditis | 142 (0.7) | 510 (0.7) | 0.254 |
| Characteristics | Odd Ratios for BPPV | |||||
|---|---|---|---|---|---|---|
| Crude † | p-Value | Model 1 †,‡ | p-Value | Model 2 †,§ | p-Value | |
| Total participants (n = 95,355) | ||||||
| Levothyroxine | 1.20 (1.10–1.30) | <0.001 * | 1.13 (1.03–1.24) | 0.014 * | 0.85 (0.75–0.96) | 0.011 * |
| Goiter | 1.37 (1.27–1.47) | <0.001 * | 1.31 (1.21–1.42) | <0.001 * | 1.28 (1.17–1.39) | <0.001 * |
| Hypothyroidism | 1.30 (1.20–1.40) | <0.001 * | 1.24 (1.13–1.35) | <0.001 * | 1.23 (1.10–1.37) | <0.001 * |
| Thyroiditis | 1.30 (1.16–1.46) | <0.001 * | 1.28 (1.13–1.44) | <0.001 * | 1.16 (1.02–1.32) | 0.025 * |
| Hyperthyroidism | 1.26 (1.14–1.38) | <0.001 * | 1.21 (1.09–1.34) | <0.001 * | 1.13 (1.02–1.26) | 0.023 * |
| Autoimmune thyroiditis | 1.12 (0.93–1.34) | 0.254 | 1.15 (0.94–1.41) | 0.169 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, H.G.; Song, Y.S.; Wee, J.H.; Min, C.; Yoo, D.M.; Kim, S.Y. Analyses of the Relation between BPPV and Thyroid Diseases: A Nested Case-Control Study. Diagnostics 2021, 11, 329. https://doi.org/10.3390/diagnostics11020329
Choi HG, Song YS, Wee JH, Min C, Yoo DM, Kim SY. Analyses of the Relation between BPPV and Thyroid Diseases: A Nested Case-Control Study. Diagnostics. 2021; 11(2):329. https://doi.org/10.3390/diagnostics11020329
Chicago/Turabian StyleChoi, Hyo Geun, Young Shin Song, Jee Hye Wee, Chanyang Min, Dae Myoung Yoo, and So Young Kim. 2021. "Analyses of the Relation between BPPV and Thyroid Diseases: A Nested Case-Control Study" Diagnostics 11, no. 2: 329. https://doi.org/10.3390/diagnostics11020329






