The Genetics of Primary Ciliary Dyskinesia in Puerto Rico
Abstract
:1. Introduction
2. Materials and Methods
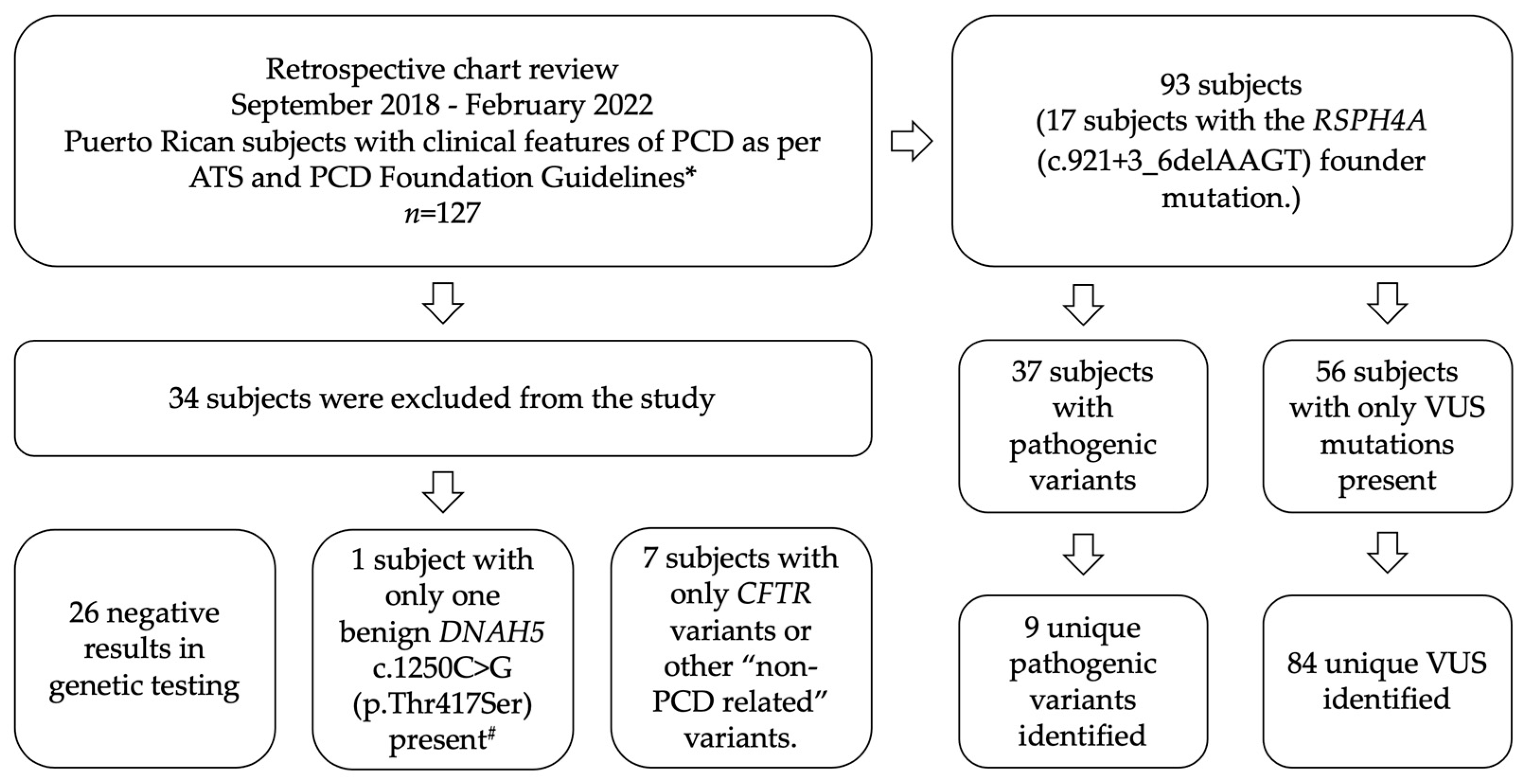
3. Results
3.1. Distribution of PCD Genetic Variants in Puerto Rico
3.2. Frequency Percentage by Zygosity
3.2.1. Pathogenic Frequency in PCD Implicated Genes
3.2.2. Homozygous Subjects with the RSPH4A (c.921+3_6delAAGT) Founder Mutation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Genes | Transcript Reference ^ |
|---|---|
| AK7 | NM_152327.3 |
| ARMC4 * | NM_018076.2 |
| C11orf70 | NM_032930.2 |
| CCDC103 | NM_213607.2 |
| CCDC114 | NM_144577.3 |
| CCDC151 | NM_145045.4 |
| CCDC39 | NM_181426.1 |
| CCDC40 | NM_017950.3 |
| CCDC65 | NM_033124.4 |
| CCNO | NM_021147.4 |
| CEP164 | NM_014956.4 |
| CFAP298 | NM_021254.2 |
| DNAAF1 | NM_178452.4 |
| DNAAF2 | NM_018139.2 |
| DNAAF3 | NM_001256714.1 |
| DNAAF4 | NM_130810.3 |
| DNAAF5 | NM_017802.3 |
| DNAH1 | NM_015512.4 |
| DNAH11 | NM_001277115.1 |
| DNAH5 | NM_001369.2 |
| DNAH8 | NM_001206927.1 |
| DNAH9 | NM_001372.3 |
| DNAI1 | NM_012144.3; NM_001281428.1 |
| DNAI2 | NM_023036.4 |
| DNAJB13 | NM_153614.3 |
| DNAL1 | NM_031427.3 |
| DRC1 | NM_145038.3 |
| GAS8 | NM_001481.2 |
| LRRC56 | NM_198075.3 |
| LRRC6 | NM_012472.4 |
| MCIDAS | NM_001190787.1 |
| NOTCH2 * | NM_024408.3 |
| OFD1 | NM_003611.2 |
| PIH1D3 | NM_001169154.1 |
| RPGR * | NM_000328.2 |
| RSPH1 | NM_080860.3 |
| RSPH3 | NM_031924.4 |
| RSPH4A | NM_001010892.2 |
| RSPH9 | NM_152732.4 |
| SPAG1 | NM_172218.2 |
| ZMYND10 | NM_015896.2 |
| PCD Genes | Unique Variants |
|---|---|
| DNAAF2 | c.58G > C (p.Val20Leu) |
| DNAAF4 | c.186C > G (p.Ser62Arg) |
| DNAH11 | c.8888C > A (p.Ser2963Tyr) |
| DNAAF5 | c.969C > A (p.Asp323Glu) |
| DNAAF3 | c.1672G > T (p.Glu558*) |
| SPAG1 | c.2717A > G (p.Asp906Gly) |
| CCDC103 | c.82C > T (p.Arg28Trp) |
| CCDC40 | c.1109G > A (p.Arg370His) |
| DNAI2 | c.1153G > A (p.Asp385Asn) |
| CCNO | c.685G > A (p.Gly229Ser) |
| RSPH4A | c.902A > C (p.Gln301Pro) |
| CFAP298 | c.803C > T (p.Pro268Leu) |
| RSPH1 | c.824G > A (p.Arg275Gln) |
| RSPH1 | c.673C > T (p.Pro225Ser) |
| CCDC103 | c.146G > C (p.Gly49Ala) |
| DNAH5 | c.6409C > A (p.Leu2137Ile) |
| DNAH5 | c.5413C > T (p.Arg1805Cys) |
| DNAH5 | c.2577+5T > G (Intronic) |
| DNAH5 | c.477C > T (Silent) |
| DNAH5 | c.8398G > A (p.Val2800Ile) |
| DNAAF5 | c.1471-3C > T (Intronic) |
| DNAH11 | c.6273 + 12C > G (Intronic) |
| DNAH11 | c.10379C > T (p.Thr3460Met) |
| DNAH11 | c.11098G > C (p.Glu3700Gln) |
| DNAH11 | c.3739A > C (p.Ile1247Leu) |
| DNAH11 | c.5434G > A (p.Val1812Met) |
| DNAH11 | c.3288G > A (p.Met1096Ile) |
| DNAH11 | c.6154C > T (p.Leu2052Phe) |
| DNAH11 | c.11678C > T (p.Thr3893Met) |
| DNAH11 | c.13243A > C (p.Lys4415Gln) |
| DNAH9 | c.3128A > T (p.His1043Leu) |
| DNAH9 | c.3593G > C (p.Trp1198Ser) |
| DNAH9 | c.4070C > A (p.Thr1357Lys) |
| DNAH9 | c.1562A > T (p.Asp521Val) |
| DNAH9 | c.1603G > A (p.Asp535Asn) |
| DNAAF2 | c.10G > A (p.Ala4Thr) |
| DNAAF2 | c.700C > T (p.Pro234Ser) |
| DNAAF2 | c.1082_1087dup (p.Val361_Ala362dup) |
| DNAH1 | c.7064C > T (p.Pro2355Leu) |
| DNAH1 | c.3877G > A (p.Asp1293Asn) |
| DNAH1 | c.1610G > A (p.Ser537Asn) |
| DNAH1 | c.6650T > C (p.Leu2217Pro) |
| DNAH1 | c.10208G > T (p.Gly3403Val) |
| DNAH1 | c.7905G > C (p.Gln2635His) |
| DNAH1 | c.9646C > G (p.Leu3216Val) |
| DNAH1 | c.8491C > A (p.Arg2831Ser) |
| DNAAF5 | c.412T > G (p.Cys138Gly) |
| DNAAF5 | c.221C > A (p.Ala74Glu) |
| DNAAF5 | c.1195G > A (p.Glu399Lys) |
| DNAAF5 | c.454G > A (p.Ala152Thr) |
| DNAH5 | c.12709G > T (p.Val4237Phe) |
| DNAH5 | c.10731C > G (p.Asn3577Lys) |
| DNAH5 | c.1438C > T (p.Leu480Phe) |
| DNAH5 | c.12523G > A (p.Val4175Met) |
| CCDC103 | c.355C > T (p.Arg119Cys) |
| CCDC103 | c.197G > A (p.Gly66Glu) |
| CCDC103 | c.301A > G (p.Thr101Ala) |
| RSPH1 | c.835C > T (p.Arg279Trp) |
| RSPH1 | c.635C > T (p.Thr212Ile) |
| DNAH8 | c.8801T > C (p.Val2934Ala) |
| DNAH8 | c.644G > A (p.Gly215Glu) |
| DNAH8 | c.7114A > G (p.Met2372Val) |
| DNAH8 | c.13607T > C (p.Leu4536Pro) |
| DNAH8 | c.2096G > A (p.Arg699His) |
| DNAH8 | c.9839A > T (p.Gln3280Leu) |
| DNAH8 | c.9811G > T (p.Ala3271Ser) |
| CFAP298 | c.534+4C > T (Intronic) |
| DNAAF3 | c.623T > C (p.Leu208Pro) |
| DNAAF3 | c.1615G > A (p.Gly539Arg) |
| CCNO | c.16C > A (p.Pro6Thr) |
| CCDC40 | c.1076G > A (p.Arg359His) |
| DNAI2 | c.487A > G (p.Arg163Gly) |
| CCDC39 | c.1229A > G (p.Gln410Arg) |
| CCDC39 | c.692T > C (p.Ile231Thr) |
| CCDC39 | c.2057A > G (p.Asn686Ser) |
| CCDC151 | c.619C > T (p.Arg207Trp) |
| CCDC65 | c.1067T > C (p.Phe356Ser) |
| CCDC65 | c.771G > T (p.Glu257Asp) |
| RSPH3 | c.895G > C (p.Ala299Pro) |
| NME8 | c.88A > G (p.Thr30Ala) |
| GAS8 | c.1255G > A (p.Asp419Asn) |
| LRRC56 | c.52G > T (p.Val18Phe) |
| OFD1 | c.2584T > G (p.Ser862Ala) |
| RSPH9 | c.98T > C (p.Met33Thr) |
References
- Zariwala, M.A.; Knowles, M.R.; Leigh, M.W. Primary Ciliary Dyskinesia. In GeneReviews((R)); University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Knowles, M.R.; Daniels, L.A.; Davis, S.D.; Zariwala, M.A.; Leigh, M.W. Primary ciliary dyskinesia. Recent advances in diagnostics, genetics, and characterization of clinical disease. Am. J. Respir. Crit. Care Med. 2013, 188, 913–922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Connor, M.G.; Griffiths, A.; Iyer, N.P.; Shapiro, A.J.; Wilson, K.C.; Thomson, C.C. Summary for Clinicians: Diagnosis of Primary Ciliary Dyskinesia. Ann. Am. Thorac. Soc. 2019, 16, 171–174. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, M.G.; Horani, A.; Shapiro, A.J. Progress in Diagnosing Primary Ciliary Dyskinesia: The North American Perspective. Diagnostics 2021, 11, 1278. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, A.J.; Davis, S.D.; Polineni, D.; Manion, M.; Rosenfeld, M.; Dell, S.D.; Chilvers, M.A.; Ferkol, T.W.; Zariwala, M.A.; Sagel, S.D.; et al. Diagnosis of Primary Ciliary Dyskinesia. An Official American Thoracic Society Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018, 197, e24–e39. [Google Scholar] [CrossRef] [PubMed]
- Hannah, W.B.; Seifert, B.A.; Truty, R.; Zariwala, M.A.; Ameel, K.; Zhao, Y.; Nykamp, K.; Gaston, B. The global prevalence and ethnic heterogeneity of primary ciliary dyskinesia gene variants: A genetic database analysis. Lancet Respir. Med. 2022, 10, 459–468. [Google Scholar] [CrossRef]
- De Jesús-Rojas, W.; Reyes-De Jesús, D.; Mosquera, R.A. Primary Ciliary Dyskinesia Diagnostic Challenges: Understanding the Clinical Phenotype of the Puerto Rican. Diagnostics 2021, 11, 281. [Google Scholar] [CrossRef] [PubMed]
- Daniels, M.L.; Leigh, M.W.; Davis, S.D.; Armstrong, M.C.; Carson, J.L.; Hazucha, M.; Dell, S.D.; Eriksson, M.; Collins, F.S.; Knowles, M.R.; et al. Founder mutation in RSPH4A identified in patients of Hispanic descent with primary ciliary dyskinesia. Hum. Mutat. 2013, 34, 1352–1356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Via, M.; Gignoux, C.R.; Roth, L.A.; Fejerman, L.; Galanter, J.; Choudhry, S.; Toro-Labrador, G.; Viera-Vera, J.; Oleksyk, T.K.; Beckman, K.; et al. History shaped the geographic distribution of genomic admixture on the island of Puerto Rico. PLoS ONE 2011, 6, e16513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Jesus Rojas, W.; Young, L.R. Hermansky-Pudlak Syndrome. Semin. Respir. Crit. Care Med. 2020, 41, 238–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Jesus-Rojas, W.; Reyes De Jesus, D.; Nieves, A.M.; Mosquera, R.A.; Martinez-Cruzado, J.C. Primary Ciliary Dyskinesia: Ancestral Haplotypes Analysis of the RSPH4A Founder Mutation in Puerto Rico. Cureus 2021, 13, e17673. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, R.; Carr, S.B.; Hogg, C. How insignificant are genetic variants of unknown significance in Primary Ciliary Dyskinesia? Eur. Respir. J. 2020, 56, 325. [Google Scholar] [CrossRef]
- Fretzayas, A.; Moustaki, M. Clinical spectrum of primary ciliary dyskinesia in childhood. World J. Clin. Pediatr. 2016, 5, 57–62. [Google Scholar] [CrossRef]
- Nykamp, K.; Anderson, M.; Powers, M.; Garcia, J.; Herrera, B.; Ho, Y.Y.; Kobayashi, Y.; Patil, N.; Thusberg, J.; Westbrook, M.; et al. Sherloc: A comprehensive refinement of the ACMG-AMP variant classification criteria. Genet. Med. 2017, 19, 1105–1117. [Google Scholar] [CrossRef] [Green Version]
- Karczewski, K.J.; Francioli, L.C.; Tiao, G.; Cummings, B.B.; Alföldi, J.; Wang, Q.; Collins, R.L.; Laricchia, K.M.; Ganna, A.; Birnbaum, D.P.; et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 2020, 581, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Goutaki, M.; Shoemark, A. Diagnosis of Primary Ciliary Dyskinesia. Clin. Chest Med. 2022, 43, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Ghandourah, H.; Dell, S.D. Severe disease due to CCDC40 gene variants and the perils of late diagnosis in primary ciliary dyskinesia. BMJ Case Rep. 2018, 2018, bcr-2018-224964. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Estrada, A.; Gravel, S.; Zakharia, F.; McCauley, J.L.; Byrnes, J.K.; Gignoux, C.R.; Ortiz-Tello, P.A.; Martínez, R.J.; Hedges, D.J.; Morris, R.W.; et al. Reconstructing the population genetic history of the Caribbean. PLoS Genet. 2013, 9, e1003925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Miyata, H.; Gao, Y.; Sha, Y.; Tang, S.; Xu, Z.; Whitfield, M.; Patrat, C.; Wu, H.; Dulioust, E.; et al. Bi-allelic DNAH8 Variants Lead to Multiple Morphological Abnormalities of the Sperm Flagella and Primary Male Infertility. Am. J. Hum. Genet. 2020, 107, 330–341. [Google Scholar] [CrossRef] [PubMed]



| Allelic Frequency | In Silico Evidence # | |||||
|---|---|---|---|---|---|---|
| PCD Genes | Pathogenic Variants | Puerto Rico | General * | Latino * | SIFT | PolyPhen-2 |
| RSPH4A | c.921+3_6delAAGT (Intronic) | 0.667 | 0.00003204 | 0.0001738 | ^ Aberrant splicing; absent or disrupted protein product. | |
| ZMYND10 | c.85T > C (p.Ser29Pro) | 0.077 | 0.00002682 | 0.0000586 | Deleterious | Probably damaging |
| CCNO | c.875_897del (p.Asp292Alafs*71) | 0.051 | 0.000003991 | 0.00 | ^ C-terminus disruption of the CCNO Protein. Likely to be disease-causative. | |
| DNAH1 | c.10468_10471del (p.Arg3490Glnfs*4) | 0.051 | 0.0000856 | 0.000198 | ^ Created a premature stop signal. Results in absence of disruptive protein. | |
| DNAH9 | c.308dup (p.Leu104Profs*45) | 0.051 | 0.0002849 | 0.0001783 | ^ Created a premature stop signal. Results in absence of disruptive protein. | |
| RSPH4A | c.1103T > G (p.Val368Gly) | 0.026 | 0.000003978 | 0.00 | Deleterious | Probably damaging |
| DNAH11 | c.3133C > T (p.Arg1045*) | 0.026 | 0.00001209 | 0.00 | ^ Created a premature stop signal. Results in absence of disruptive protein. | |
| DNAH5 | c.2431+5G > A (Intronic) | 0.026 | 0.00009564 | 0.00 | ^ Aberrant splicing | |
| DNAI1 | c.370C > T (p.Arg124Cys) | 0.026 | 0.000403 | 0.0003386 | Tolerated | Probably damaging |
| Allelic Frequency | In Silico Evidence *** | |||||
|---|---|---|---|---|---|---|
| PCD Genes | Variants of Unknown Significance (VUS) * | Puerto Rico | General # | Latino # | SIFT | PolyPhen-2 |
| DNAAF2 | c.58G > C (p.Val20Leu) | 0.053 | 0.00004059 | 0.0002017 | Tolerated | Probably damaging |
| DNAAF4 | c.186C > G (p.Ser62Arg) | 0.045 | 0.00001193 | 0.00002895 | Deleterious | Probably damaging |
| DNAH11 | c.8888C > A (p.Ser2963Tyr) | 0.038 | ** | ** | Deleterious | Probably damaging |
| DNAAF5 | c.969C > A (p.Asp323Glu) | 0.038 | 0.00004926 | 0.0003222 | Likely tolerated | Likely tolerated |
| DNAAF3 | c.1672G > T (p.Glu558*) | 0.038 | 0.00002413 | 0.00002899 | ^ Created a premature stop signal. Results in absence of disruptive protein. | |
| SPAG1 | c.2717A > G (p.Asp906Gly) | 0.023 | 0.00005179 | 0.0001737 | Deleterious | Benign |
| CCDC103 | c.82C > T (p.Arg28Trp) | 0.023 | 0.00005142 | 0.0000315 | Likely disruptive | Likely disruptive |
| CCDC40 | c.1109G > A (p.Arg370His) | 0.015 | 0.00009654 | 0.00006263 | Deleterious | Probably damaging |
| DNAI2 | c.1153G > A (p.Asp385Asn) | 0.015 | 0.00003889 | 0.0001693 | Deleterious | Probably damaging |
| CCNO | c.685G > A (p.Gly229Ser) | 0.015 | 0.0002135 | 0.00007137 | Deleterious | Benign |
| RSPH4A | c.902A > C (p.Gln301Pro) | 0.015 | 0.00001598 | 0.00008691 | Tolerated | Benign |
| CFAP298 | c.803C > T (p.Pro268Leu) | 0.015 | 0.00001768 | 0.0001129 | Likely tolerated | Likely tolerated |
| RSPH1 | c.824G > A (p.Arg275Gln) | 0.015 | 0.00002831 | 0.00 | Tolerated | Benign |
| RSPH1 | c.673C > T (p.Pro225Ser) | 0.015 | 0.0000283 | 0.0001411 | Tolerated | Benign |
| CCDC103 | c.146G > C (p.Gly49Ala) | 0.015 | 0.00002829 | 0.00002822 | Tolerated | Benign |
| DNAH5 | c.6409C > A (p.Leu2137Ile) | 0.015 | 0.00003537 | 0.0002259 | Deleterious | Probably damaging |
| DNAH5 | c.5413C > T (p.Arg1805Cys) | 0.015 | 0.0001203 | 0.0001129 | Deleterious | Probably damaging |
| DNAH5 | c.2577+5T > G (Intronic) | 0.015 | 0.00001993 | 0.00005791 | Not reportable: aberrant splicing | |
| DNAH5 | c.477C > T (Silent) | 0.015 | ** | ** | ^ Create or strengthen a splice site. | |
| DNAH5 | c.8398G > A (p.Val2800Ile) | 0.015 | 0.00002786 | 0.00005793 | Tolerated | Benign |
| DNAAF5 | c.1471-3C > T (Intronic) | 0.015 | 0.00000442 | 0.0000308 | ^ Aberrant splicing; absent or disrupted protein product. | |
| Other Genes | 0.525 | |||||
| Characteristics | Children (n = 7) | Adults (n = 10) | Total (n = 17) |
|---|---|---|---|
| Gender (female), n (%) | 3 (42) | 8 (80) | 11 (64) |
| Age, median+SD, (years) | 11+11.5 | 38+13.3 | 23+17.4 |
| Ethnicity, n (%), Hispanic, Puerto Rican | 7 (100) | 10 (100) | 17 (100) |
| Year-round wet cough, n (%) | 7 (100) | 10 (100) | 17 (100) |
| Year-round, daily nasal congestion, n (%) | 7 (100) | 10 (100) | 17 (100) |
| History of neonatal respiratory distress, n (%) | 4 (57) | 4 (40) | 8 (47) |
| Hearing loss, n (%) | 6 (85) | 6 (60) | 12 (71) |
| Bronchiectasis on HRCT, n (%) | 5 (71) | 10 (100) | 15 (88) |
| Chronic secretory otitis media, n (%) | 2 (29) | 5 (100) | 7 (41) |
| Laterality defects, n (%) | 0 (0) | 0 (0) | 0 (0) |
| Forced expiratory volume in 1 s (FEV1), median + SD of % of predicted | 67 + 25.2 | 44 + 8.8 | 49 + 21.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Jesús-Rojas, W.; Muñiz-Hernández, J.; Alvarado-Huerta, F.; Meléndez-Montañez, J.M.; Santos-López, A.J.; Mosquera, R.A. The Genetics of Primary Ciliary Dyskinesia in Puerto Rico. Diagnostics 2022, 12, 1127. https://doi.org/10.3390/diagnostics12051127
De Jesús-Rojas W, Muñiz-Hernández J, Alvarado-Huerta F, Meléndez-Montañez JM, Santos-López AJ, Mosquera RA. The Genetics of Primary Ciliary Dyskinesia in Puerto Rico. Diagnostics. 2022; 12(5):1127. https://doi.org/10.3390/diagnostics12051127
Chicago/Turabian StyleDe Jesús-Rojas, Wilfredo, José Muñiz-Hernández, Francisco Alvarado-Huerta, Jesús M. Meléndez-Montañez, Arnaldo J. Santos-López, and Ricardo A. Mosquera. 2022. "The Genetics of Primary Ciliary Dyskinesia in Puerto Rico" Diagnostics 12, no. 5: 1127. https://doi.org/10.3390/diagnostics12051127








