Preoperative Magnetic Resonance Cholangiopancreatography for Detecting Difficult Laparoscopic Cholecystectomy in Acute Cholecystitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. MRI Assessment
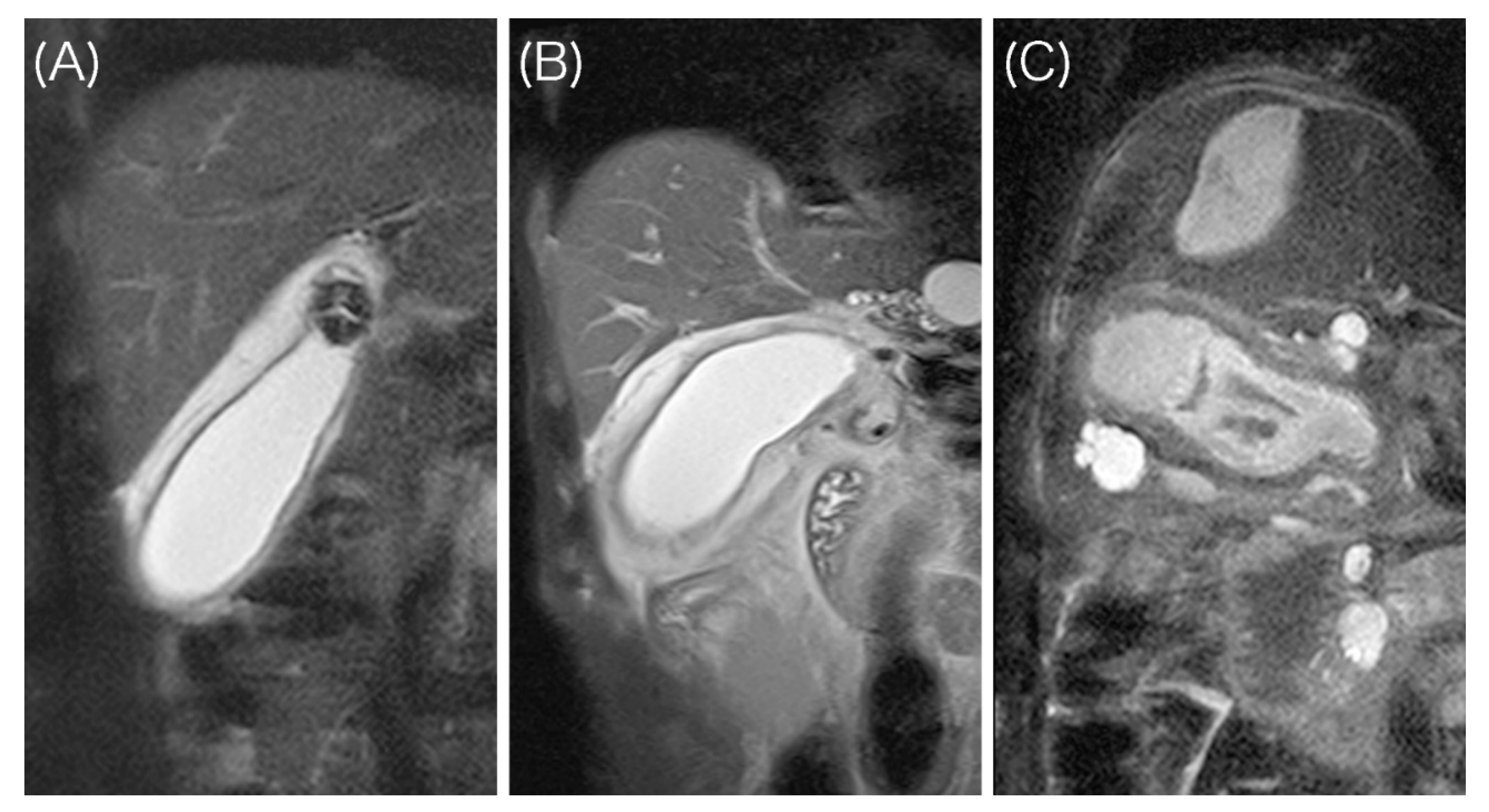
- A high signal intensity (HSI) group having two layers with a discrete margin composed of a thin inner layer (≤3 mm) with a low signal and a relatively thick outer layer with a high signal;
- An intermediate signal intensity (ISI) group having two layers with a partially ill-defined margin composed of a partially thickened inner layer (>3 mm) with a low signal and an outer layer with a high or partially heterogeneous intermediate signal;
- A low signal intensity (LSI) group having ill-defined layers composed of a diffusely thickened inner layer (>3 mm) with a low signal and an outer layer with an intermediate to low signal.
2.3. Outcomes
2.3.1. Primary Outcomes
2.3.2. Secondary Outcomes
2.4. Identification of Predictors for Increased Surgical Difficulty
2.5. Statistical Analysis
3. Results
3.1. Patient Selection and MRI Assessment
3.2. Patient Characteristics in Each MRI Group
3.3. Outcomes in Each MRI Group
3.4. Identification of Risk Factors for Bailout Procedures
3.5. Identification of Risk Factors for Prolonged Operation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Okamoto, K.; Suzuki, K.; Takada, T.; Strasberg, S.M.; Asbun, H.J.; Endo, I.; Yamamoto, M. Tokyo Guidelines 2018: Flowchart for the manage-ment of acute cholecystitis. J. Hepato Biliary Pancreatic Sci. 2018, 2, 55–72. [Google Scholar] [CrossRef]
- Wakabayashi, G.; Iwashita, Y.; Hibi, T.; Takada, T.; Strasberg, S.M.; Asbun, H.J.; Endo, I.; Umezawa, A.; Asai, K.; Suzuki, K.; et al. Tokyo Guidelines 2018: Surgical management of acute cholecystitis: Safe steps in laparoscopic cholecystectomy for acute cholecystitis (with videos). J. Hepato Biliary Pancreatic Sci. 2018, 25, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Flum, D.R.; Dellinger, E.P.; Cheadle, A.; Chan, L.; Koepsell, T. Intraoperative Cholangiography and Risk of Common Bile Duct Injury during Cholecystectomy. JAMA 2003, 289, 1639–1644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seibert, D.; Matulis, S.R.; Griswold, F. A rare right hepatic duct anatomical variant discovered after laparoscopic bile duct transection. Surg. Laparosc. Endosc. 1996, 2, 61–64. [Google Scholar] [CrossRef]
- Adamsen, S.; Hansen, O.H.; Funch-Jensen, P.; Schulze, S.; Stage, J.G.; Wara, P. Bile duct injury during laparoscopic cholecystecto-my: A prospective nationwide series. J. Am. Coll. Surg. 1997, 2, 571–578. [Google Scholar]
- Hugh, T.B. New strategies to prevent laparoscopic bile duct injury—Surgeons can learn from pilots. Surgery 2002, 132, 826–835. [Google Scholar] [CrossRef]
- Peng, W.K.; Sheikh, Z.; Nixon, S.J.; Paterson-Brown, S. Role of laparoscopic cholecystectomy in the early management of acute gallbladder disease. BJS 2005, 92, 586–591. [Google Scholar] [CrossRef]
- Nebiker, C.A.; Baierlein, S.A.; Beck, S.; Von Flüe, M.; Ackermann, C.; Peterli, R. Is routine MR cholangiopancreatography (MRCP) justified prior to cholecystectomy? Langenbeck’s Arch. Surg. 2008, 394, 1005–1010. [Google Scholar] [CrossRef]
- Wong, H.-P.; Chiu, Y.-L.; Shiu, B.-H.; Ho, L.-C. Preoperative MRCP to detect choledocholithiasis in acute calculous cholecystitis. J. Hepato Biliary Pancreatic Sci. 2012, 19, 458–464. [Google Scholar] [CrossRef] [Green Version]
- Kurata, M.; Honda, G.; Okuda, Y.; Kobayashi, S.; Sakamoto, K.; Iwasaki, S.; Chiba, K.; Tabata, T.; Kuruma, S.; Kamisawa, T. Preoperative detection and handling of aberrant right posterior sectoral hepatic duct during laparoscopic cholecystectomy. J. Hepato Biliary Pancreatic Sci. 2015, 22, 558–562. [Google Scholar] [CrossRef]
- Jung, S.E.; Lee, J.M.; Lee, K.; Rha, S.E.; Choi, B.G.; Kim, E.K.; Hahn, S.T. Gallbladder wall thickening: MR imaging and pathologic correla-tion with emphasis on layered pattern. Eur. Radiol. 2005, 2, 694–701. [Google Scholar] [CrossRef]
- Omiya, K.; Hiramatsu, K.; Kato, T.; Shibata, Y.; Yoshihara, M.; Aoba, T.; Arimoto, A.; Ito, A. Preoperative MRI for predicting pathological changes associated with surgical difficulty during laparoscopic cholecystectomy for acute cholecystitis. BJS Open 2020, 4, 1137–1145. [Google Scholar] [CrossRef]
- Hirota, M.; Takada, T.; Kawarada, Y.; Nimura, Y.; Miura, F.; Hirata, K.; Mayumi, T.; Yoshida, M.; Strasberg, S.; Pitt, H.; et al. Diagnostic criteria and severity assessment of acute cholecystitis: Tokyo Guidelines. J. Hepato Biliary Pancreatic Surg. 2007, 14, 78–82. [Google Scholar] [CrossRef] [Green Version]
- Yokoe, M.; Takada, T.; Strasberg, S.M.; Solomkin, J.S.; Mayumi, T.; Gomi, H.; Pitt, H.A.; Garden, O.J.; Kiriyama, S.; Hata, J.; et al. TG13 diagnostic criteria and severity grading of acute cholecystitis (with videos). J. Hepato Biliary Pancreatic Sci. 2013, 20, 35–46. [Google Scholar] [CrossRef]
- Yokoe, M.; Hata, J.; Takada, T.; Strasberg, S.M.; Bun, T.A.Y.; Wakabayashi, G.; Kozaka, K.; Endo, I.; DeZiel, D.J.; Miura, F.; et al. Tokyo Guidelines 2018: Diagnostic criteria and severity grading of acute cholecystitis (with videos). J. Hepato Biliary Pancreatic Sci. 2018, 25, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Takada, T.; Strasberg, S.M.; Pitt, H.A.; Gouma, D.J.; Garden, O.J.; Supe, A.N. TG13 surgical management of acute cholecystitis. J. Hepato Biliary Pancreatic Sci. 2013, 2, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Zhang, Z.; Wang, Y.; Gong, K.; Lu, Y.; Zhang, N. Comparison of Laparoscopic Cholecystectomy for Acute Cholecystitis within and Beyond 72 h of Symptom Onset During Emergency Admissions. World J. Surg. 2012, 36, 2654–2658. [Google Scholar] [CrossRef]
- Terho, P.M.; Leppäniemi, A.K.; Mentula, P.J. Laparoscopic cholecystectomy for acute calculous cholecystitis: A retrospective study assessing risk factors for conversion and complications. World J. Emerg. Surg. 2016, 11, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shamiyeh, A.; Danis, J.; Wayand, W.; Zehetner, J. A 14-year analysis of laparoscopic cholecystectomy: Conversion—When and why? Surg. Laparosc. Endosc. Percutan. Tech. 2007, 2, 271–276. [Google Scholar] [CrossRef]
- Sakuramoto, S.; Sato, S.; Okuri, T.; Sato, K.; Hiki, Y.; Kakita, A. Preoperative evaluation to predict technical difficulties of laparo-scopic cholecystectomy on the basis of histological inflammation findings on resected gallbladder. Am. J. Surg. 2000, 2, 114–121. [Google Scholar] [CrossRef]
- Philip Rothman, J.; Burcharth, J.; Pommergaard, H.C.; Viereck, S.; Rosenberg, J. Preoperative Risk Factors for Conversion of Laparoscopic Cholecystectomy to Open Surgery—A Systematic Review and Meta-Analysis of Observational Studies. Dig. Surg. 2016, 2, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Low, J.K.; Barrow, P.; Owera, A.; Ammori, B.J. Timing of laparoscopic cholecystectomy for acute cholecystitis: Evidence to sup-port a proposal for an early interval surgery. Am. Surg. 2007, 2, 1188–1192. [Google Scholar] [CrossRef]
- Hiromatsu, T.; Hasegawa, H.; Sakamoto, E.; Komatsu, S.; Kawai, K.; Tabata, T. Preoperative evaluation of difficulty on laparo-scopic cholecystectomy (in Japanese). Jpn. Gastroenterol. Surg. 2007, 40, 1449–1455. [Google Scholar] [CrossRef] [Green Version]
- Ćwik, G.; Skoczylas, T.; Wyroślak-Najs, J.; Wallner, G. The value of percutaneous ultrasound in predicting conversion from laparoscopic to open cholecystectomy due to acute cholecystitis. Surg. Endosc. 2013, 27, 2561–2568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, K.S.; Baek, S.Y.; Kang, B.C.; Choi, H.-Y.; Han, H.-S. Evaluation of preoperative sonography in acute cholecystitis to predict technical difficulties during laparoscopic cholecystectomy. J. Clin. Ultrasound 2004, 32, 115–122. [Google Scholar] [CrossRef]
- Cho, J.Y.; Han, H.-S.; Yoon, Y.-S.; Ahn, K.S.; Lee, S.H.; Hwang, J.-H. Hepatobiliary Scan for Assessing Disease Severity in Patients with Cholelithiasis. Arch. Surg. 2011, 146, 169. [Google Scholar] [CrossRef] [Green Version]
- Ambe, P.C.; Christ, H.; Wassenberg, D. Does the Tokyo guidelines predict the extent of gallbladder inflammation in patients with acute cholecystitis? A single center retrospective analysis. BMC Gastroenterol. 2015, 15, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Bennett, G.L.; Rusinek, H.; Lisi, V.; Israel, G.M.; Krinsky, G.A.; Slywotzky, C.M.; Megibow, A. CT Findings in Acute Gangrenous Cholecystitis. Am. J. Roentgenol. 2002, 178, 275–281. [Google Scholar] [CrossRef]
- Utsumi, M.; Aoki, H.; Kunitomo, T.; Mushiake, Y.; Yasuhara, I.; Taniguchi, F.; Arata, T.; Katsuda, K.; Tanakaya, K.; Takeuchi, H. Preoperative Risk Factors for Conversion of Laparoscopic Cholecystectomy to Open Cholecystectomy and the Usefulness of the 2013 Tokyo Guidelines. Acta Med. Okayama 2017, 71, 419–425. [Google Scholar]
- Sato, N.; Yabuki, K.; Shibao, K.; Mori, Y.; Tamura, T.; Higure, A.; Yamaguchi, K. Risk factors for a prolonged operative time in a sin-gle-incision laparoscopic cholecystectomy. HPB 2014, 16, 177–182. [Google Scholar] [CrossRef] [Green Version]
- Bickel, A.; Rappaport, A.; Hazani, E.; Eitan, A. Laparoscopic Cholecystectomy for Acute Cholecystitis Performed by Residents in Surgery: A Risk Factor for Conversion to Open Laparotomy? J. Laparoendosc. Adv. Surg. Tech. 1998, 8, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.M.; Liu, C.L.; Fan, S.T.; Lai, E.C.; Wong, J. Prospective randomized study of early versus delayed laparoscopic cholecystec-tomy for acute cholecystitis. Ann. Surg. 1998, 2, 461–467. [Google Scholar] [CrossRef]
- Lai, P.B.; Kwong, K.H.; Leung, K.L.; Kwok, S.P.; Chan, A.C.; Chung, S.C.; Lau, W.Y. Randomized trial of early versus delayed laparoscopic cholecystectomy for acute cholecystitis. BJS 1998, 85, 764–767. [Google Scholar] [CrossRef] [PubMed]
- Kolla, S.B.; Aggarwal, S.; Kumar, A.; Kumar, R.; Chumber, S.; Parshad, R.; Seenu, V. Early versus delayed laparoscopic cholecystecto-my for acute cholecystitis: A prospective randomized trial. Surg Endosc. 2004, 2, 1323–1327. [Google Scholar] [CrossRef]
- Gutt, C.N.; Encke, J.; Koninger, J.; Harnoss, J.C.; Weigand, K.; Kipfmuller, K.; Büchler, M.W. Acute cholecystitis: Early versus delayed chol-ecystectomy, a multicenter randomized trial (ACDC study, NCT00447304). Ann. Surg. 2013, 258, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Pogorelic, Z.; Aralica, M.; Jukic, M.; Zitko, V.; Despot, R.; Juric, I. Gallbladder Disease in Children: A 20-year Single-center Expe-rience. Indian Pediatr. 2019, 2, 384–386. [Google Scholar] [CrossRef]
- Ansaloni, L.; Pisano, M.; Coccolini, F.; Peitzmann, A.B.; Fingerhut, A.; Catena, F.; Agresta, F.; Allegri, A.; Bailey, I.; Balogh, Z.J.; et al. 2016 WSES guidelines on acute calculous cholecystitis. World J. Emerg. Surg. 2016, 11, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, Y.; Nagayama, M.; Okumura, A.; Amoh, Y.; Katsube, T.; Suga, T.; Koyama, S.; Nakatani, K.; Dodo, Y. MR Imaging of Acute Biliary Disorders. Radiographics 2007, 27, 477–495. [Google Scholar] [CrossRef] [PubMed]
- Regan, F.; Schaefer, D.C.; Smith, D.P.; Petronis, J.D.; Bohlman, M.E.; Magnuson, T.H. The Diagnostic Utility of HASTE MRI in the Evaluation of Acute Cholecystitis. J. Comput. Assist. Tomogr. 1998, 22, 638–642. [Google Scholar] [CrossRef]

| Signal Intensity of the Gallbladder Wall on MRI | |||||
|---|---|---|---|---|---|
| All (n = 321) | HSI (n = 103) | ISI (n = 116) | LSI (n = 102) | p Value | |
| Age (years) * | 62 (49–72) | 60 (46–71) | 62 (50–72) | 64 (56–75) | 0.061 |
| Sex | |||||
| Male | 214 (66.7) | 60 (58.3) | 84 (72.4) | 70 (68.6) | 0.079 |
| Female | 107 (33.3) | 43 (41.7) | 32 (27.6) | 32 (31.4) | |
| BMI (kg/m2) * | 24.7 (22.4–27.3) | 24.9 (22.5–28.1) | 24.6 (22.3–27.6) | 24.6 (22.5–26.8) | 0.953 |
| ASA physical status | |||||
| I | 106 (33.0) | 39 (37.9) | 36 (31.0) | 31 (30.4) | 0.953 |
| II | 192 (59.8) | 60 (58.3) | 70 (60.3) | 62 (60.8) | |
| III | 23 (7.2) | 4 (3.9) | 10 (9.8) | 9 (8.8) | |
| Diabetes mellitus | 54 (16.8) | 17 (16.5) | 19 (16.4) | 18 (17.6) | 0.964 |
| Previous upper abdominal surgery | 11 (3.4) | 3 (2.9) | 3 (2.6) | 5 (4.9) | 0.691 |
| Body temperature * | 36.9 (36.5–37.5) | 36.8 (36.4–37.2) | 36.9 (36.5–37.5) | 37.1 (36.6–37.7) | 0.014 |
| Severe inflammation findings on CT | 28 (8.7) | 0 (0.0) | 10 (8.6) | 18 (17.6) | <0.001 |
| Thickness of gallbladder wall on MRI (mm) * | 7 (5–8) | 6 (4–8) | 7 (6–8) | 7 (6–9) | <0.001 |
| Incarcerated stones in the gallbladder neck on MRI | 151 (47.0) | 51 (49.5) | 53 (45.7) | 47 (46.1) | 0.831 |
| Fluid retention around the gallbladder on MRI | 108 (33.6) | 17 (16.5) | 37 (31.9) | 54 (52.9) | <0.001 |
| WBC (/μL) * | 12,140 (9500–15,630) | 10,560 (8310–14,225) | 11,130 (8817–14,995) | 13,115 (10,760–16,100) | 0.001 |
| CRP (mg/dL) * | 1.24 (0.18–7.6) | 0.49 (0.10–1.41) | 0.63 (0.13–6.28) | 6.5 (1.51–16.09) | <0.001 |
| AST (U/L) * | 23 (18–32) | 23 (18–31) | 23 (18–32) | 23 (19–38) | 0.542 |
| ALT (U/L) * | 24 (16–40) | 24 (16–38) | 22 (16–41) | 27 (17–46) | 0.404 |
| T-Bil (mg/dL) * | 0.9 (0.6–1.5) | 0.7 (0.6–1.1) | 1.0 (0.6–1.6) | 1.1 (0.8–1.7) | <0.001 |
| Alb (g/dL) * | 4.1 (3.8–4.4) | 4.2 (4.0–4.4) | 4.1 (3.8–4.4) | 4.0 (3.6–4.2) | <0.001 |
| Tokyo Guidelines Severity Grade | |||||
| I | 250 (77.9) | 93 (90.3) | 93 (80.2) | 64 (62.7) | <0.001 |
| II | 56 (17.4) | 7 (6.8) | 20 (17.2) | 29 (28.4) | |
| III | 9 (4.7) | 3 (2.9) | 3 (2.6) | 9 (8.9) | |
| Time between onset of disease and surgery | |||||
| ≤48 h | 243 (75.7) | 90 (87.4) | 89 (76.7) | 64 (62.7) | <0.001 |
| >48 h | 78 (24.3) | 13 (12.6) | 27 (23.3) | 38 (37.3) | |
| Experience of the operator | |||||
| ≤4 years | 87 (27.1) | 28 (27.2) | 32 (27.6) | 27 (26.5) | 0.987 |
| ≥5 years | 234 (72.9) | 75 (72.8) | 84 (72.4) | 75 (73.5) | |
| Signal Intensity of the Gallbladder Wall on MRI | |||||
|---|---|---|---|---|---|
| ALL (n = 321) | HSI (n = 103) | ISI (n = 116) | LSI (n = 102) | p Value | |
| Surgical outcomes | |||||
| Bailout procedures | 79 (24.6) | 7 (6.8) | 31 (26.7) | 41 (40.2) | <0.001 † |
| Conversion to open surgery | 37 (11.5) | 0 (0.0) | 10 (8.6) | 27 (26.5) | <0.001 ‡ |
| Laparoscopic subtotal cholecystectomy | 42 (13.1) | 7 (6.8) | 21 (18.1) | 14 (13.7) | 0.041 § |
| Operating time (minutes) * | 114 (88–144) | 95 (80–117) | 110 (81–132) | 138 (116–171) | <0.001 || |
| Blood loss (mL) * | 0 (0–50) | 0 (0–0) | 0 (0–50) | 23 (0–221) | <0.001 ¶ |
| Biliary injury | 2 (0.6) | 0 (0.0) | 0 (0.0) | 2 (2.0) | 0.100 |
| Accidental gallbladder injury | 195 (60.7) | 52 (50.5) | 66 (56.9) | 77 (75.5) | <0.001 # |
| Intraoperative findings | |||||
| Degree of inflammation assessed by the surgeon | |||||
| Mild to moderate | 116 (36.1) | 63 (61.2) | 40 (34.5) | 13 (12.7) | <0.001 ** |
| Severe | 205 (63.9) | 40 (38.8) | 76 (65.5) | 89 (87.3) | |
| Postoperative outcomes | |||||
| Overall complications | 18 (5.6) | 4 (3.9) | 5 (4.3) | 9 (8.8) | 0.259 |
| Major complications | 9 (2.8) | 1 (1.0) | 4 (3.4) | 4 (3.9) | 0.441 |
| Reoperation | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | - |
| Postoperative hospital stay * | 3 (3–4) | 3 (3–3) | 3 (3–4) | 3 (3–6) | <0.001 †† |
| Readmission | 1 (0.3) | 0 (0.0) | 1 (0.9) | 0 (0.0) | 1.000 |
| Number (%) with Bailout Procedures | Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|
| Variable | Category | p Value | OR (95% CI) | p Value | |
| Age (years) | ≥72 | 24/81 (29.6) | 0.235 | ||
| <72 | 55/240 (22.9) | ||||
| Sex | Male | 57/214 (26.6) | 0.272 | ||
| Female | 22/107 (20.6) | ||||
| BMI (kg/m2) | ≥30 | 10/40 (25.0) | 1.000 | ||
| <30 | 69/281 (24.6) | ||||
| ASA physical status | ≥III | 3/23 (13.0) | 0.218 | ||
| ≤II | 76/298 (25.5 | ||||
| Diabetes mellitus | Yes | 10/54 (18.5) | 0.301 | ||
| No | 69/267 (25.8) | ||||
| Past acute cholecystitis | Yes | 6/19 (31.6) | 0.425 | ||
| No | 73/302 (24.2) | ||||
| Previous upper abdominal surgery | Yes | 3/11 (27.3) | 0.735 | ||
| No | 76/310 (24.5) | ||||
| Body temperature (°C) | ≥37.5 | 23/83 (27.7) | 0.461 | ||
| <37.5 | 56/238 (23.5) | ||||
| Severe inflammation findings on CT * | Yes | 15/28 (53.6) | 0.001 | 1.92 (0.66–5.52) | 0.233 |
| No | 64/293 (21.8) | ||||
| Thickness of the gallbladder wall on MRI (mm) | ≥8 | 43/121 (35.5) | <0.001 | 1.87 (1.07–3.29) | 0.029 |
| <8 | 36/200 (18.0) | ||||
| Incarcerated stones in the gallbladder neck on MRI | Yes | 37/151 (24.5) | 1.000 | ||
| No | 24/170 (23.5) | ||||
| Fluid retention around the gallbladder on MRI | Yes | 38/108 (35.2) | 0.002 | 1.24 (0.67–2.28) | 0.480 |
| No | 41/213 (19.2) | ||||
| Signal intensity of the gallbladder wall on MRI | HSI | 7/103 (6.8) | <0.001 | 1 | |
| ISI | 31/116 (26.7) | 3.71 (1.51–9.10) | 0.004 | ||
| LSI | 41/102 (40.2) | 5.30 (2.11–13.3) | <0.001 | ||
| WBC (/μL) | ≥15,000 | 28/95 (29.5) | 0.203 | ||
| <15,000 | 51/226 (22.6) | ||||
| CRP (mg/dL) | ≥8.0 | 22/78 (28.2) | <0.001 | 1.18 (0.58–2.40) | 0.641 |
| <8.0 | 15/243 (6.2) | ||||
| T-Bil (mg/dL) | ≥1.5 | 26/88 (29.5) | 0.245 | ||
| <1.5 | 53/233 (23.8) | ||||
| Alb (g/dL) | ≤3.8 | 31/90 (34.4) | 0.014 | 1.21 (0.65–2.25) | 0.557 |
| >3.8 | 48/231 (20.8) | ||||
| Tokyo Guidelines Severity Grade | II, III | 28/71 (39.4) | 0.002 | 0.985 (0.43–2.28) | 0.972 |
| I | 51/250 (20.4) | ||||
| Time between onset and surgery (hours) | >24 | 64/205 (31.2) | <0.001 | 2.19 (1.12–4.29) | 0.022 |
| ≤24 | 15/116 (12.9) | ||||
| Experience of the operator (years) | ≤4 | 22/87 (25.3) | 0.885 | ||
| ≥ 5 | 57/234 (24.4) | ||||
| Number (%) with Prolonged Surgery | Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|
| Variable | Category | p Value | OR (95% CI) | p Value | |
| Age (years) | ≥72 | 22/81 (27.2) | 0.659 | ||
| <72 | 59/240 (24.6) | ||||
| Sex | Male | 57/214 (26.6) | 0.496 | ||
| Female | 24/107 (22.4) | ||||
| BMI (kg/m2) | ≥30 | 9/40 (22.5) | 0.846 | ||
| <30 | 72/281 (25.6) | ||||
| ASA physical status | ≥III | 6/23 (26.1) | 1.000 | ||
| ≤II | 75/298 (25.2) | ||||
| Diabetes mellitus | Yes | 9/54 (16.7) | 0.125 | ||
| No | 72/267 (27.0) | ||||
| Past acute cholecystitis | Yes | 3/19 (15.8) | 0.422 | ||
| No | 78/302 (25.8) | ||||
| Previous upper abdominal surgery | Yes | 1/11 (9.1) | 0.302 | ||
| No | 80/310 (25.8) | ||||
| Body temperature (°C) | ≥37.5 | 27/88 (30.7) | 0.195 | ||
| <37.5 | 54/233 (23.2) | ||||
| Severe inflammation findings on CT * | Yes | 12/28 (42.9) | 0.038 | 0.90 (0.31–2.65) | 0.854 |
| No | 69/293 (23.5) | ||||
| Thickness of the gallbladder wall on MRI (mm) | ≥8 | 40/121 (33.1) | 0.017 | 1.64 (0.93–2.89) | 0.087 |
| <8 | 41/200 (20.5) | ||||
| Incarcerated stones in the gallbladder neck on MRI | Yes | 36/151 (23.8) | 0.609 | ||
| No | 45/170 (26.5) | ||||
| Fluid retention around the gallbladder on MRI | Yes | 37/108 (34.3) | 0.010 | 1.17 (0.64–2.15) | 0.612 |
| No | 44/214 (20.7) | ||||
| Signal intensity of the gallbladder wall on MRI | HSI | 11/103 (10.7) | <0.001 | 1 | |
| ISI | 22/116 (19.0) | 1.72 (0.77–3.85) | 0.184 | ||
| LSI | 48/102 (47.1) | 6.10 (2.74–13.60) | <0.001 | ||
| WBC (/μL) | ≥15,000 | 27/95 (28.4) | 0.401 | ||
| <15,000 | 54/226 (23.9) | ||||
| CRP (mg/dL) | ≥8.0 | 29/78 (37.2) | 0.007 | 1.08 (0.54–2.18) | 0.828 |
| <8.0 | 52/243 (21.4) | ||||
| T-Bil (mg/dL) | ≥1.5 | 22/88 (25.0) | 1.000 | ||
| <1.5 | 59/233 (25.3) | ||||
| Alb (g/dL) | ≤3.8 | 29/90 (32.2) | 0.086 | 1.05 (0.55–1.98) | 0.888 |
| >3.8 | 52/231 (22.5) | ||||
| Tokyo Guidelines Severity Grade | II, III | 27/71 (38.0) | 0.008 | 1.31 (0.57–2.99) | 0.514 |
| I | 54/250 (21.6) | ||||
| Time between onset and surgery (hours) | >24 | 29/116 (25.0) | 1.000 | ||
| ≤24 | 52/205 (25.4) | ||||
| Experience of the operator (years) | ≤4 | 29/87 (33.3) | 0.045 | 2.03 (1.11–3.71) | 0.022 |
| ≥5 | 52/234 (22.2) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omiya, K.; Hiramatsu, K.; Shibata, Y.; Fukaya, M.; Fujii, M.; Aoba, T.; Arimoto, A.; Yamaguchi, T.; Kato, T. Preoperative Magnetic Resonance Cholangiopancreatography for Detecting Difficult Laparoscopic Cholecystectomy in Acute Cholecystitis. Diagnostics 2021, 11, 383. https://doi.org/10.3390/diagnostics11030383
Omiya K, Hiramatsu K, Shibata Y, Fukaya M, Fujii M, Aoba T, Arimoto A, Yamaguchi T, Kato T. Preoperative Magnetic Resonance Cholangiopancreatography for Detecting Difficult Laparoscopic Cholecystectomy in Acute Cholecystitis. Diagnostics. 2021; 11(3):383. https://doi.org/10.3390/diagnostics11030383
Chicago/Turabian StyleOmiya, Kojiro, Kazuhiro Hiramatsu, Yoshihisa Shibata, Masahide Fukaya, Masahiro Fujii, Taro Aoba, Atsuki Arimoto, Takayuki Yamaguchi, and Takehito Kato. 2021. "Preoperative Magnetic Resonance Cholangiopancreatography for Detecting Difficult Laparoscopic Cholecystectomy in Acute Cholecystitis" Diagnostics 11, no. 3: 383. https://doi.org/10.3390/diagnostics11030383






