Magnetic Resonance Imaging in (Near-)Term Infants with Hypoxic-Ischemic Encephalopathy
Abstract
1. Introduction
2. Brain Injury Patterns in HIE
2.1. Basal Ganglia and Thalami (BGT) Predominant Pattern of Injury
2.2. White Matter/Watershed (WM/WS) Predominant Pattern of Injury
2.3. Near Total Injury
2.4. Other Injury Associated with HIE
3. Magnetic Resonance Imaging in Infants with HIE
3.1. Conventional MRI
3.2. Diffusion Weighted Imaging
3.3. Susceptibility Weighted Imaging
4. Advanced Imaging Modalities
4.1. Diffusion Tensor Imaging
4.2. Arterial Spin Labeling
5. Magnetic Resonance Spectroscopy
6. HIE Mimics
7. Scoring Systems
8. Pitfalls and Recommendations for the Assessment of Brain Injury on MRI
8.1. Optimal Timing of MRI
8.2. Repeat MRI
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahearne, C.E.; Boylan, G.B.; Murray, D.M. Short and long term prognosis in perinatal asphyxia: An update. World J. Clin. Pediatr. 2016, 5, 67–74. [Google Scholar] [CrossRef]
- Kurinczuk, J.J.; White-Koning, M.; Badawi, N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum. Dev. 2010, 86, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.C.; Kozuki, N.; Blencowe, H.; Vos, T.; Bahalim, A.; Darmstadt, G.L.; Niermeyer, S.; Ellis, M.; Robertson, N.J.; Cousens, S.; et al. Intrapartum-related neonatal encephalopathy incidence and impairment at regional and global levels for 2010 with trends from 1990. Pediatr Res. 2013, 74, 50–72. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, S.E.; Berg, M.; Hunt, R.; Tarnow-Mordi, W.O.; Inder, T.E.; Davis, P.G. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst. Rev. 2013, 2013, CD003311. [Google Scholar] [CrossRef] [PubMed]
- Azzopardi, D.; Strohm, B.; Marlow, N.; Brocklehurst, P.; Deierl, A.; Eddama, O.; Goodwin, J.; Halliday, H.L.; Juszczak, E.; Kapellou, O.; et al. Effects of hypothermia for perinatal asphyxia on childhood outcomes. N. Engl. J. Med. 2014, 371, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Conway, J.M.; Walsh, B.H.; Boylan, G.B.; Murray, D.M. Mild hypoxic ischaemic encephalopathy and long term neurodevelopmental outcome—A systematic review. Early Hum. Dev. 2018, 120, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Karamian, A.S.; Mercimek-Andrews, S.; Mohammad, K.; Molloy, E.; Chang, T.; Chau, V.; Murray, D.; Wusthoff, C.J. Neonatal encephalopathy: Etiologies other than hypoxic-ischemic encephalopathy. Semin. Fetal Neonatal Med. 2021, 26, 101272. [Google Scholar] [CrossRef]
- Pressler, R.M.; Cilio, M.R.; Mizrahi, E.M.; Moshé, S.L.; Nunes, M.L.; Plouin, P.; Vanhatalo, S.; Yozawitz, E.; de Vries, L.S.; Vinayan, K.P.; et al. The ILAE classification of seizures and the epilepsies: Modification for seizures in the neonate. Position paper by the ILAE task force on neonatal seizures. Epilepsia 2021, 62, 615–628. [Google Scholar] [CrossRef]
- Annink, K.V.; De Vries, L.S.; Groenendaal, F.; Vijlbrief, D.C.; Weeke, L.C.; Roehr, C.C.; Lequin, M.; Reiss, I.; Govaert, P.; Benders, M.J.N.L.; et al. The development and validation of a cerebral ultrasound scoring system for infants with hypoxic-ischaemic encephalopathy. Pediatr. Res. 2020, 87 (Suppl. 1), 59–66. [Google Scholar] [CrossRef]
- Epelman, M.; Daneman, A.; Kellenberger, C.J.; Aziz, A.; Konen, O.; Moineddin, R.; Whyte, H.; Blaser, S. Neonatal encephalopathy: A prospective comparison of head US and MRI. Pediatr. Radiol. 2010, 40, 1640–1650. [Google Scholar] [CrossRef]
- Cheong, J.L.; Coleman, L.; Hunt, R.W.; Lee, K.J.; Doyle, L.W.; Inder, T.E.; Jacobs, S.E.; Infant Cooling Evaluation Collaboration. Prognostic utility of magnetic resonance imaging in neonatal hypoxic-ischemic encephalopathy substudy of a randomized trial. Arch. Pediat. Adol. Med. 2012, 166, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, M.; Ramenghi, L.A.; Edwards, A.D.; Brocklehurst, P.; Halliday, H.; Levene, M.; Strohm, B.; Thoresen, M.; Whitelaw, A.; Azzopardi, D. Assessment of brain tissue injury after moderate hypothermia in neonates with hypoxic-ischaemic encephalopathy: A nested substudy of a randomised controlled trial. Lancet Neurol. 2010, 9, 39–45. [Google Scholar] [CrossRef]
- Alderliesten, T.; de Vries, L.S.; Benders, M.J.; Koopman, C.; Groenendaal, F. MR imaging and outcome of term neonates with perinatal asphyxia: Value of diffusion-weighted MR imaging and (1)H MR spectroscopy. Radiology 2011, 261, 235–242. [Google Scholar] [CrossRef]
- Lally, P.J.; Montaldo, P.; Oliveira, V.; Soe, A.; Swamy, R.; Bassett, P.; Mendoza, J.; Atreja, G.; Kariholu, U.; Pattnayak, S.; et al. Magnetic resonance spectroscopy assessment of brain injury after moderate hypothermia in neonatal encephalopathy: A prospective multicentre cohort study. Lancet Neurol. 2019, 18, 35–45. [Google Scholar] [CrossRef]
- Thayyil, S.; Chandrasekaran, M.; Taylor, A.; Bainbridge, A.; Cady, E.B.; Chong, W.K.K.; Murad, S.; Omar, R.Z.; Robertson, N.J. Cerebral magnetic resonance biomarkers in neonatal encephalopathy: A meta-analysis. Pediatrics 2010, 125, e382–e395. [Google Scholar] [CrossRef] [PubMed]
- De Vis, J.B.; Hendrikse, J.; Petersen, E.T.; de Vries, L.S.; van Bel, F.; Alderliesten, T.; Negro, S.; Groenendaal, F.; Benders, M.J. Arterial spin-labelling perfusion MRI and outcome in neonates with hypoxic-ischemic encephalopathy. Eur. Radiol. 2015, 25, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Dibble, M.; O’Dea, M.I.; Hurley, T.; Byrne, A.; Colleran, G.; Molloy, E.J.; Bokde, A.L.W. Diffusion tensor imaging in neonatal encephalopathy: A systematic review. Arch. Dis. Child Fetal. 2020, 105, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Tusor, N.; Wusthoff, C.; Smee, N.; Merchant, N.; Arichi, T.; Allsop, J.M.; Cowan, F.M.; Azzopardi, D.; Edwards, A.D.; Counsell, S.J. Prediction of neurodevelopmental outcome after hypoxic-ischemic encephalopathy treated with hypothermia by diffusion tensor imaging analyzed using tract-based spatial statistics. Pediatr. Res. 2012, 72, 63–69. [Google Scholar] [CrossRef]
- Massaro, A.N.; Evangelou, I.; Fatemi, A.; Vezina, G.; McCarter, R.; Glass, P.; Limperopoulos, C. White matter tract integrity and developmental outcome in newborn infants with hypoxic-ischemic encephalopathy treated with hypothermia. Dev. Med. Child Neurol. 2015, 57, 441–448. [Google Scholar] [CrossRef]
- Gunn, A.J.; Bennet, L. Fetal hypoxia insults and patterns of brain injury: Insights from animal models. Clin. Perinatol. 2009, 36, 579–593. [Google Scholar] [CrossRef]
- Okereafor, A.; Allsop, J.; Counsell, S.J.; Fitzpatrick, J.; Azzopardi, D.; Rutherford, M.A.; Cowan, F.M. Patterns of brain injury in neonates exposed to perinatal sentinel events. Pediatrics 2008, 121, 906–914. [Google Scholar] [CrossRef]
- Martinez-Biarge, M.; Diez-Sebastian, J.; Kapellou, O.; Gindner, D.; Allsop, J.M.; Rutherford, M.A.; Cowan, F.M. Predicting motor outcome and death in term hypoxic-ischemic encephalopathy. Neurology 2011, 76, 2055–2061. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Biarge, M.; Diez-Sebastian, J.; Rutherford, M.A.; Cowan, F.M. Outcomes after central grey matter injury in term perinatal hypoxic-ischaemic encephalopathy. Early Hum. Dev. 2010, 86, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Volpe, J.J.; Pasternak, J.F. Parasagittal cerebral injury in neonatal hypoxic-ischemic encephalopathy: Clinical and neuroradiologic features. J. Pediatr. 1977, 91, 472–476. [Google Scholar] [CrossRef]
- Miller, S.; Ramaswamy, V.; Michelson, D.; Barkovich, A.J.; Holshouser, B.; Wycliffe, N.; Glidden, D.; Deming, D.; Partridge, J.C.; Wu, Y.W.; et al. Patterns of brain injury in term neonatal encephalopathy. J. Pediatr. 2005, 146, 453–460. [Google Scholar] [CrossRef]
- Sato, Y.; Hayakawa, M.; Iwata, O.; Okumura, A.; Kato, T.; Hayakawa, F.; Kubota, T.; Maruyama, K.; Hasegawa, M.; Sato, M.; et al. Delayed neurological signs following isolated parasagittal injury in asphyxia at term. Eur. J. Paediatr. Neurol. 2008, 12, 359–365. [Google Scholar] [CrossRef]
- Martinez-Biarge, M.; Bregant, T.; Wusthoff, C.; Chew, A.T.; Diez-Sebastian, J.; Rutherford, M.A.; Cowan, F.M. White matter and cortical injury in hypoxic-ischemic encephalopathy: Antecedent factors and 2-year outcome. J. Pediatr. 2012, 161, 799–807. [Google Scholar] [CrossRef]
- Harteman, J.C.; Groenendaal, F.; Toet, M.C.; Benders, M.J.; Van Haastert, I.C.; Nievelstein, R.A.; Koopman-Esseboom, C.; De Vries, L.S. Diffusion-weighted imaging changes in cerebral watershed distribution following neonatal encephalopathy are not invariably associated with an adverse outcome. Dev. Med. Child Neurol. 2013, 55, 642–653. [Google Scholar] [CrossRef]
- Perez, A.; Ritter, S.; Brotschi, B.; Werner, H.; Caflisch, J.; Martin, E.; Latal, B. Long-term neurodevelopmental outcome with hypoxic-ischemic encephalopathy. J. Pediatr. 2013, 163, 454–459. [Google Scholar] [CrossRef]
- Lee, B.L.; Gano, D.; Rogers, E.E.; Xu, D.; Cox, S.; Barkovich, A.J.; Li, Y.; Ferriero, D.M.; Glass, H.C. Long-term cognitive outcomes in term newborns with watershed injury caused by neonatal encephalopathy. Pediatr. Res. 2021. Online ahead of print. [Google Scholar] [CrossRef]
- Hayman, M.; van Wezel-Meijler, G.; van Straaten, H.; Brilstra, E.; Groenendaal, F.; de Vries, L.S. Punctate white-matter lesions in the full-term newborn: Underlying aetiology and outcome. Eur. J. Paediatr. Neurol. 2019, 23, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Li, A.M.; Chau, V.; Poskitt, K.J.; Sargent, M.A.; Lupton, B.A.; Hill, A.; Roland, E.; Miller, S.P. White matter injury in term newborns with neonatal encephalopathy. Pediatr. Res. 2009, 65, 85–89. [Google Scholar] [CrossRef]
- Vermeulen, R.J.; Fetter, W.P.; Hendrikx, L.; Van Schie, P.E.; van der Knaap, M.S.; Barkhof, F. Diffusion-weighted MRI in severe neonatal hypoxic ischaemia: The white cerebrum. Neuropediatrics 2003, 34, 72–76. [Google Scholar] [PubMed]
- Annink, K.V.; Meerts, L.; van der Aa, N.E.; Alderliesten, T.; Nikkels, P.G.J.; Nijboer, C.H.A.; Groenendaal, F.; de Vries, L.S.; Benders, M.J.N.L.; Hoebeek, F.E.; et al. Cerebellar injury in term neonates with hypoxic-ischemic encephalopathy is underestimated. Pediatr. Res. 2021, 89, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Lemmon, M.E.; Wagner, M.W.; Bosemani, T.; Carson, K.A.; Northington, F.J.; Huisman, T.A.; Poretti, A. Diffusion tensor imaging detects occult cerebellar injury in severe neonatal hypoxic-ischemic encephalopathy. Dev. Neurosci. 2017, 39, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Radicioni, M.; Bini, V.; Chiarini, P.; Fantauzzi, A.; Leone, F.; Scattoni, R.; Camerini, P.G. Cerebral sinovenous thrombosis in the asphyxiated cooled infants: A prospective observational study. Pediatr. Neurol. 2017, 66, 63–68. [Google Scholar] [CrossRef]
- Cowan, F.; Rutherford, M.; Groenendaal, F.; Eken, P.; Mercuri, E.; Bydder, G.M.; Meiners, L.C.; Dubowitz, L.M.; de Vries, L.S. Origin and timing of brain lesions in term infants with neonatal encephalopathy. Lancet 2003, 361, 736–742. [Google Scholar] [CrossRef]
- Ramaswamy, V.; Miller, S.P.; Barkovich, A.J.; Partridge, J.C.; Ferriero, D.M. Perinatal stroke in term infants with neonatal encephalopathy. Neurology 2004, 62, 2088–2091. [Google Scholar] [CrossRef]
- Adhami, F.; Liao, G.; Morozov, Y.M.; Schloemer, A.; Schmithorst, V.J.; Lorenz, J.N.; Dunn, R.S.; Vorhees, C.V.; Wills-Karp, M.; Degen, J.L.; et al. Cerebral ischemia-hypoxia induces intravascular coagulation and autophagy. Am. J. Pathol. 2006, 169, 566–583. [Google Scholar] [CrossRef]
- Michoulas, A.; Basheer, S.N.; Roland, E.H.; Poskitt, K.; Miller, S.; Hill, A. The role of hypoxia-ischemia in term newborns with arterial stroke. Pediatr. Neurol. 2011, 44, 254–258. [Google Scholar] [CrossRef]
- Harbert, M.J.; Tam, E.W.Y.; Glass, H.C.; Bonifacio, S.L.; Haeusslein, L.A.; Barkovich, A.J.; Jeremy, R.J.; Rogers, E.E.; Glidden, D.; Ferriero, D.M. Hypothermia is correlated with seizure absence in perinatal stroke. J. Child Neurol. 2011, 26, 1126–1130. [Google Scholar] [CrossRef]
- Alderliesten, T.; Nikkels, P.G.; Benders, M.J.; de Vries, L.S.; Groenendaal, F. Antemortem cranial MRI compared with postmortem histopathologic examination of the brain in term infants with neonatal encephalopathy following perinatal asphyxia. Arch. Dis. Child. Fetal Neonatal Ed. 2012, 98, F304–F309. [Google Scholar] [CrossRef] [PubMed]
- Annink, K.V.; De Vries, L.S.; Groenendaal, F.; Heuvel, M.P.V.D.; Van Haren, N.E.M.; Swaab, H.; Van Handel, M.; Jongmans, M.J.; Benders, M.J.; Van Der Aa, N.E. The long-term effect of perinatal asphyxia on hippocampal volumes. Pediatr. Res. 2019, 85, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Molavi, M.; Vann, S.D.; de Vries, L.S.; Groenendaal, F.; Lequin, M. Signal change in the mammillary bodies after perinatal asphyxia. AJNR Am. J. Neuroradiol. 2019, 40, 1829–1834. [Google Scholar] [CrossRef] [PubMed]
- Lequin, M.; Steggerda, S.; Severino, M.; Tortora, D.; Parodi, A.; Ramenghi, L.A.; Groenendaal, F.; Meys, K.M.; Benders, M.J.; de Vries, L.S.; et al. Mammillary body injury in neonatal encephalopathy: A multicentre, retrospective study. Pediatr. Res. 2021. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Annink, K.V.; de Vries, L.S.; Groenendaal, F.; Eijsermans, R.M.J.C.; Mocking, M.; van Schooneveld, M.M.J.; Dudink, J.; van Straaten, H.L.M.; Benders, M.J.N.L.; Lequin, M.; et al. Mammillary body atrophy and other MRI correlates of school-age outcome following neonatal hypoxic-ischemic encephalopathy. Sci. Rep. 2021, 11, 5017. [Google Scholar] [CrossRef] [PubMed]
- Barkovich, A.J.; Truwit, C.L. Brain damage from perinatal asphyxia: Correlation of MR findings with gestational age. AJNR Am. J. Neuroradiol. 1990, 11, 1087–1096. [Google Scholar] [PubMed]
- Shroff, M.M.; Soares-Fernandes, J.P.; Whyte, H.; Raybaud, C. MR imaging for diagnostic evaluation of encephalopathy in the newborn. Radiographics 2010, 30, 763–780. [Google Scholar] [CrossRef]
- Barkovich, A.J.; Westmark, K.; Partridge, C.; Sola, A.; Ferriero, D.M. Perinatal asphyxia—MR findings in the first 10 days. Am. J. Neuroradiol. 1995, 16, 427–438. [Google Scholar]
- Rutherford, M.A.; Pennock, J.M.; Counsell, S.J.; Mercuri, E.; Cowan, F.M.; Dubowitz, L.M.S.; Edwards, A.D. Abnormal magnetic resonance signal in the internal capsule predicts poor neurodevelopmental outcome in infants with hypoxic-ischemic encephalopathy. Pediatrics 1998, 102 Pt 1, 323–328. [Google Scholar] [CrossRef]
- Sener, R.N. Diffusion MRI: Apparent diffusion coefficient (ADC) values in the normal brain and a classification of brain disorders based on ADC values. Comput. Med. Imaging Graph. 2001, 25, 299–326. [Google Scholar] [CrossRef]
- Cowan, F.M.; Pennock, J.M.; Hanrahan, J.D.; Manji, K.P.; Edwards, A.D. Early detection of cerebral infarction and hypoxic ischemic encephalopathy in neonates using diffusion-weighted magnetic resonance imaging. Neuropediatrics 1994, 25, 172–175. [Google Scholar] [CrossRef]
- Robertson, R.L.; Ben-Sira, L.; Barnes, P.D.; Mulkern, R.V.; Robson, C.D.; Maier, S.E.; Rivkin, M.J.; Du Plessis, A.J. MR line-scan diffusion-weighted imaging of term neonates with perinatal brain ischemia. AJNR Am. J. Neuroradiol. 1999, 20, 1658–1670. [Google Scholar] [PubMed]
- Bednarek, N.; Mathur, A.; Inder, T.; Wilkinson, J.; Neil, J.; Shimony, J. Impact of therapeutic hypothermia on MRI diffusion changes in neonatal encephalopathy. Neurology 2012, 78, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- Haacke, E.M.; Mittal, S.; Wu, Z.; Neelavalli, J.; Cheng, Y.C. Susceptibility-weighted imaging: Technical aspects and clinical applications, part 1. AJNR Am. J. Neuroradiol. 2009, 30, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Bosemani, T.; Poretti, A.; Huisman, T.A. Susceptibility-weighted imaging in pediatric neuroimaging. J. Magn. Reson. Imaging 2014, 40, 530–544. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, G.; Kido, D.; Wycliffe, N.; Jacobson, J.P.; Oyoyo, U.; Ashwal, S. Hypoxic-ischemic injury: Utility of susceptibility-weighted imaging. Pediatr. Neurol. 2011, 45, 220–224. [Google Scholar] [CrossRef]
- Messina, S.A.; Poretti, A.; Tekes, A.; Robertson, C.; Johnston, M.V.; Huisman, T.A. Early predictive value of susceptibility weighted imaging (SWI) in pediatric hypoxic-ischemic injury. J. Neuroimaging 2014, 24, 528–530. [Google Scholar] [CrossRef]
- Smith, S.M.; Jenkinson, M.; Johansen-Berg, H.; Rueckert, D.; Nichols, T.E.; Mackay, C.; Watkins, K.; Ciccarelli, O.; Cader, M.Z.; Matthews, P.M.; et al. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. Neuroimage 2006, 31, 1487–1505. [Google Scholar] [CrossRef]
- Porter, E.J.; Counsell, S.J.; Edwards, A.D.; Allsop, J.; Azzopardi, D. Tract-based spatial statistics of magnetic resonance images to assess disease and treatment effects in perinatal asphyxial encephalopathy. Pediatr. Res. 2010, 68, 205–209. [Google Scholar] [CrossRef]
- Petersen, E.T.; Zimine, I.; Ho, Y.C.; Golay, X. Non-invasive measurement of perfusion: A critical review of arterial spin labelling techniques. Br. J. Radiol. 2006, 79, 688–701. [Google Scholar] [CrossRef]
- Kleuskens, D.G.; Goncalves Costa, F.; Annink, K.V.; van den Hoogen, A.; Alderliesten, T.; Groenendaal, F.; Benders, M.J.; Dudink, J. Pathophysiology of cerebral hyperperfusion in term neonates with hypoxic-ischemic encephalopathy: A systematic review for future research. Front. Pediatr. 2021, 9, 631258. [Google Scholar] [CrossRef] [PubMed]
- Wintermark, P.; Hansen, A.; Gregas, M.C.; Soul, J.; Labrecque, M.; Robertson, R.L.; Warfield, S.K. Brain perfusion in asphyxiated newborns treated with therapeutic hypothermia. AJNR Am. J. Neuroradiol. 2011, 32, 2023–2029. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Wang, Q.; Li, Y.; Ma, X.; Li, W.; Wang, Q. Diagnostic performance of arterial spin-labeled perfusion imaging and diffusion-weighted imaging in full-term neonatal hypoxic-ischemic encephalopathy. J. Integr. Neurosci. 2021, 20, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Proisy, M.; Corouge, I.; Legouhy, A.; Nicolas, A.; Charon, V.; Mazille, N.; Leroux, S.; Bruneau, B.; Barillot, C.; Ferré, J.-C. Changes in brain perfusion in successive arterial spin labeling MRI scans in neonates with hypoxic-ischemic encephalopathy. Neuroimage Clin. 2019, 24, 101939. [Google Scholar] [CrossRef]
- Peden, C.J.; Cowan, F.M.; Bryant, D.J.; Sargentoni, J.; Cox, I.J.; Menon, D.K.; Gadian, D.G.; Bell, J.D.; Dubowitz, L.M. Proton MR spectroscopy of the brain in infants. J. Comput. Assist. Tomogr. 1990, 14, 886–894. [Google Scholar] [CrossRef]
- Schmitz, B.; Wang, X.; Barker, P.B.; Pilatus, U.; Bronzlik, P.; Dadak, M.; Kahl, K.G.; Lanfermann, H.; Ding, X.-Q. Effects of aging on the human brain: A proton and phosphorus MR spectroscopy study at 3T. J. Neuroimaging 2018, 28, 416–421. [Google Scholar] [CrossRef]
- Shibasaki, J.; Aida, N.; Morisaki, N.; Tomiyasu, M.; Nishi, Y.; Toyoshima, K. Changes in brain metabolite concentrations after neonatal hypoxic-ischemic encephalopathy. Radiology 2018, 288, 840–848. [Google Scholar] [CrossRef]
- Toft, P.B.; Leth, H.; Lou, H.C.; Pryds, O.; Henriksen, O. Metabolite concentrations in the developing brain estimated with proton MR spectroscopy. J. Magn. Reson. Imaging 1994, 4, 674–680. [Google Scholar] [CrossRef]
- Roelants-Van Rijn, A.M.; Van der Grond, J.; De Vries, L.S.; Groenendaal, F. Value of H-1-MRS using different echo times in neonates with cerebral hypoxia-ischemia. Pediatr. Res. 2001, 49, 356–362. [Google Scholar] [CrossRef]
- Wu, T.W.; Tamrazi, B.; Hsu, K.H.; Ho, E.; Reitman, A.J.; Borzage, M.; Blüml, S.; Wisnowski, J.L. Cerebral lactate concentration in neonatal hypoxic-ischemic encephalopathy: In relation to time, characteristic of injury, and serum lactate concentration. Front. Neurol. 2018, 9, 293. [Google Scholar] [CrossRef] [PubMed]
- Shibasaki, J.; Niwa, T.; Piedvache, A.; Tomiyasu, M.; Morisaki, N.; Fujii, Y.; Toyoshima, K.; Aida, N. Comparison of predictive values of magnetic resonance biomarkers based on scan timing in neonatal encephalopathy following therapeutic hypothermia. J. Pediatr. 2021, 239, 101–109.e4. [Google Scholar] [CrossRef] [PubMed]
- Leth, H.; Toft, P.B.; Pryds, O.; Peitersen, B.; Lou, H.C.; Henriksen, O. Brain lactate in preterm and growth-retarded neonates. Acta Paediatr. 1995, 84, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Roelants-van Rijn, A.M.; van der Grond, J.; Stigter, R.H.; de Vries, L.S.; Groenendaal, F. Cerebral structure and metabolism and long-term outcome in small-for-gestational-age preterm neonates. Pediatr. Res. 2004, 56, 285–290. [Google Scholar] [CrossRef]
- Moorcraft, J.; Bolas, N.M.; Ives, N.K.; Ouwerkerk, R.; Smyth, J.; Rajagopalan, B.; Hope, P.L.; Radda, G.K. Global and depth resolved phosphorus magnetic resonance spectroscopy to predict outcome after birth asphyxia. Arch. Dis. Child. 1991, 66, 1119–1123. [Google Scholar] [CrossRef]
- Roth, S.C.; Edwards, A.D.; Cady, E.B.; Delpy, D.T.; Wyatt, J.S.; Azzopardi, D.; Baudin, J.; Townsend, J.; Stewart, A.L.; Reynolds, E.O.R. Relation between cerebral oxidative-metabolism following birth asphyxia, and neurodevelopmental outcome and brain growth at one year. Dev. Med. Child Neurol. 1992, 34, 285–295. [Google Scholar] [CrossRef]
- Poretti, A.; Blaser, S.I.; Lequin, M.H.; Fatemi, A.; Meoded, A.; Northington, F.J.; Boltshauser, E.; Huisman, T.A. Neonatal neuroimaging findings in inborn errors of metabolism. J. Magn. Reson. Imaging 2012, 37, 294–312. [Google Scholar] [CrossRef]
- Sener, R.N. Maple syrup urine disease: Diffusion MRI, and proton MR spectroscopy findings. Comput. Med. Imaging Graph. 2007, 31, 106–110. [Google Scholar] [CrossRef]
- McAdams, R.M.; Richards, T.L. Detection of nonketotic hyperglycinemia in a neonate using proton magnetic resonance spectroscopy. Radiol. Case Rep. 2009, 4, 310. [Google Scholar] [CrossRef][Green Version]
- Vijayakumar, K.; Gunny, R.; Grunewald, S.; Carr, L.; Chong, K.W.; DeVile, C.; Robinson, R.; McSweeney, N.; Prabhakar, P. Clinical neuroimaging features and outcome in molybdenum cofactor deficiency. Pediatr. Neurol. 2011, 45, 246–252. [Google Scholar] [CrossRef]
- Durmaz, M.S.; Ozbakir, B. Molybdenum cofactor deficiency: Neuroimaging findings. Radiol. Case Rep. 2018, 13, 592–595. [Google Scholar] [CrossRef] [PubMed]
- Luptakova, D.; Baciak, L.; Pluhacek, T.; Skriba, A.; Sediva, B.; Havlicek, V.; Juranek, I. Membrane depolarization and aberrant lipid distributions in the neonatal rat brain following hypoxic-ischaemic insult. Sci. Rep. 2018, 8, 6952. [Google Scholar] [CrossRef] [PubMed]
- Barkovich, A.J.; Hajnal, B.L.; Vigneron, D.; Sola, A.; Partridge, J.C.; Allen, F.; Ferriero, D.M. Prediction of neuromotor outcome in perinatal asphyxia: Evaluation of MR scoring systems. AJNR Am. J. Neuroradiol. 1998, 19, 143–149. [Google Scholar] [PubMed]
- Shankaran, S.; Barnes, P.D.; Hintz, S.R.; Laptook, A.R.; Zaterka-Baxter, K.M.; McDonald, S.A.; Ehrenkranz, R.A.; Walsh, M.C.; Tyson, J.E.; Donovan, E.F.; et al. Brain injury following trial of hypothermia for neonatal hypoxic-ischaemic encephalopathy. Arch. Dis. Child. Fetal Neonatal Ed. 2012, 97, F398–F404. [Google Scholar] [PubMed]
- Trivedi, S.B.; Vesoulis, Z.; Rao, R.; Liao, S.M.; Shimony, J.S.; McKinstry, R.C.; Mathur, A.M. A validated clinical MRI injury scoring system in neonatal hypoxic-ischemic encephalopathy. Pediatr. Radiol. 2017, 47, 1491–1499. [Google Scholar] [CrossRef]
- Weeke, L.C.; Groenendaal, F.; Mudigonda, K.; Blennow, M.; Lequin, M.H.; Meiners, L.C.; van Haastert, I.C.; Benders, M.J.; Hallberg, B.; de Vries, L.S. A novel magnetic resonance imaging score predicts neurodevelopmental outcome after perinatal asphyxia and therapeutic hypothermia. J. Pediatr. 2018, 192, 33–40.e2. [Google Scholar] [CrossRef]
- Bhroin, M.N.; Kelly, L.; Sweetman, D.; Aslam, S.; O'Dea, M.I.; Hurley, T.; Slevin, M.; Murphy, J.; Byrne, A.T.; Colleran, G.; et al. Relationship between MRI scoring systems and neurodevelopmental outcome at two years in infants with neonatal encephalopathy. Pediatr. Neurol. 2022, 126, 35–42. [Google Scholar] [CrossRef]
- Barkovich, A.; Miller, S.; Bartha, A.; Newton, N.; Hamrick, S.; Mukherjee, P.; Glenn, O.; Xu, D.; Partridge, J.; Ferriero, D.; et al. MR imaging, MR spectroscopy, and diffusion tensor imaging of sequential studies in neonates with encephalopathy. AJNR Am. J. Neuroradiol. 2006, 27, 533–547. [Google Scholar]
- Skranes, J.H.; Cowan, F.M.; Stiris, T.; Fugelseth, D.; Thoresen, M.; Server, A. Brain imaging in cooled encephalopathic neonates does not differ between four and 11 days after birth. Acta Paediatr. 2015, 104, 752–758. [Google Scholar] [CrossRef]
- Wintermark, P.; Hansen, A.; Soul, J.; Labrecque, M.; Robertson, R.L.; Warfield, S.K. Early versus late MRI in asphyxiated newborns treated with hypothermia. Arch. Dis. Child. Fetal Neonatal Ed. 2010, 96, F36–F44. [Google Scholar] [CrossRef]
- O’Kane, A.; Vezina, G.; Chang, T.; Bendush, N.; Ridore, M.; Gai, J.; Bost, J.; Glass, P.; Massaro, A.N. Early versus late brain magnetic resonance imaging after neonatal hypoxic ischemic encephalopathy treated with therapeutic hypothermia. J. Pediatr. 2021, 232, 73–79.e2. [Google Scholar] [CrossRef]
- Barkovich, A.J.; Baranski, K.; Vigneron, D.; Partridge, J.C.; Hallam, D.K.; Hajnal, B.L.; Ferriero, D.M. Proton MR spectroscopy for the evaluation of brain injury in asphyxiated, term neonates. AJNR Am J Neuroradiol. 1999, 20, 1399–1405. [Google Scholar] [PubMed]
- Penrice, J.; Lorek, A.; Cady, E.B.; Amess, P.N.; Wylezinska, M.; Cooper, C.E.; D’Souza, P.; Brown, G.C.; Kirkbride, V.; Edwards, A.D.; et al. Proton magnetic resonance spectroscopy of the brain during acute hypoxia-ischemia and delayed cerebral energy failure in the newborn piglet. Pediatr. Res. 1997, 41, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Wintermark, P.; Labrecque, M.; Warfield, S.K.; DeHart, S.; Hansen, A. Can induced hypothermia be assured during brain MRI in neonates with hypoxic-ischemic encephalopathy? Pediatr. Radiol. 2010, 40, 1950–1954. [Google Scholar] [CrossRef]
- Wu, T.W.; McLean, C.; Friedlich, P.; Grimm, J.; Bluml, S.; Seri, I. Maintenance of whole-body therapeutic hypothermia during patient transport and magnetic resonance imaging. Pediatr. Radiol. 2014, 44, 613–617. [Google Scholar] [CrossRef]
- Wezel-Meijler, G.; de Vries, L.S. Cranial ultrasound—Optimizing utility in the NICU. Curr. Pediatr. Rev. 2014, 10, 16–27. [Google Scholar] [CrossRef]
- Hall, P.; Adami, H.-O.; Trichopoulos, D.; Pedersen, N.L.; Lagiou, P.; Ekbom, A.; Ingvar, M.; Lundell, M.; Granath, F. Effect of low doses of ionising radiation in infancy on cognitive function in adulthood: Swedish population based cohort study. BMJ 2004, 328, 19. [Google Scholar] [CrossRef]
- Pearce, M.S.; Salotti, J.A.; Little, M.P.; McHugh, K.; Lee, C.; Kim, K.P.; Howe, N.L.; Ronckers, C.M.; Rajaraman, P.; Craft, A.W.; et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: A retrospective cohort study. Lancet 2012, 380, 499–505. [Google Scholar] [CrossRef]
- Executive summary: Neonatal encephalopathy and neurologic outcome, second edition. Report of the American college of obstetricians and gynecologists’ task force on neonatal encephalopathy. Obstet. Gynecol. 2014, 123, 896–901. [CrossRef]
- Wisnowski, J.L.; Wintermark, P.; Bonifacio, S.L.; Smyser, C.D.; Barkovich, A.J.; Edwards, A.D.; de Vries, L.S.; Inder, T.E.; Chau, V. Neuroimaging in the term newborn with neonatal encephalopathy. Semin. Fetal Neonatal Med. 2021, 26, 101304. [Google Scholar] [CrossRef]
- de Vries, L.S.; Alderliesten, T.; Benders, M.J.; Groenendaal, F. Serial 1- and 2-dimensional cerebral MRI measurements in full-term infants after perinatal asphyxia. Neonatology 2016, 110, 27–32. [Google Scholar]
- Byrne, P.; Welch, R.; Johnson, M.A.; Darrah, J.; Piper, M. Serial magnetic resonance imaging in neonatal hypoxic-ischemic encephalopathy. J. Pediatr. 1990, 117, 694–700. [Google Scholar] [CrossRef]
- Millet, V.; Bartoli, J.M.; Lacroze, V.; Raybaud, C.; Unal, D.; Girard, N. Predictive significance of magnetic resonance imaging at 4 months of adjusted age in infants after a perinatal neurologic insult. Biol. Neonate 1998, 73, 207–219. [Google Scholar] [CrossRef]
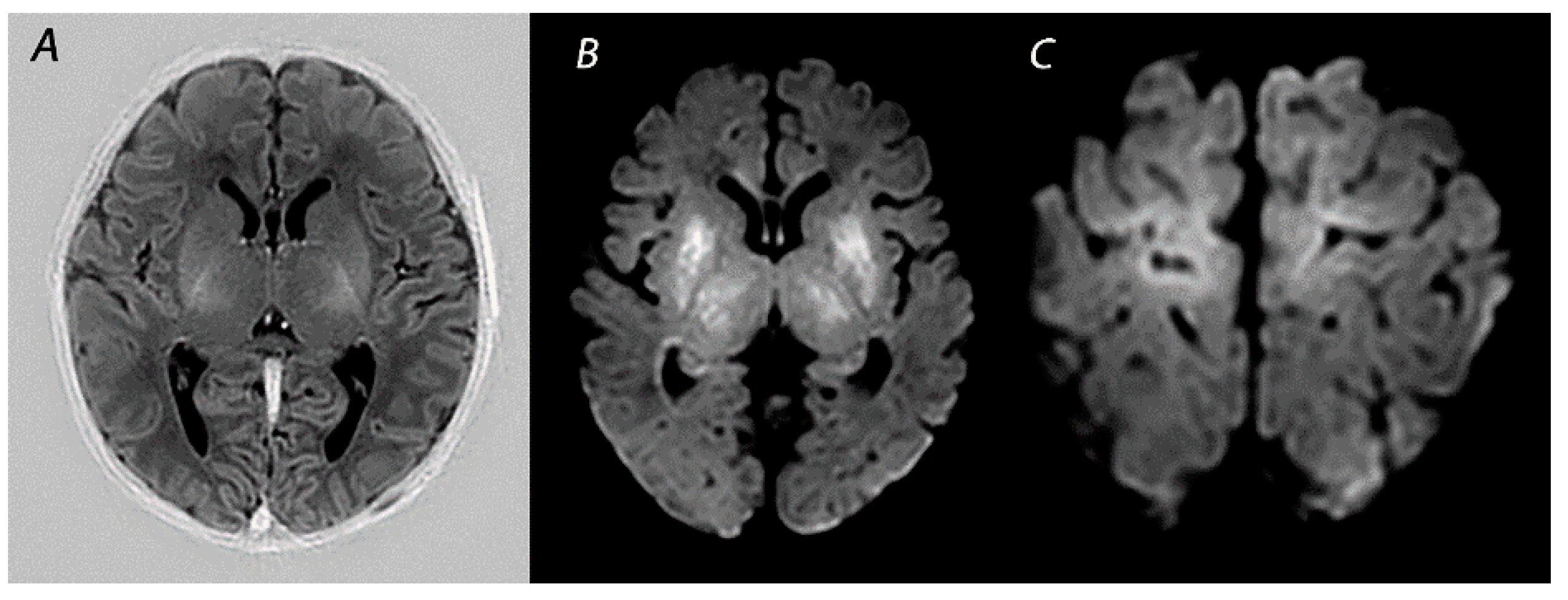

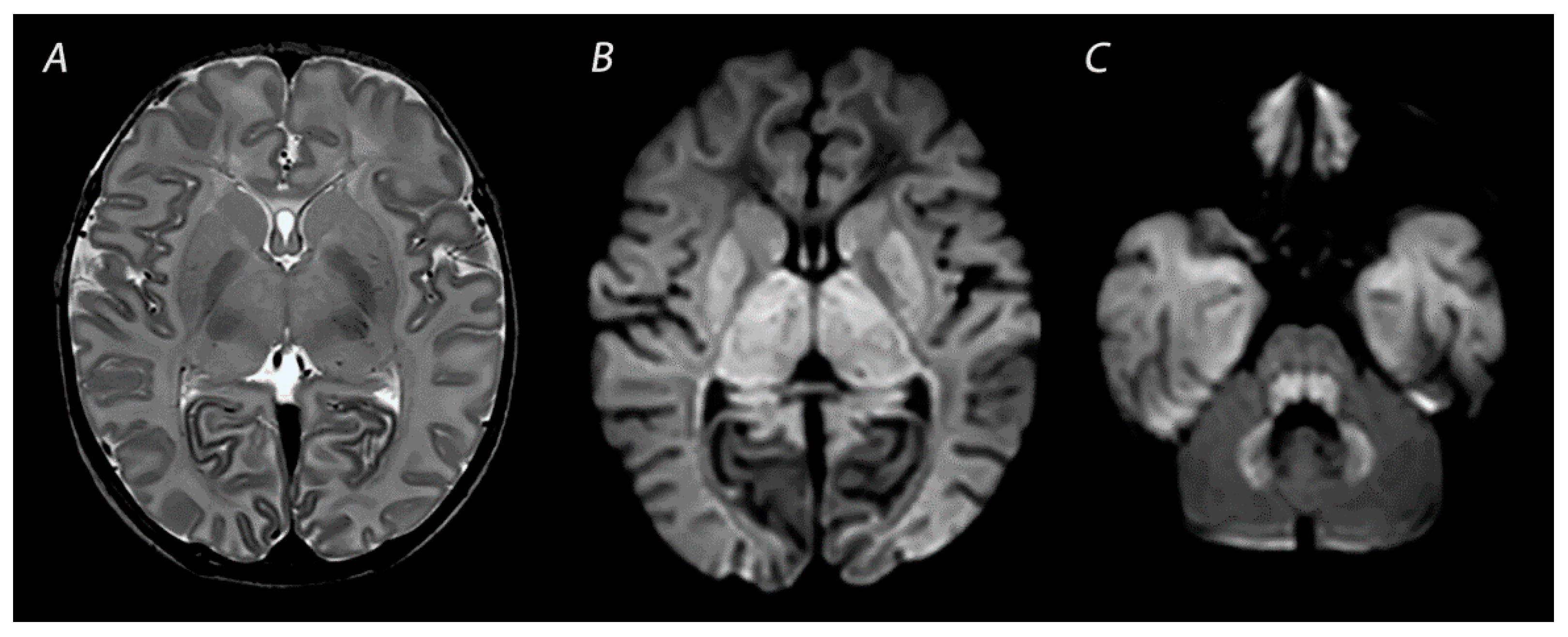
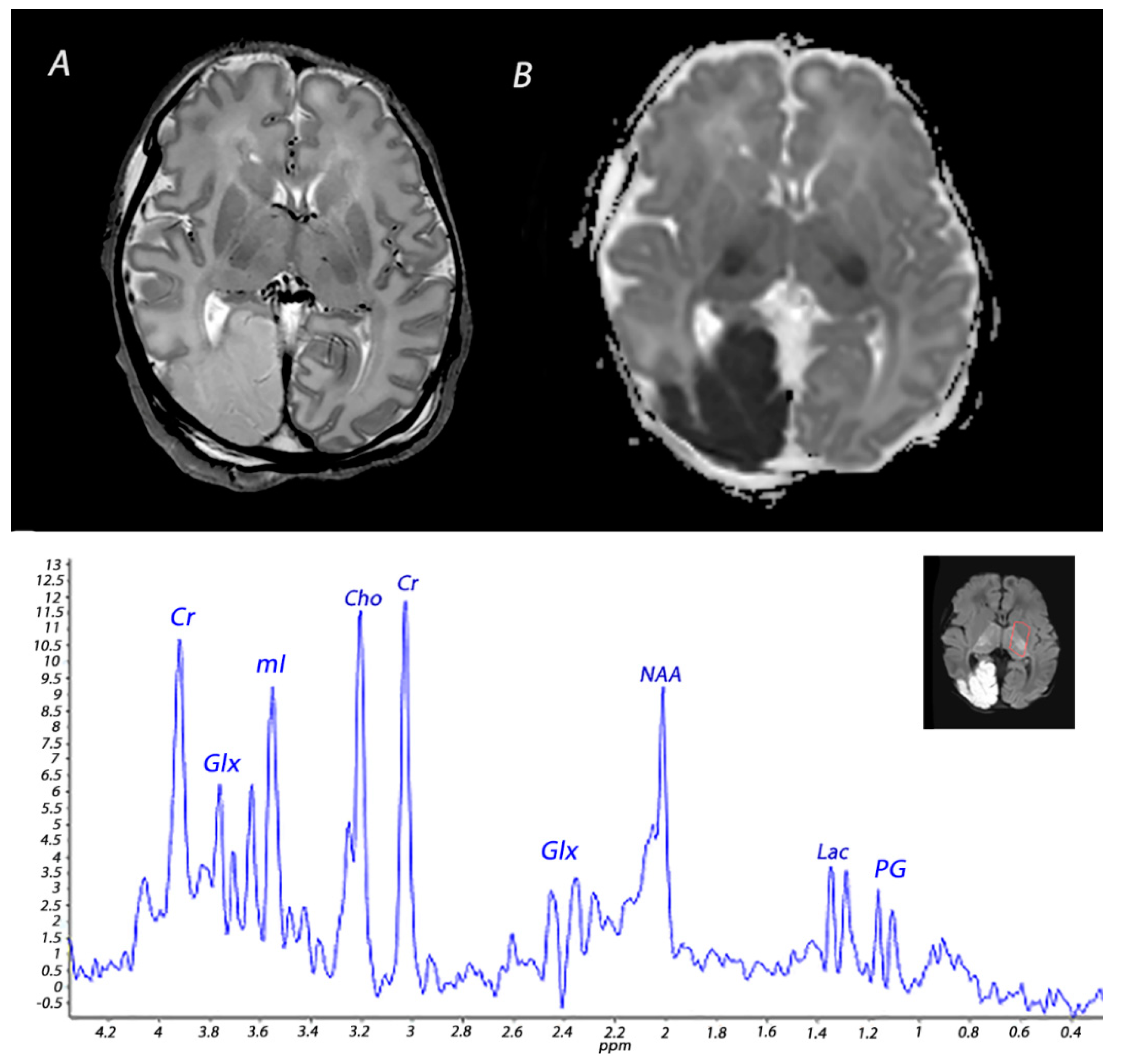

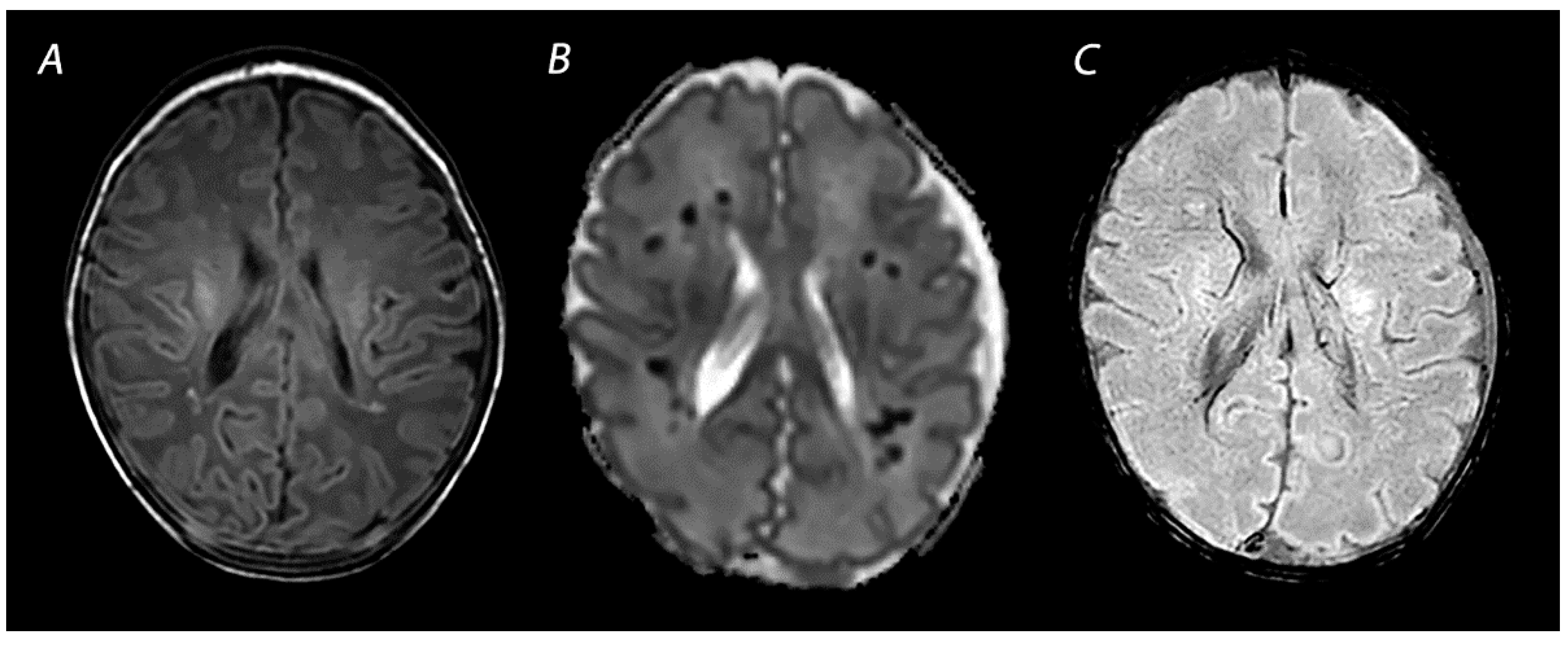

| Pattern of Injury | Imaging Modality | Imaging Findings | Time Frame of Abnormalities |
|---|---|---|---|
| WM/WS | T1WI/T2WI | Abnormal signal intensity in the white matter of the watershed areas of the cerebral arteries, and also the overlying cortex in severely affected infants. T2WI may show loss of gray-white matter differentiation at the cortex. | Inconspicuous or subtle abnormalities in the first days, which become gradually more apparent by the latter half of the first week following the insult. MRI obtained beyond 1 month can show cortical thinning, white matter volume loss, cysts and gliosis of the cortex and white matter. |
| DWI | High signal intensity on isotropic DWI with low ADC values in the affected areas. | Abnormalities peak at 3–5 days after the insult. Pseudo-normalization occurs after approximately 11–12 days for infants treated with therapeutic hypothermia, and 6–8 days in non-cooled infants. | |
| 1H-MRS | Increased lactate and decreased NAA in the affected white matter. | Lactate in general increases <24 h and subsequently normalizes by the end of the first week, but persistent elevation has been reported. 1 NAA declines <24 h and remains low during the first 2 weeks after the insult, although some studies have reported that NAA levels do not significantly diminish until approximately 48 h after the insult. 2 | |
| SWI | Prominent hypo-intense veins, low signal intensity at the site of hemorrhagic lesions. | Prominent hypo-intense veins have been observed as early as 18 h after birth, but current literature is limited. Low signal intensity at the site of hemorrhagic lesions is seen immediately and can persists for many months. | |
| BGT | T1WI/T2WI | Abnormal signal intensity in the basal ganglia, thalami and the perirolandic cortex. Absence of a normal high-signal intensity of the PLIC. | Inconspicuous or subtle abnormalities in the first days, which become gradually more apparent by the latter half of the first week following the insult. MRI obtained beyond 1 month can show volume loss, cysts, gliosis and impaired myelination of the central gray matter and perirolandic cortex. |
| DWI | High signal intensity on isotropic DWI with low ADC values in affected areas. | Abnormalities peak at 3–5 days after the insult. Pseudo-normalization occurs after approximately 11–12 days for infants treated with therapeutic hypothermia, and 6–8 days in non-cooled infants. | |
| 1H-MRS | Increased lactate and decreased NAA in basal ganglia and thalami. | Lactate in general increases <24 h and subsequently normalizes by the end of the first week, but persistent elevation has been reported. 1 NAA declines <24 h and remains low during the first 2 weeks after the insult, although some studies have reported that NAA levels do not significantly diminish until approximately 48 h after the insult. 2 | |
| SWI | Prominent hypo-intense veins | Prominent hypo-intense veins have been observed as early as 18 h after birth, but current literature is limited. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parmentier, C.E.J.; de Vries, L.S.; Groenendaal, F. Magnetic Resonance Imaging in (Near-)Term Infants with Hypoxic-Ischemic Encephalopathy. Diagnostics 2022, 12, 645. https://doi.org/10.3390/diagnostics12030645
Parmentier CEJ, de Vries LS, Groenendaal F. Magnetic Resonance Imaging in (Near-)Term Infants with Hypoxic-Ischemic Encephalopathy. Diagnostics. 2022; 12(3):645. https://doi.org/10.3390/diagnostics12030645
Chicago/Turabian StyleParmentier, Corline E. J., Linda S. de Vries, and Floris Groenendaal. 2022. "Magnetic Resonance Imaging in (Near-)Term Infants with Hypoxic-Ischemic Encephalopathy" Diagnostics 12, no. 3: 645. https://doi.org/10.3390/diagnostics12030645
APA StyleParmentier, C. E. J., de Vries, L. S., & Groenendaal, F. (2022). Magnetic Resonance Imaging in (Near-)Term Infants with Hypoxic-Ischemic Encephalopathy. Diagnostics, 12(3), 645. https://doi.org/10.3390/diagnostics12030645






