Kabat Rehabilitation in Facial Nerve Palsy after Parotid Gland Tumor Surgery: A Case-Control Study
Abstract
:1. Introduction
2. Materials and Methods
- Upper fulcrum: includes the corrugators, frontalis, and orbicularis;
- Intermediate fulcrum: includes the common elevator muscle of the upper lip and wing of nose, the dilator naris, and the mitriform;
- Lower fulcrum: includes the risorius, zygomaticus major, the orbicularis, the zygomaticus minor, the triangular of the lower lip, buccinator, chin muscle, and square muscle of the chin [22].
Statistical Analysis
- People with a mild grading (from I to II) of HB registered at 3 months;
- People with a severe grading (from III to V) of HB registered at 3 months.
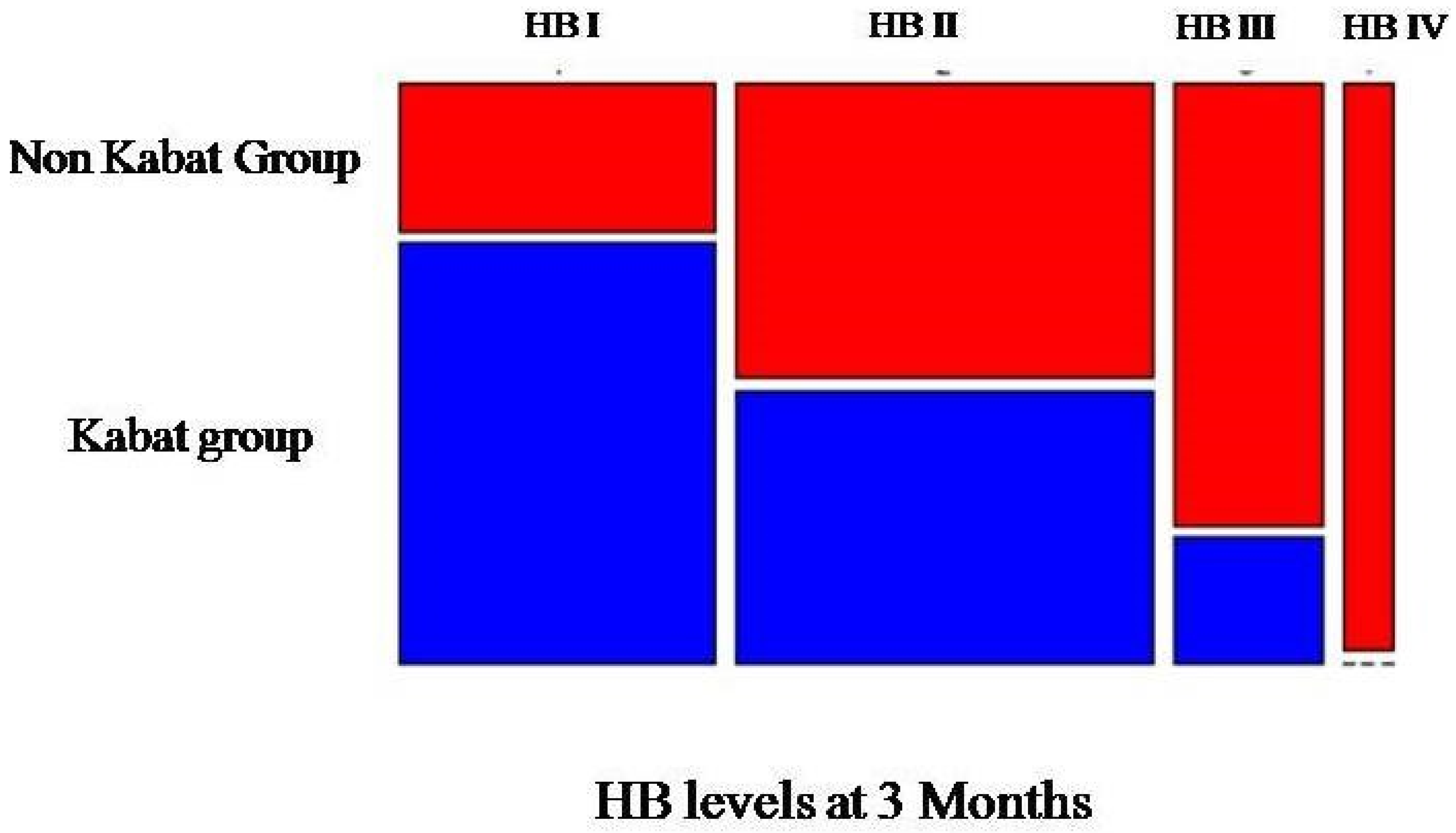
3. Results
3.1. Descriptive Statistics
3.2. Multiple Linear Regression Model
Generalized Linear Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haring, C.T.; Ellsperman, S.E.; Edwards, B.M.; Kileny, P.; Kovatch, D.; Mannarelli, G.R.; Meloch, M.A.; Miller, C.; Pitts, C.; Prince, M.E.P.; et al. Assessment of Intraoperative Nerve Monitoring Parameters Associated With Facial Nerve Outcome in Parotidectomy for Benign Disease. JAMA Otolaryngol. Head Neck Surg. 2019, 145, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Eren, S.B.; Dogan, R.; Ozturan, O.; Veyseller, B.; Hafiz, A.M. How Deleterious Is Facial Nerve Dissection for the Facial Nerve in Parotid Surgery: An Electrophysiological Evaluation. J. Craniofac. Surg. 2017, 28, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Kim, B.Y.; Kim, H.; Lee, E.; Park, W.; Choi, S.; Chung, M.K.; Son, Y.I.; Baek, C.H.; Jeong, H.S. Incidence of postoperative facial weakness in parotid tumor surgery: A tumor subsite analysis of 794 parotidectomies. BMC Surg. 2019, 19, 199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogart, K.R. Socioemotional functioning with facial paralysis: Is there a congenital or acquired advantage? Health Psychol. 2020, 39, 345–354. [Google Scholar] [CrossRef] [PubMed]
- House, J.W.; Brackmann, D.E. Facial nerve grading system. Otolaryngol. Head Neck Surg. 1985, 93, 146–147. [Google Scholar] [CrossRef]
- Berg, T.; Jonsson, L.; Engström, M. Agreement between the Sunnybrook, House-Brackmann, and Yanagihara Facial Nerve Grading Systems in Bell’s Palsy. Otol. Neurotol. 2004, 25, 1020–1026. [Google Scholar] [CrossRef]
- Luijmes, R.E.; Pouwels, S.; Beurskens, C.H.; Kleiss, I.J.; Siemann, I.; Ingels, K.J. Quality of life before and after different treatment modalities in peripheral facial palsy: A systematic review. Laryngoscope 2017, 127, 1044–1051. [Google Scholar] [CrossRef]
- Min-Jung, O.T. Medical Management of Acute Facial Paralysis. Otolaryngol. Clin. N. Am. 2018, 51, 1051–1075. [Google Scholar] [CrossRef]
- Gagyor, I.; Madhok, V.B.; Daly, F.; Sullivan, F. Antiviral treatment for Bell’s palsy (idiopathic facial paralysis). Cochrane Database Syst. Rev. 2019, 9, CD001869. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.J.; Lee, H.Y. Acute Peripheral Facial Palsy: Recent Guidelines and a Systematic Review of the Literature. J. Korean Med. Sci. 2020, 35, e245. [Google Scholar] [CrossRef]
- Varadharajan, K.; Beegun, I.; Daly, N. Use of steroids for facial nerve paralysis after parotidectomy: A systematic review. World J. Clin. Cases 2015, 3, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Infante-Cossio, P.; Prats-Golczer, V.E.; Lopez-Martos, R.; Montes-Latorre, E.; Exposito-Tirado, J.A.; Gonzalez-Cardero, E. Effectiveness of facial exercise therapy for facial nerve dysfunction after superficial parotidectomy: A randomized controlled trial. Clin. Rehabil. 2016, 30, 1097–1107. [Google Scholar] [CrossRef] [PubMed]
- Monini, S.; Buffoni, A.; Romeo, M.; Di Traglia, M.; Filippi, C.; Atturo, F.; Barbara, M. Kabat rehabilitation for Bell’s palsy in the elderly. Acta Otolaryngol. 2017, 137, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Di Stadio, A.; Gambacorta, V.; Ralli, M.; Pagliari, J.; Longari, F.; Greco, A.; Ricci, G. Facial taping as biofeedback to improve the outcomes of physical rehab in Bell’s palsy: Preliminary results of a randomized case-control study. Eur. Arch. Otorhinolaryngol. 2021, 278, 1693–1698. [Google Scholar] [CrossRef]
- Javaherian, M.; Attarbashi Moghaddam, B.; Bashardoust Tajali, S.; Dabbaghipour, N. Efficacy of low-level laser therapy on management of Bell’s palsy: A systematic review. Lasers Med. Sci. 2020, 35, 1245–1252. [Google Scholar] [CrossRef]
- Maddali-Bongi, S.; Landi, G.; Galluccio, F.; Del Rosso, A.; Miniati, I.; Conforti, M.L.; Casale, R.; Matucci-Cerinic, M. The rehabilitation of facial involvement in systemic sclerosis: Efficacy of the combination of connective tissue massage, Kabat’s technique and kinesitherapy: A randomized controlled trial. Rheumatol. Int. 2011, 31, 895–901. [Google Scholar] [CrossRef]
- Morreale, M.; Marchione, P.; Pili, A.; Lauta, A.; Castiglia, S.F.; Spallone, A.; Pierelli, F.; Giacomini, P. Early versus delayed rehabilitation treatment in hemiplegic patients with ischemic stroke: Proprioceptive or cognitive approach? Eur. J. Phys. Rehabil. Med. 2016, 52, 81–89. [Google Scholar]
- Guiu-Tula, F.X.; Cabanas-Valdes, R.; Sitja-Rabert, M.; Urrutia, G.; Gomara-Toldra, N. The Efficacy of the proprioceptive neuromuscular facilitation (PNF) approach in stroke rehabilitation to improve basic activities of daily living and quality of life: A systematic review and meta-analysis protocol. BMJ Open 2017, 7, e016739. [Google Scholar] [CrossRef] [Green Version]
- Tedla, J.S.; Sangadala, D.R. Proprioceptive neuromuscular facilitation techniques in adhesive capsulitis: A systematic review and meta-analysis. J. Musculoskelet. Neuronal Interact. 2019, 19, 482–491. [Google Scholar]
- Gunning, E.; Uszynski, M.K. Effectiveness of the Proprioceptive Neuromuscular Facilitation Method on Gait Parameters in Patients With Stroke: A Systematic Review. Arch. Phys. Med. Rehabil. 2019, 100, 980–986. [Google Scholar] [CrossRef] [Green Version]
- Kabat, H.; Knott, M. Proprioceptive facilitation therapy for paralysis. Physiotherapy 1954, 40, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Giacalone, A.; Sciarrillo, T.; Rocco, G.; Ruberti, E. Kabat rehabilitation for facial nerve paralysis: Perspective on neurokinetic recovery and review of clinical evaluation tools. Int. J. Acad. Sci. Res. 2018, 6, 38–46. [Google Scholar]
- Robinson, M.W.; Baiungo, J. Facial Rehabilitation: Evaluation and Treatment Strategies for the Patient with Facial Palsy. Otolaryngol. Clin. N. Am. 2018, 51, 1151–1167. [Google Scholar] [CrossRef]
- Ishii, L.E.; Godoy, A.; Encarnacion, C.O.; Byrne, P.J.; Boahene, K.D.; Ishii, M. What faces reveal: Impaired affect display in facial paralysis. Laryngoscope 2011, 121, 1138–1143. [Google Scholar] [CrossRef]
- Bogart, K.; Tickle-Degnen, L.; Ambady, N. Communicating without the Face: Holistic Perception of Emotions of People with Facial Paralysis. Basic Appl. Soc. Psych. 2014, 36, 309–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jack, K.; McLean, S.M.; Moffett, J.K.; Gardiner, E. Barriers to treatment adherence in physiotherapy outpatient clinics: A systematic review. Man Ther. 2010, 15, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Lonsdale, C.; Hall, A.M.; Williams, G.C.; McDonough, S.M.; Ntoumanis, N.; Murray, A.; Hurley, D.A. Communication style and exercise compliance in physiotherapy (CONNECT). A cluster randomized controlled trial to test a theory-based intervention to increase chronic low back pain patients’ adherence to physiotherapists’ recommendations: Study rationale, design, and methods. BMC Musculoskelet. Disord. 2012, 13, 104. [Google Scholar] [CrossRef] [Green Version]
- Banks, C.A.; Jowett, N.; Azizzadeh, B.; Beurskens, C.; Bhama, P.; Borschel, G.; Coombs, C.; Coulson, S.; Croxon, G.; Diels, J.; et al. Worldwide Testing of the eFACE Facial Nerve Clinician-Graded Scale. Plast. Reconstr. Surg. 2017, 139, 491e–498e. [Google Scholar] [CrossRef]
- Vrabec, J.T.; Backous, D.D.; Djalilian, H.R.; Gidley, P.W.; Leonetti, J.P.; Marzo, S.J.; Morrison, D.; Ng, M.; Ramsey, M.J.; Schaitkin, B.M.; et al. Facial Nerve Grading System 2.0. Otolaryngol Head Neck Surg 2009, 140, 445–450. [Google Scholar] [CrossRef]
- Fattah, A.Y.; Gurusinghe, A.D.R.; Gavilan, J.; Hadlock, T.A.; Marcus, J.R.; Marres, H.; Nduka, C.C.; Slattery, W.H.; Snyder-Warwick, A.K.; Sir Charles Bell, S. Facial nerve grading instruments: Systematic review of the literature and suggestion for uniformity. Plast. Reconstr. Surg. 2015, 135, 569–579. [Google Scholar] [CrossRef]



| No. (%) | Total Patients (n = 425) | HB IV (n = 34) | HB V (n = 22) | |
|---|---|---|---|---|
| Age (Years, Mean, Range) | 46.7 (12–86) | 47.4 (14–84) | 63.9 (36–82) | |
| Gender | Male | 254 | 24 | 16 |
| Female | 171 | 10 | 6 | |
| Pathology | Benign tumors | 352 | 29 | 20 |
| Malignant tumors | 73 | 5 | 2 | |
| Tumor subsite | Superficial to the facial nerve | 293 | 21 | 7 |
| Deep to the facial nerve | 47 | 6 | 6 | |
| Superficial and deep location | 85 | 7 | 9 | |
| Extent of surgery | Extracapsular dissection | 184 | 7 | 1 |
| Partial parotidectomy | 122 | 9 | 3 | |
| Superficial parotidectomy | 73 | 15 | 7 | |
| Total parotidectomy | 46 | 3 | 11 | |
| Patient | Gender | Age | Exent of Surgery (E.D a, S.P b, P.P c, P.T d) | HB (7 D) | HB (1 M) | HB (3 M) | Hb (6 M) |
|---|---|---|---|---|---|---|---|
| Kabat Group | |||||||
| 1 | M | 49 | T.P | V | III | I | I |
| 2 | M | 33 | S.P | IV | III | II | I |
| 3 | M | 52 | P.P | V | IV | II | II |
| 4 | M | 84 | P.P | IV | IV | III | I |
| 5 | M | 69 | E.D | V | II | I | I |
| 6 | M | 14 | S.P | IV | IV | II | I |
| 7 | M | 72 | T.P | V | V | II | II |
| 8 | M | 51 | T.P | V | IV | III | III |
| 9 | M | 37 | S.P | IV | II | I | I |
| 10 | M | 18 | E.D | IV | I | I | I |
| 11 | M | 76 | S.P | V | IV | II | I |
| 12 | M | 37 | P.P | IV | I | I | I |
| 13 | M | 41 | T.P | IV | III | I | I |
| 14 | M | 73 | S.P | IV | IV | II | II |
| 15 | M | 61 | T.P | V | II | I | I |
| 16 | M | 53 | S.P | IV | II | I | I |
| 17 | M | 76 | E.D | IV | I | I | I |
| 18 | M | 41 | S.P | IV | III | II | II |
| 19 | M | 55 | S.P | IV | II | II | I |
| 20 | M | 46 | P.P | V | III | II | II |
| 21 | F | 37 | E.D | IV | II | I | I |
| 22 | F | 68 | T.P | IV | IV | II | I |
| 23 | F | 71 | S.P | V | II | I | I |
| 24 | F | 48 | P.P | IV | I | I | I |
| 25 | F | 46 | P.P | IV | II | I | I |
| 26 | F | 47 | S.P | IV | II | II | I |
| 27 | F | 78 | S.P | V | III | II | II |
| 28 | F | 73 | T.P | V | II | I | I |
| Non-Kabat Group | |||||||
| 1 | M | 45 | P.P | IV | IV | III | I |
| 2 | M | 36 | S.P | V | V | III | II |
| 3 | M | 65 | P.P | IV | III | II | I |
| 4 | M | 82 | S.P | V | III | II | II |
| 5 | M | 73 | S.P | IV | IV | IV | I |
| 6 | M | 16 | E.D | IV | II | I | I |
| 7 | M | 78 | T.P | V | IV | II | II |
| 8 | M | 54 | T.P | V | III | III | III |
| 9 | M | 39 | S.P | IV | III | I | I |
| 10 | M | 15 | S.P | IV | II | I | I |
| 11 | M | 78 | T.P | V | IV | II | I |
| 12 | M | 47 | S.P | IV | II | II | I |
| 13 | M | 29 | S.P | IV | III | II | I |
| 14 | M | 68 | P.P | V | IV | III | I |
| 15 | M | 57 | T.P | V | III | II | II |
| 16 | M | 66 | S.P | IV | II | I | I |
| 17 | M | 81 | E.D | IV | III | II | I |
| 18 | M | 33 | P.P | IV | III | II | I |
| 19 | M | 32 | E.D | IV | IV | II | I |
| 20 | M | 64 | T.P | V | IV | III | III |
| 21 | F | 19 | E.D | IV | III | II | I |
| 22 | F | 77 | T.P | V | V | IV | II |
| 23 | F | 65 | S.P | V | IV | III | I |
| 24 | F | 51 | P.P | IV | III | II | I |
| 25 | F | 32 | S.P | IV | III | II | I |
| 26 | F | 82 | T.P | IV | IV | III | II |
| 27 | F | 49 | S.P | V | IV | IV | II |
| 28 | F | 78 | P.P | IV | II | I | I |
| Parameter | Estimate | St.Error | t-Value | Pr (>|t|) | Level of Significance |
|---|---|---|---|---|---|
| Intercept | 1.405911 | 0.423367 | 3.321 | 0.001701 | ** |
| Kabat | −0.712216 | 0.202041 | −3.525 | 0.000929 | *** |
| Age | 0.008280 | 0.005551 | 1.492 | 0.142208 | |
| Gender | −0.049406 | 0.225573 | −0.219 | 0.827539 | |
| EoSPP 1 | 0.449229 | 0.350219 | 1.283 | 0.205628 | |
| EoSSP 1 | 0.567987 | 0.314315 | 1.807 | 0.076894 | |
| EoSTP 1 | 0.593099 | 0.354328 | 1.674 | 0.100529 |
| Parameter | Estimate | St.Error | t-Value | Pr (>|t|) | Level of Significance |
|---|---|---|---|---|---|
| Intercept | 1.70734 | 0.31214 | 5.470 | 1.24 × 10−6 | *** |
| Kabat | −0.71161 | 0.20144 | −3.533 | 0.000862 | *** |
| Age | 0.01070 | 0.00514 | 2.082 | 0.042151 | * |
| Parameter | Estimate | St.Error | Z Value | Pr (>|z|) | Level of Significance |
|---|---|---|---|---|---|
| Intercept | −2.37459 | 1.19865 | −1.981 | 0.0476 | * |
| Kabat | −2.03370 | 0.85060 | −2.391 | 0.0168 | * |
| Age | 0.03181 | 0.01929 | 1.649 | 0.0992 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boschetti, C.E.; Lo Giudice, G.; Spuntarelli, C.; Apice, C.; Rauso, R.; Santagata, M.; Tartaro, G.; Colella, G. Kabat Rehabilitation in Facial Nerve Palsy after Parotid Gland Tumor Surgery: A Case-Control Study. Diagnostics 2022, 12, 565. https://doi.org/10.3390/diagnostics12030565
Boschetti CE, Lo Giudice G, Spuntarelli C, Apice C, Rauso R, Santagata M, Tartaro G, Colella G. Kabat Rehabilitation in Facial Nerve Palsy after Parotid Gland Tumor Surgery: A Case-Control Study. Diagnostics. 2022; 12(3):565. https://doi.org/10.3390/diagnostics12030565
Chicago/Turabian StyleBoschetti, Ciro Emiliano, Giorgio Lo Giudice, Chiara Spuntarelli, Carmine Apice, Raffaele Rauso, Mario Santagata, Gianpaolo Tartaro, and Giuseppe Colella. 2022. "Kabat Rehabilitation in Facial Nerve Palsy after Parotid Gland Tumor Surgery: A Case-Control Study" Diagnostics 12, no. 3: 565. https://doi.org/10.3390/diagnostics12030565








