Salivary Neurotrophins Brain-Derived Neurotrophic Factor and Nerve Growth Factor Associated with Childhood Obesity: A Multiplex Magnetic Luminescence Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Anthropometric and Blood Pressure Measurements
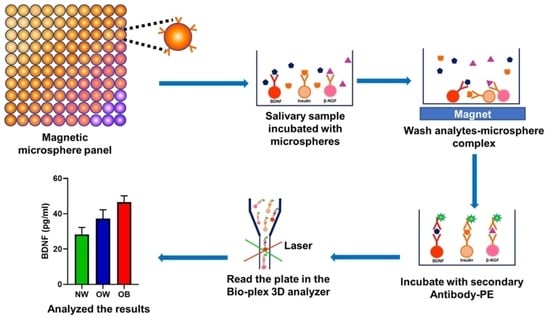
2.3. Measurement of Salivary BDNF, NGF, and Insulin by Multiplex Analysis
2.4. Statistical Analysis
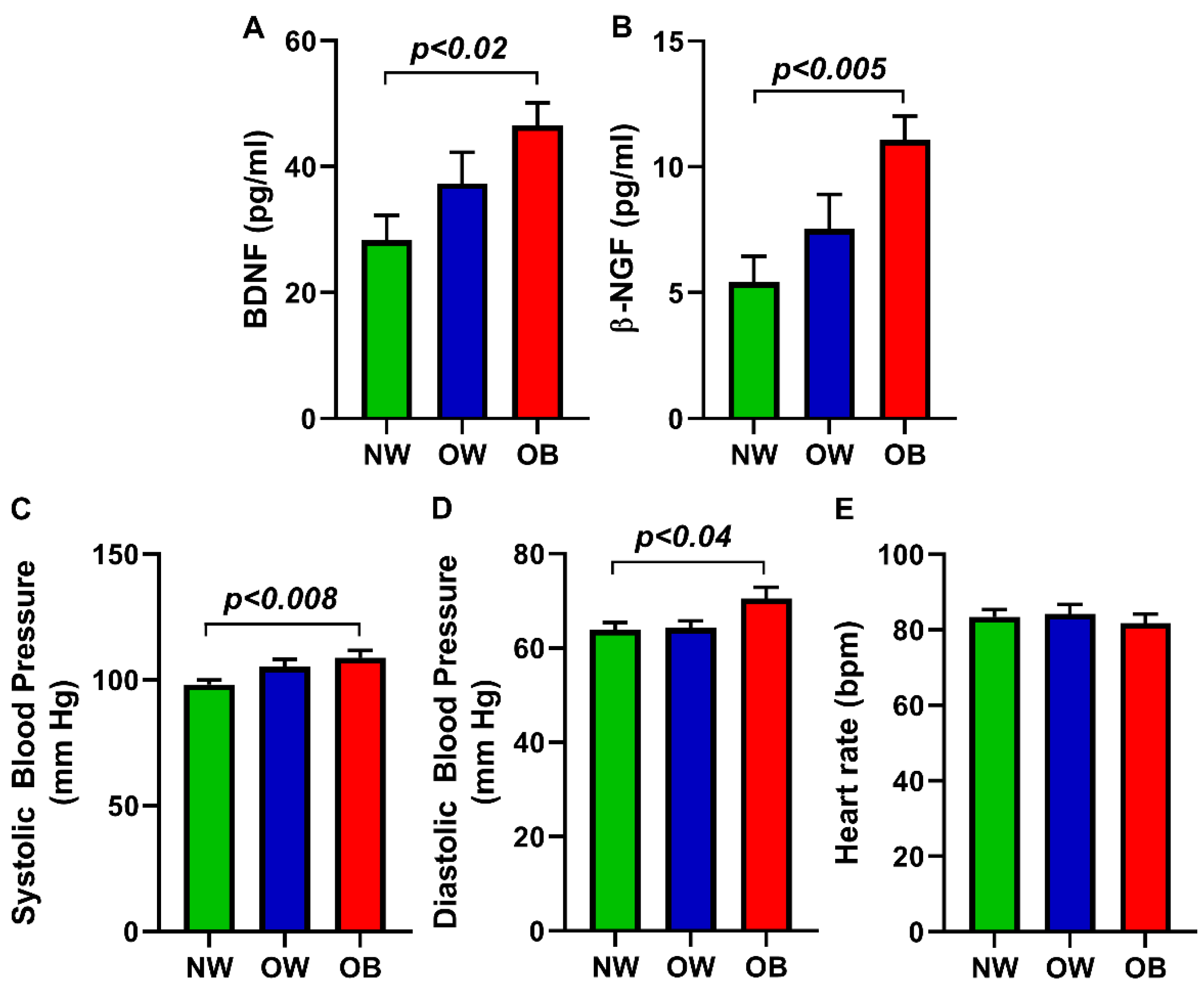
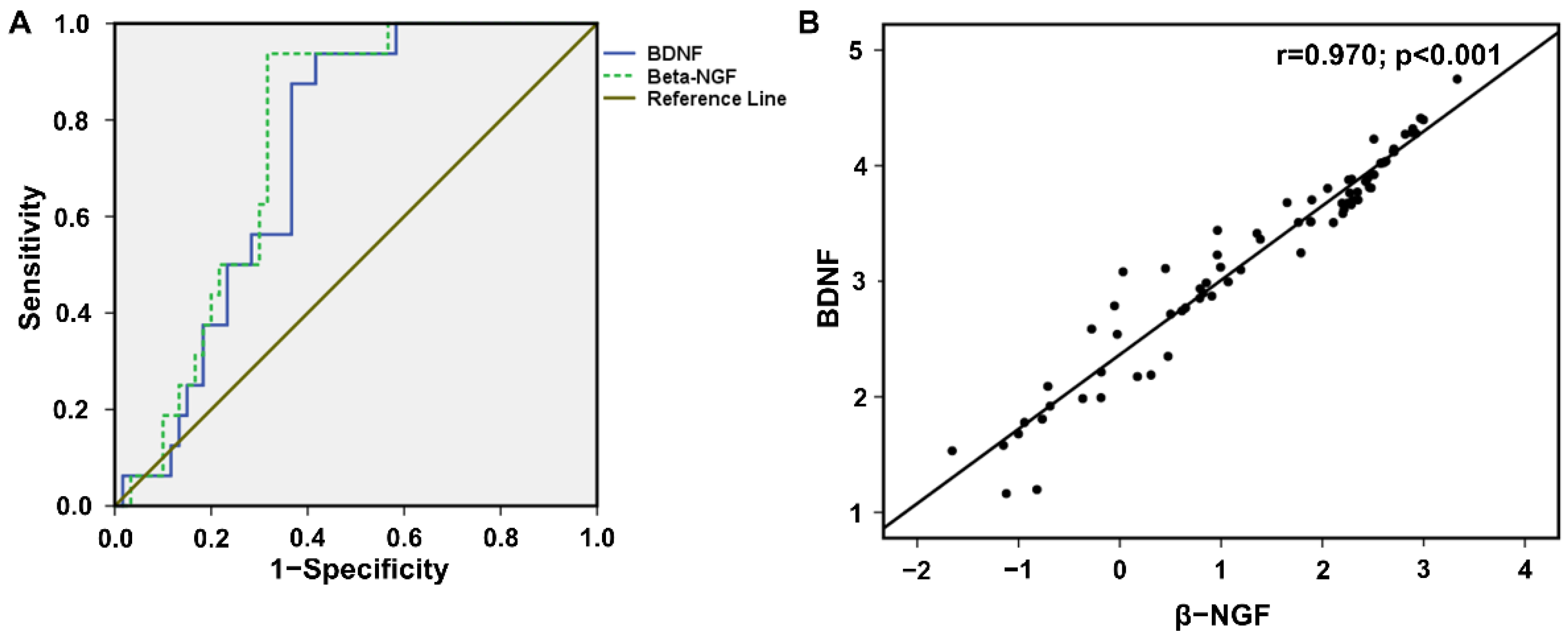
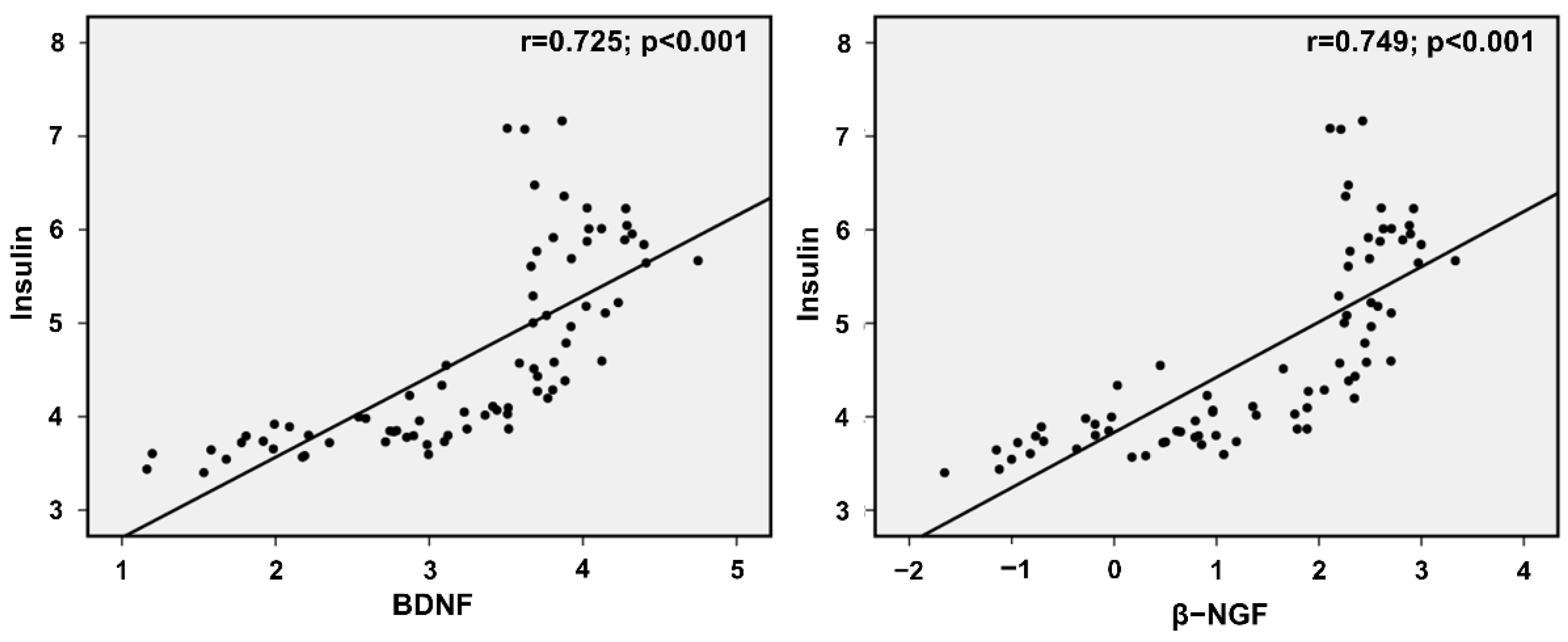
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hruby, A.; Hu, F.B. The Epidemiology of Obesity: A Big Picture. Pharmacoeconomics 2015, 33, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Loo, J.A.; Wong, D.T. Human body fluid proteome analysis. Proteomics 2006, 6, 6326–6353. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Preventio (CDC). Prevalence of Obesity among Adults and Youth: United States, 2015–2016. Available online: https://www.cdc.gov/nchs/products/databriefs/db288.htm#:~:text=The%20prevalence%20of%20obesity%20among%20U.S.%20youth%20was%2018.5%25%20in,5%20years)%20(13.9%25 (accessed on 15 April 2022).
- Mayeux, R. Biomarkers: Potential uses and limitations. NeuroRx 2004, 1, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Myette-Cote, E.; Baba, K.; Brar, R.; Little, J.P. Detection of Salivary Insulin Following Low versus High Carbohydrate Meals in Humans. Nutrients 2017, 9, 1204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pico, C.; Serra, F.; Rodriguez, A.M.; Keijer, J.; Palou, A. Biomarkers of Nutrition and Health: New Tools for New Approaches. Nutrients 2019, 11, 1092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selvaraju, V.; Babu, J.R.; Geetha, T. Multiplexed measurements of salivary fetuin-A, insulin, and adiponectin as potential non-invasive biomarkers in childhood obesity. Cytokine 2022, 153, 155843. [Google Scholar] [CrossRef]
- Srinivasan, M.; Meadows, M.L.; Maxwell, L. Assessment of Salivary Adipokines Resistin, Visfatin, and Ghrelin as Type 2 Diabetes Mellitus Biomarkers. Biochem. Res. Int. 2018, 2018, 7463796. [Google Scholar] [CrossRef] [Green Version]
- Yoshizawa, J.M.; Schafer, C.A.; Schafer, J.J.; Farrell, J.J.; Paster, B.J.; Wong, D.T. Salivary biomarkers: Toward future clinical and diagnostic utilities. Clin. Microbiol. Rev. 2013, 26, 781–791. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, A.; Yadav, S.S.; Dwivedi, P.; Lal, N.; Usman, K.; Khattri, S. Correlation of Serum and Salivary Cytokines Level With Clinical Parameters in Metabolic Syndrome With Periodontitis. J. Clin. Lab. Anal. 2016, 30, 649–655. [Google Scholar] [CrossRef] [Green Version]
- Ladgotra, A.; Verma, P.; Raj, S.S. Estimation of Salivary and Serum Biomarkers in Diabetic and Non Diabetic Patients—A Comparative Study. J. Clin. Diagn. Res. 2016, 10, ZC56–ZC61. [Google Scholar] [CrossRef]
- Rosas-Vargas, H.; Martinez-Ezquerro, J.D.; Bienvenu, T. Brain-derived neurotrophic factor, food intake regulation, and obesity. Arch. Med. Res. 2011, 42, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Bullo, M.; Peeraully, M.R.; Trayhurn, P.; Folch, J.; Salas-Salvado, J. Circulating nerve growth factor levels in relation to obesity and the metabolic syndrome in women. Eur. J. Endocrinol. 2007, 157, 303–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lebrun, B.; Bariohay, B.; Moyse, E.; Jean, A. Brain-derived neurotrophic factor (BDNF) and food intake regulation: A minireview. Auton. Neurosci. 2006, 126–127, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Rios, M. BDNF and the central control of feeding: Accidental bystander or essential player? Trends Neurosci. 2013, 36, 83–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, S.; Watanabe, K.; Hashimoto, E.; Saito, T. Low serum BDNF and food intake regulation: A possible new explanation of the pathophysiology of eating disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 33, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Krabbe, K.S.; Nielsen, A.R.; Krogh-Madsen, R.; Plomgaard, P.; Rasmussen, P.; Erikstrup, C.; Fischer, C.P.; Lindegaard, B.; Petersen, A.M.; Taudorf, S.; et al. Brain-derived neurotrophic factor (BDNF) and type 2 diabetes. Diabetologia 2007, 50, 431–438. [Google Scholar] [CrossRef]
- Lommatzsch, M.; Zingler, D.; Schuhbaeck, K.; Schloetcke, K.; Zingler, C.; Schuff-Werner, P.; Virchow, J.C. The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiol. Aging 2005, 26, 115–123. [Google Scholar] [CrossRef]
- Alomari, M.A.; Khabour, O.F.; Alawneh, K.; Alzoubi, K.H.; Maikano, A.B. The importance of physical fitness for the relationship of BDNF with obesity measures in young normal-weight adults. Heliyon 2020, 6, e03490. [Google Scholar] [CrossRef]
- Glud, M.; Christiansen, T.; Larsen, L.H.; Richelsen, B.; Bruun, J.M. Changes in Circulating BDNF in relation to Sex, Diet, and Exercise: A 12-Week Randomized Controlled Study in Overweight and Obese Participants. J. Obes. 2019, 2019, 4537274. [Google Scholar] [CrossRef]
- Katuri, R.B.; Gaur, G.S.; Sahoo, J.P.; Bobby, Z.; Shanmugavel, K. Association of Circulating Brain-Derived Neurotrophic Factor with Cognition among Adult Obese Population. J. Obes. Metab. Syndr. 2021, 30, 163–172. [Google Scholar] [CrossRef]
- Sandrini, L.; Di Minno, A.; Amadio, P.; Ieraci, A.; Tremoli, E.; Barbieri, S.S. Association between Obesity and Circulating Brain-Derived Neurotrophic Factor (BDNF) Levels: Systematic Review of Literature and Meta-Analysis. Int. J. Mol. Sci. 2018, 19, 2281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, I.T.; Wang, J.S.; Fu, C.P.; Lin, S.Y.; Sheu, W.H. Relationship between body weight and the increment in serum brain-derived neurotrophic factor after oral glucose challenge in men with obesity and metabolic syndrome: A prospective study. Medicine 2016, 95, e5260. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, H.N.; MacCallum, P.R. The obesity, metabolic syndrome, and type 2 diabetes mellitus pandemic: Part I. Increased cardiovascular disease risk and the importance of atherogenic dyslipidemia in persons with the metabolic syndrome and type 2 diabetes mellitus. J. Cardiometab. Syndr. 2009, 4, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Borelli, V.; Marchioli, A.; Di Taranto, R.; Romano, M.; Chiandussi, S.; Di Lenarda, R.; Biasotto, M.; Zabucchi, G. Neuropeptides in saliva of subjects with burning mouth syndrome: A pilot study. Oral Dis. 2010, 16, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.U.; Park, J.W.; Kho, H.S.; Chung, S.C.; Chung, J.W. Plasma and saliva levels of nerve growth factor and neuropeptides in chronic migraine patients. Oral Dis. 2011, 17, 187–193. [Google Scholar] [CrossRef]
- Saruta, J.; Sato, S.; Tsukinoki, K. The role of neurotrophins related to stress in saliva and salivary glands. Histol. Histopathol. 2010, 25, 1317–1330. [Google Scholar] [CrossRef] [PubMed]
- Selvaraju, V.; Babu, J.R.; Geetha, T. Association of salivary C-reactive protein with the obesity measures and markers in children. Diabetes Metab. Syndr. Obes. 2019, 12, 1239–1247. [Google Scholar] [CrossRef] [Green Version]
- Kuczmarski, R.J.; Ogden, C.L.; Guo, S.S.; Grummer-Strawn, L.M.; Flegal, K.M.; Mei, Z.; Wei, R.; Curtin, L.R.; Roche, A.F.; Johnson, C.L. 2000 CDC Growth Charts for the United States: Methods and Development; Vital Health Stat. 11; Department of Health and Human Services; Centers for Disease Control and Prevention; National Center for Health Statistics: Atlanta, GA, USA, 2002; pp. 1–190.
- Butte, N.F.; Garza, C.; de Onis, M. Evaluation of the feasibility of international growth standards for school-aged children and adolescents. J. Nutr. 2007, 137, 153–157. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.K.; Metzger, D.L.; Daymont, C.; Hadjiyannakis, S.; Rodd, C.J. LMS tables for waist-circumference and waist-height ratio Z-scores in children aged 5-19 y in NHANES III: Association with cardio-metabolic risks. Pediatr. Res. 2015, 78, 723–729. [Google Scholar] [CrossRef]
- Vachharajani, V.; Granger, D.N. Adipose tissue: A motor for the inflammation associated with obesity. IUBMB Life 2009, 61, 424–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Alameey, I.R.; Ahmed, H.H.; Abushady, M.M. Role of Lifestyle Intervention Program in Regulating Brain Derived Neurotrophic Factor in Obese Children with Metabolic Syndrome Components. Biomed. Pharmacol. J. 2019, 12, 1317–1328. [Google Scholar] [CrossRef]
- Roth, C.L.; Elfers, C.; Gebhardt, U.; Muller, H.L.; Reinehr, T. Brain-derived neurotrophic factor and its relation to leptin in obese children before and after weight loss. Metabolism 2013, 62, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Villalobos Gutierrez, P.T.; Delgado, G.G.; Renteria, C.T.; Orozco, E.R.; Coronado, O.G. Obesity and Overweight Influence BDNF Serum Levels in the Pediatric Population. Metab.—Clin. Exp. 2020, 104, 19–20. [Google Scholar] [CrossRef] [Green Version]
- Ha, J.; Cohen, J.I.; Tirsi, A.; Convit, A. Association of obesity-mediated insulin resistance and hypothalamic volumes: Possible sex differences. Dis. Markers 2013, 35, 249–259. [Google Scholar] [CrossRef] [Green Version]
- Noble, E.E.; Billington, C.J.; Kotz, C.M.; Wang, C. The lighter side of BDNF. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R1053–R1069. [Google Scholar] [CrossRef]
- Mazor-Aronovitch, K.; Lotan, D.; Modan-Moses, D.; Fradkin, A.; Pinhas-Hamiel, O. Blood pressure in obese and overweight children and adolescents. Isr. Med. Assoc. J. 2014, 16, 157–161. [Google Scholar]
- Schiel, R.; Beltschikow, W.; Kramer, G.; Stein, G. Overweight, obesity and elevated blood pressure in children and adolescents. Eur. J. Med. Res. 2006, 11, 97–101. [Google Scholar]
- Golden, E.; Emiliano, A.; Maudsley, S.; Windham, B.G.; Carlson, O.D.; Egan, J.M.; Driscoll, I.; Ferrucci, L.; Martin, B.; Mattson, M.P. Circulating brain-derived neurotrophic factor and indices of metabolic and cardiovascular health: Data from the Baltimore Longitudinal Study of Aging. PLoS ONE 2010, 5, e10099. [Google Scholar] [CrossRef]
- Han, J.C.; Liu, Q.R.; Jones, M.; Levinn, R.L.; Menzie, C.M.; Jefferson-George, K.S.; Adler-Wailes, D.C.; Sanford, E.L.; Lacbawan, F.L.; Uhl, G.R.; et al. Brain-derived neurotrophic factor and obesity in the WAGR syndrome. N. Engl. J. Med. 2008, 359, 918–927. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Shi, P.; Luo, C.Y.; Zhou, Y.F.; Yu, H.T.; Guo, C.Y.; Wu, F. Prevalence of hypertension in overweight and obese children from a large school-based population in Shanghai, China. BMC Public Health 2013, 13, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pazin, D.C.; Rosaneli, C.F.; Olandoski, M.; Oliveira, E.R.N.; Baena, C.P.; Figueredo, A.S.; Baraniuk, A.O.; Kaestner, T.; Guarita-Souza, L.C.; Faria-Neto, J.R. Waist Circumference is Associated with Blood Pressure in Children with Normal Body Mass Index: A Cross-Sectional Analysis of 3417 School Children. Arq. Bras. Cardiol. 2017, 109, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.A.; Chen, Y.J.; Tsao, Y.C.; Yeh, W.C.; Li, W.C.; Tzeng, I.S.; Chen, J.Y. Relationship between obesity indices and hypertension among middle-aged and elderly populations in Taiwan: A community-based, cross-sectional study. BMJ Open 2019, 9, e031660. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Tang, D.D.; Yin, E.G.; Wei, L.L.; Chen, P.; Deng, S.P.; Tu, L.L. Diagnostic Significance of Serum Levels of Nerve Growth Factor and Brain Derived Neurotrophic Factor in Diabetic Peripheral Neuropathy. Med. Sci. Monit. 2018, 24, 5943–5950. [Google Scholar] [CrossRef] [PubMed]
- Tuyet, L.T.; Nhung, B.T.; Dao, D.T.A.; Hanh, N.T.H.; Tuyen, L.D.; Binh, T.Q.; Thuc, V.T.M. The Brain-Derived Neurotrophic Factor Val66Met Polymorphism, Delivery Method, Birth Weight, and Night Sleep Duration as Determinants of Obesity in Vietnamese Children of Primary School Age. Child Obes. 2017, 13, 392–399. [Google Scholar] [CrossRef]
- Levinger, I.; Goodman, C.; Matthews, V.; Hare, D.L.; Jerums, G.; Garnham, A.; Selig, S. BDNF, metabolic risk factors, and resistance training in middle-aged individuals. Med. Sci. Sports Exerc. 2008, 40, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, N.H.; Tarp, J.; Andersen, L.B.; Gejl, A.K.; Huang, T.; Peijs, L.; Bugge, A. The association between serum brain-derived neurotrophic factor and a cluster of cardiovascular risk factors in adolescents: The CHAMPS-study DK. PLoS ONE 2017, 12, e0186384. [Google Scholar] [CrossRef] [Green Version]


| Parameters | Crude β Coefficient (SE) | Adjusted β Coefficient (SE) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OW | OB | OW | OB | |||||||||
| β | OR | p | β | OR | p | β | OR | p | β | OR | p | |
| BDNF | 0.020 (0.013) | 1.020 | 0.128 | 0.035 (0.014) | 1.036 | 0.011 | 0.013 (0.014) | 1.01 | 0.35 | 0.066 (0.022) | 1.068 | 0.002 |
| β-NGF | 0.066 (0.048) | 1.069 | 0.171 | 0.151 (0.053) | 1.163 | 0.004 | 0.041 (0.052) | 1.04 | 0.43 | 0.273 (0.086) | 1.314 | 0.001 |
| Insulin | 0.004 (0.002) | 1.004 | 0.041 | 0.006 (0.002) | 1.006 | 0.002 | 0.004 (0.002) | 1.004 | 0.075 | 0.008 (0.003) | 1.008 | 0.002 |
| Systolic blood pressure | 0.060 (0.027) | 1.062 | 0.027 | 0.081 (0.029) | 1.085 | 0.005 | 0.105 (0.039) | 1.111 | 0.007 | 0.113 (0.042) | 1.120 | 0.007 |
| Diastolic blood pressure | 0.005 (0.032) | 1.005 | 0.883 | 0.081 (0.035) | 1.084 | 0.021 | 0.004 (0.039) | 1.004 | 0.922 | 0.079 (0.043) | 1.082 | 0.063 |
| Heart rate | 0.006 (0.024) | 1.006 | 0.814 | −0.014 (0.027) | 0.986 | 0.601 | 0.007 (0.028) | 1.007 | 0.817 | −0.027 (0.032) | 0.973 | 0.405 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selvaraju, V.; Babu, J.R.; Geetha, T. Salivary Neurotrophins Brain-Derived Neurotrophic Factor and Nerve Growth Factor Associated with Childhood Obesity: A Multiplex Magnetic Luminescence Analysis. Diagnostics 2022, 12, 1130. https://doi.org/10.3390/diagnostics12051130
Selvaraju V, Babu JR, Geetha T. Salivary Neurotrophins Brain-Derived Neurotrophic Factor and Nerve Growth Factor Associated with Childhood Obesity: A Multiplex Magnetic Luminescence Analysis. Diagnostics. 2022; 12(5):1130. https://doi.org/10.3390/diagnostics12051130
Chicago/Turabian StyleSelvaraju, Vaithinathan, Jeganathan R. Babu, and Thangiah Geetha. 2022. "Salivary Neurotrophins Brain-Derived Neurotrophic Factor and Nerve Growth Factor Associated with Childhood Obesity: A Multiplex Magnetic Luminescence Analysis" Diagnostics 12, no. 5: 1130. https://doi.org/10.3390/diagnostics12051130
APA StyleSelvaraju, V., Babu, J. R., & Geetha, T. (2022). Salivary Neurotrophins Brain-Derived Neurotrophic Factor and Nerve Growth Factor Associated with Childhood Obesity: A Multiplex Magnetic Luminescence Analysis. Diagnostics, 12(5), 1130. https://doi.org/10.3390/diagnostics12051130