Analytical Evaluation of Visby Medical RT-PCR Portable Device for Rapid Detection of SARS-CoV-2
Abstract
:1. Introduction
2. Material and Methods
2.1. Patient Samples and Assay Controls
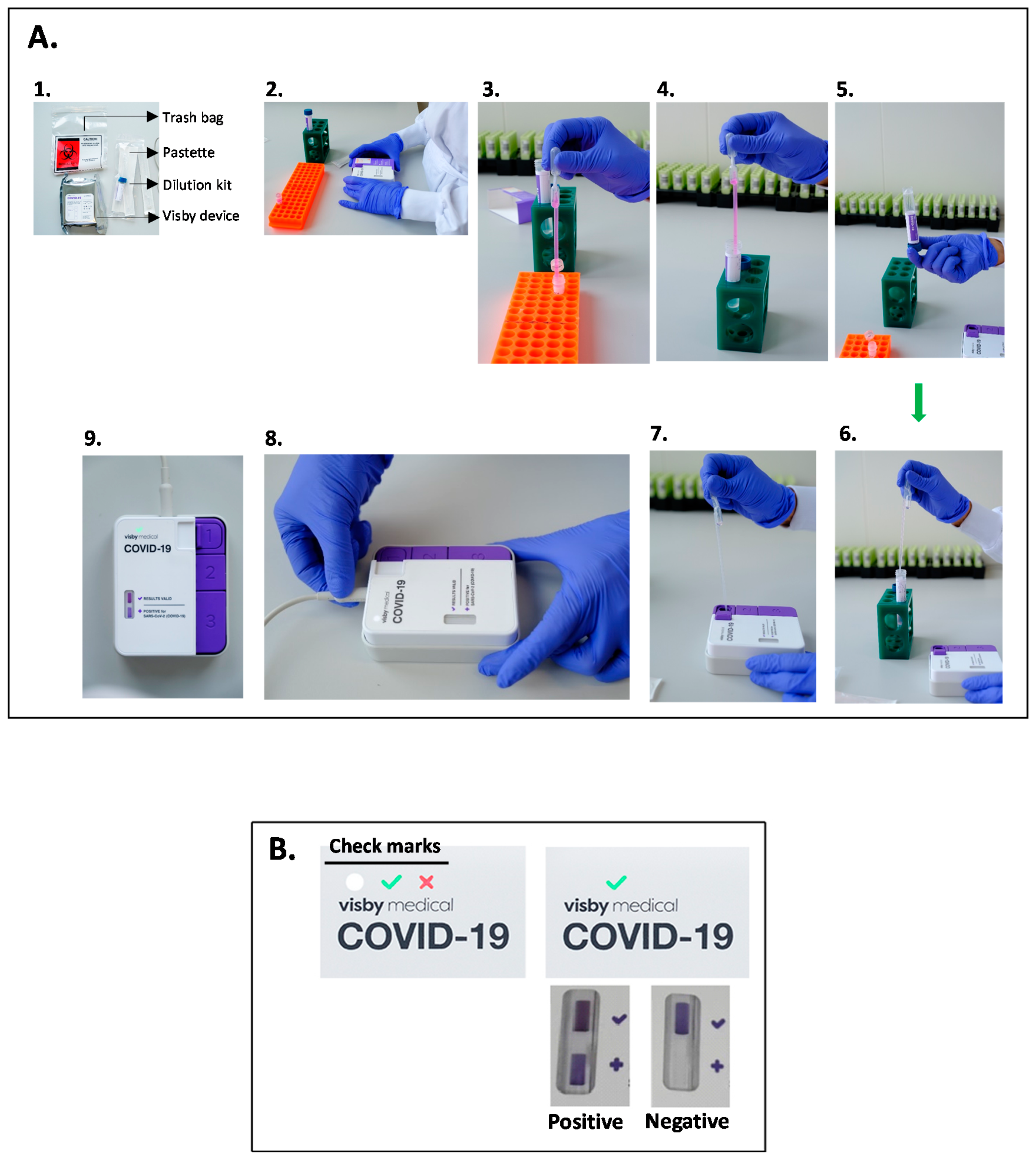
2.2. Visby Medical POC Device Testing
2.3. Comparison RT-qPCR Assay
2.4. Evaluation of Analytical Performances
2.5. Limit of Detection (LoD)
3. Results
3.1. Visby POC Device and Cobas SARS-CoV-2 Nucleic Acid Detection Concordance
3.2. Analytical Limit of Detection (LoD)
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Udugama, B.; Kadhiresan, P.; Kozlowski, H.N.; Malekjahani, A.; Osborne, M.; Li, V.Y.C.; Chen, H.; Mubareka, S.; Gubbay, J.B.; Chan, W.C.W. Diagnosing COVID-19: The Disease and Tools for Detection. ACS Nano 2020, 14, 3822–3835. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7144809/ (accessed on 10 December 2020). [CrossRef] [Green Version]
- Dinnes, J.; Deeks, J.J.; Adriano, A.; Berhane, S.; Davenport, C.; Dittrich, S.; Emperador, D.; Takwoingi, Y.; Cunningham, J.; Beese, S.; et al. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst. Rev. 2020, 8, CD013705. Available online: https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD013705/full (accessed on 10 December 2020). [PubMed]
- Joung, J.; Ladha, A.; Saito, M.; Segel, M.; Bruneau, R.; Huang, M.W.; Kim, N.-G.; Yu, X.; Li, J.; Walker, B.D.; et al. Point-of-care testing for COVID-19 using SHERLOCK diagnostics. medRxiv 2020. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7273289/ (accessed on 10 December 2020). [CrossRef]
- Gibani, M.M.; Toumazou, C.; Sohbati, M.; Sahoo, R.; Karvela, M.; Hon, T.-K.; De Mateo, S.; Burdett, A.; Leung, K.Y.F.; Barnett, J.; et al. Assessing a novel, lab-free, point-of-care test for SARS-CoV-2 (CovidNudge): A diagnostic accuracy study. Lancet Microbe 2020, 1, e300–e307. [Google Scholar] [CrossRef]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, e63. [Google Scholar] [CrossRef] [Green Version]
- Taki, K.; Yokota, I.; Fukumoto, T.; Iwasaki, S.; Fujisawa, S.; Takahashi, M.; Negishi, S.; Hayasaka, K.; Sato, K.; Oguri, S.; et al. SARS-CoV-2 detection by fluorescence loop-mediated isothermal amplification with and without RNA extraction. J. Infect. Chemother. 2021, 27, 410–412. Available online: http://www.sciencedirect.com/science/article/pii/S1341321X20303998 (accessed on 10 December 2020). [CrossRef] [PubMed]
- Nawattanapaiboon, K.; Pasomsub, E.; Prombun, P.; Wongbunmak, A.; Jenjitwanich, A.; Mahasupachai, P.; Vetcho, P.; Chayrach, C.; Manatjaroenlap, N.; Samphaongern, C.; et al. Colorimetric reverse transcription loop-mediated isothermal amplification (RT-LAMP) as a visual diagnostic platform for the detection of the emerging coronavirus SARS-CoV-2. Analyst 2021, 146, 471–477. Available online: https://pubs.rsc.org/en/content/articlelanding/2021/an/d0an01775b (accessed on 10 December 2020). [CrossRef]
- Xia, S.; Chen, X. Single-copy sensitive, field-deployable, and simultaneous dual-gene detection of SARS-CoV-2 RNA via modified RT–RPA. Cell Discov. 2020, 6, 37. [Google Scholar] [CrossRef]
- Huang, W.; Lin, D.; Wang, C.; Bao, C.; Zhang, Z.; Chen, X.; Zhang, Z.; Huang, J. The determination of release from isolation of COVID-19 patients requires ultra-high sensitivity nucleic acid test technology. J. Infect. 2021, 82, 159–198. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7330563/ (accessed on 10 December 2020). [CrossRef]
- Behrmann, O.; Bachmann, I.; Spiegel, M.; Schramm, M.; El Wahed, A.A.; Dobler, G.; Dame, G.; Hufert, F.T. Rapid detection of SARS-CoV-2 by low volume real-time single tube reverse transcription recombinase polymerase amplification using an exo probe with an internally linked quencher (exo-IQ). Clin. Chem. 2020, 66, 1047–1054. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7239256/ (accessed on 10 December 2020). [CrossRef]
- Basu, A.; Zinger, T.; Inglima, K.; Woo, K.; Atie, O.; Yurasits, L.; See, B.; Aguero-Rosenfeld, M.E. Performance of Abbott ID Now COVID-19 Rapid Nucleic Acid Amplification Test Using Nasopharyngeal Swabs Transported in Viral Transport Media and Dry Nasal Swabs in a New York City Academic Institution. J. Clin. Microbiol. 2020, 58, e01136-20. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7383552/ (accessed on 10 December 2020). [CrossRef] [PubMed]
- Harrington, A.; Cox, B.; Snowdon, J.; Bakst, J.; Ley, E.; Grajales, P.; Maggiore, J.; Kahn, S. Comparison of Abbott ID Now and Abbott m2000 Methods for the Detection of SARS-CoV-2 from Nasopharyngeal and Nasal Swabs from Symptomatic Patients. J. Clin. Microbiol. 2020, 58, e00798-20. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7383519/ (accessed on 10 December 2020). [CrossRef] [Green Version]
- Dunbar, S.; Das, S. Amplification chemistries in clinical virology. J. Clin. Virol. 2019, 115, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Ménová, P.; Raindlová, V.; Hocek, M. Scope and Limitations of the Nicking Enzyme Amplification Reaction for the Synthesis of Base-Modified Oligonucleotides and Primers for PCR. Bioconjug. Chem. 2013, 24, 1081–1093. [Google Scholar] [CrossRef]
- Morris, S.R.; Bristow, C.C.; Wierzbicki, M.R.; Sarno, M.; Asbel, L.; French, A.; Gaydos, C.A.; Hazan, L.; Mena, L.; Madhivanan, P.; et al. Performance of a single-use, rapid, point-of-care PCR device for the detection of Neisseria gonorrhoeae, Chlamydia trachomatis, and Trichomonas vaginalis: A cross-sectional study. Lancet Infect. Dis. 2021, 21, 668–676. Available online: https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(20)30734-9/abstract (accessed on 10 December 2020). [CrossRef]
- Calame, A.; Mazza, L.; Renzoni, A.; Kaiser, L.; Schibler, M. Sensitivity of nasopharyngeal, oropharyngeal, and nasal wash specimens for SARS-CoV-2 detection in the setting of sampling device shortage. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 21, 668–676. Available online: https://doi.org/10.1007/s10096-020-04039-8 (accessed on 10 December 2020).
- Clopper, C.J.; Pearson, E.S. The Use of Confidence or Fiducial Limits Illustrated in the Case of the Binomial. Biometrika 1934, 26, 404–413. [Google Scholar] [CrossRef]
- Essaidi-Laziosi, M.; Geiser, J.; Huang, S.; Constant, S.; Kaiser, L.; Tapparel, C. Interferon-Dependent and Respiratory Virus-Specific Interference in Dual Infections of Airway Epithelia. Sci. Rep. 2020, 10, 10246. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7314816/ (accessed on 10 December 2020). [CrossRef] [PubMed]
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.; Bleicker, T.; Brünink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020, 25, 2000045. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6988269/ (accessed on 18 December 2020). [CrossRef] [Green Version]
- Artesi, M.; Bontems, S.; Göbbels, P.; Franckh, M.; Maes, P.; Boreux, R.; Meex, C.; Melin, P.; Hayette, M.-P.; Bours, V.; et al. A Recurrent Mutation at Position 26340 of SARS-CoV-2 Is Associated with Failure of the E Gene Quantitative Reverse Transcription-PCR Utilized in a Commercial Dual-Target Diagnostic Assay. J. Clin. Microbiol. 2020, 58, e01598-20. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7512182/ (accessed on 10 December 2020). [CrossRef]
- CDC. Information for Laboratories about Coronavirus (COVID-19). Centers for Disease Control and Prevention, 2020. Available online: https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html (accessed on 10 December 2020).
- Bullard, J.; Dust, K.; Funk, D.; Strong, J.E.; Alexander, D.; Garnett, L.; Boodman, C.; Bello, A.; Hedley, A.; Schiffman, Z.; et al. Predicting infectious SARS-CoV-2 from diagnostic samples. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2020, 22, ciaa638. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7314198/ (accessed on 10 December 2020).
- Porte, L.; Legarraga, P.; Vollrath, V.; Aguilera, X.; Munita, J.M.; Araos, R.; Pizarro, G.; Vial, P.; Iruretagoyena, M.; Dittrich, S.; et al. Evaluation of a novel antigen-based rapid detection test for the diagnosis of SARS-CoV-2 in respiratory samples. Int. J. Infect. Dis. 2020, 99, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Scohy, A.; Anantharajah, A.; Bodéus, M.; Kabamba-Mukadi, B.; Verroken, A.; Rodriguez-Villalobos, H. Low performance of rapid antigen detection test as frontline testing for COVID-19 diagnosis. J.Clin. Virol. 2020, 129, 104455. [Google Scholar] [CrossRef] [PubMed]
- Diao, B.; Wen, K.; Zhang, J.; Chen, J.; Han, C.; Chen, Y.; Wang, S.; Deng, G.; Zhou, H.; Wu, Y. Accuracy of a nucleocapsid protein antigen rapid test in the diagnosis of SARS-CoV-2 infection. Clin. Microbiol. Infect. 2021, 27, 289.e1–289.e4. Available online: https://www.clinicalmicrobiologyandinfection.com/article/S1198-743X(20)30611-X/abstract (accessed on 10 December 2020). [CrossRef]
- Mak, G.C.K.; Lau, S.S.Y.; Wong, K.K.Y.; Chow, N.L.S.; Lau, C.S.; Lam, E.T.K.; Chan, R.C.W.; Tsang, D.N.C. Evaluation of rapid antigen detection kit from the WHO Emergency Use List for detecting SARS-CoV-2. J. Clin. Virol. 2021, 134, 104712. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7716730/ (accessed on 10 December 2020). [CrossRef] [PubMed]
- Weitzel, T.; Legarraga, P.; Iruretagoyena, M.; Pizarro, G.; Vollrath, V.; Araos, R.; Munita, J.M.; Porte, L. Head-to-head comparison of four antigen-based rapid detection tests for the diagnosis of SARS-CoV-2 in respiratory samples. bioRxiv 2020. [Google Scholar] [CrossRef]
- Gao, X.; Sun, B.; Guan, Y. Pullulan reduces the non-specific amplification of loop-mediated isothermal amplification (LAMP). Anal. Bioanal. Chem. 2019, 411, 1211–1218. [Google Scholar] [CrossRef]
- Karthik, K.; Rathore, R.; Thomas, P.; Arun, T.R.; Viswas, K.N.; Dhama, K.; Agarwal, R.K. New closed tube loop mediated isothermal amplification assay for prevention of product cross-contamination. MethodsX 2014, 1, 137–143. [Google Scholar] [CrossRef]
- Hsieh, K.; Mage, P.L.; Csordas, A.T.; Eisenstein, M.; Soh, H.T. Simultaneous elimination of carryover contamination and detection of DNA with uracil-DNA-glycosylase-supplemented loop-mediated isothermal amplification (UDG-LAMP). Chem. Commun. 2014, 50, 3747–3749. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-G.; Brewster, J.D.; Paul, M.; Tomasula, P.M. Two Methods for Increased Specificity and Sensitivity in Loop-Mediated Isothermal Amplification. Molecules 2015, 20, 6048–6059. [Google Scholar] [CrossRef] [Green Version]

| Reference RT-qPCR Cobas POS | Reference RT-qPCR Cobas NEG | Total | |
|---|---|---|---|
| Visby POS | 58 | 0 | 58 |
| Visby NEG | 3 | 17 | 20 |
| TOTAL | 61 | 17 | 78 |
| CT Values Cut-Offs | Sensitivity | 95% CI | N |
|---|---|---|---|
| 15–20 | 100% | 82–100% | 19 |
| 21–25 | 100% | 84–100% | 21 |
| 26–30 | 100% | 66–100% | 9 |
| 31–35 | 100% | 43–94.5% | 12 |
| Delay Since Onset (Days) | Sensitivity | 95% CI | N |
|---|---|---|---|
| 0 | 87.5% | 47.3–99.7% | 8 |
| 1 | 90.9% | 58.7–99.8% | 11 |
| 2–3 | 95.2% | 76.2–99.9% | 21 |
| 4–5 | 100% | 73.5–100% | 12 |
| 6–7 | 100% | 47.8–100% | 5 |
| >7 | 100% | 39.8–100% | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Renzoni, A.; Perez, F.; Ngo Nsoga, M.T.; Yerly, S.; Boehm, E.; Gayet-Ageron, A.; Kaiser, L.; Schibler, M. Analytical Evaluation of Visby Medical RT-PCR Portable Device for Rapid Detection of SARS-CoV-2. Diagnostics 2021, 11, 813. https://doi.org/10.3390/diagnostics11050813
Renzoni A, Perez F, Ngo Nsoga MT, Yerly S, Boehm E, Gayet-Ageron A, Kaiser L, Schibler M. Analytical Evaluation of Visby Medical RT-PCR Portable Device for Rapid Detection of SARS-CoV-2. Diagnostics. 2021; 11(5):813. https://doi.org/10.3390/diagnostics11050813
Chicago/Turabian StyleRenzoni, Adriana, Francisco Perez, Marie Thérèse Ngo Nsoga, Sabine Yerly, Erik Boehm, Angèle Gayet-Ageron, Laurent Kaiser, and Manuel Schibler. 2021. "Analytical Evaluation of Visby Medical RT-PCR Portable Device for Rapid Detection of SARS-CoV-2" Diagnostics 11, no. 5: 813. https://doi.org/10.3390/diagnostics11050813
APA StyleRenzoni, A., Perez, F., Ngo Nsoga, M. T., Yerly, S., Boehm, E., Gayet-Ageron, A., Kaiser, L., & Schibler, M. (2021). Analytical Evaluation of Visby Medical RT-PCR Portable Device for Rapid Detection of SARS-CoV-2. Diagnostics, 11(5), 813. https://doi.org/10.3390/diagnostics11050813






