Clinical Evaluation of an Innovative Metal-Artifact-Reduction Algorithm in FD-CT Angiography in Cerebral Aneurysms Treated by Endovascular Coiling or Surgical Clipping
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Acquisition and Postprocessing
2.2.1. FD-CTA
2.2.2. DSA
2.2.3. Postprocessing
2.3. Data Evaluation
2.3.1. Image Quality
2.3.2. Aneurysm Occlusion
2.3.3. Coil Package Diameter (CPD)
2.4. Statistical Analysis
3. Results
3.1. Patients
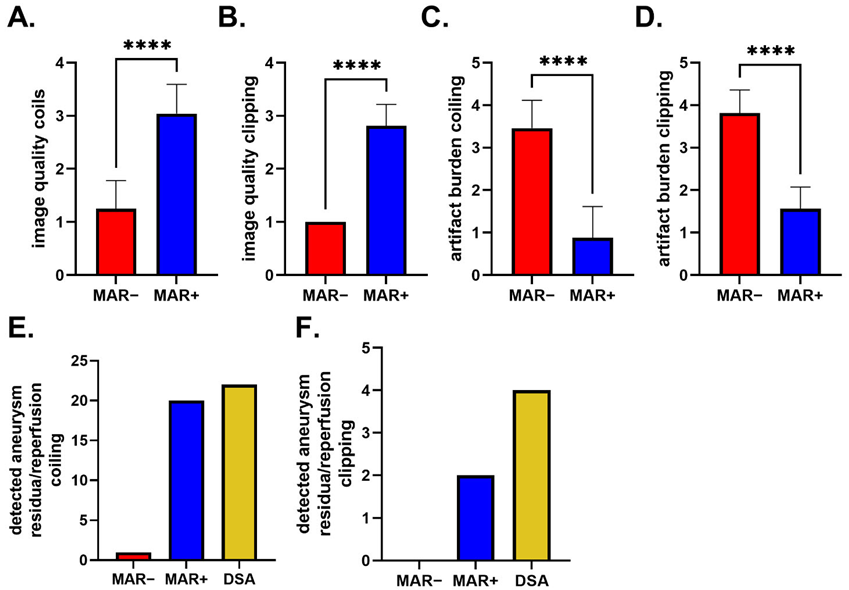
3.2. Image Quality
3.3. Aneurysm Occlusion
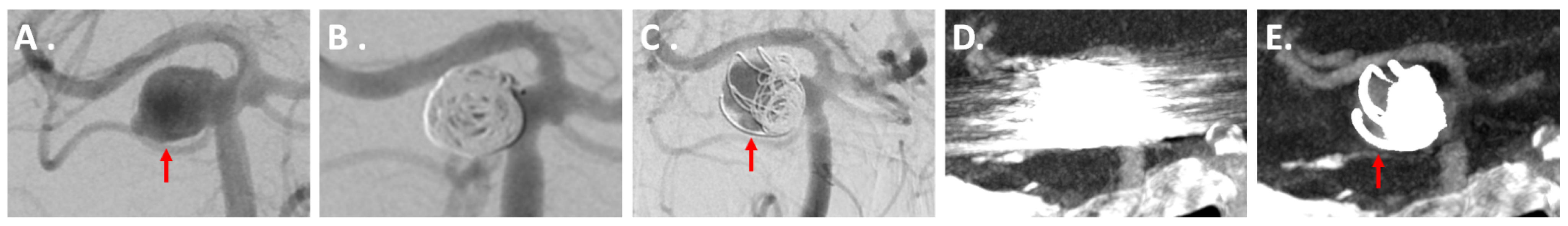
3.3.1. IAs Treated via Coiling
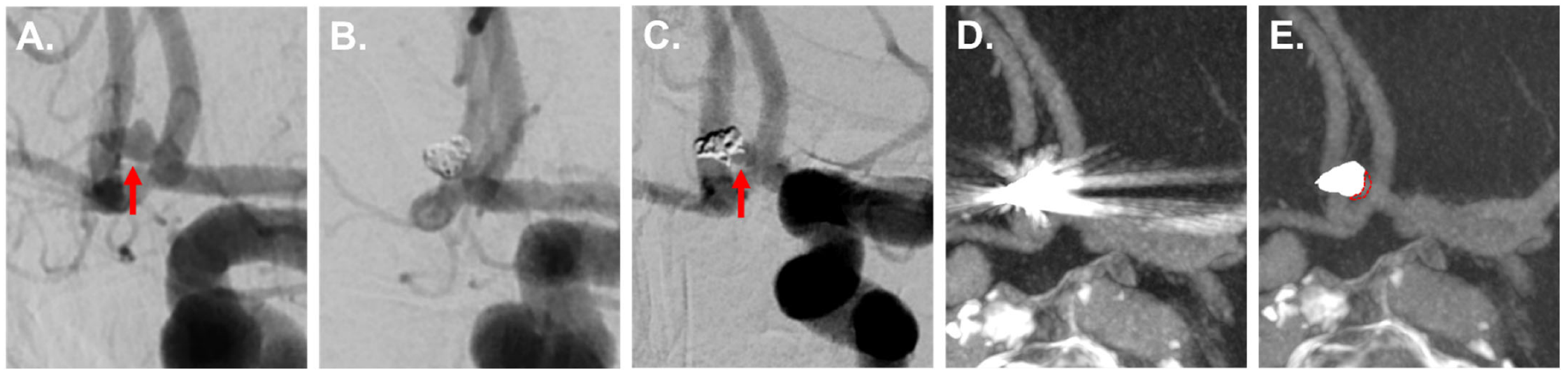
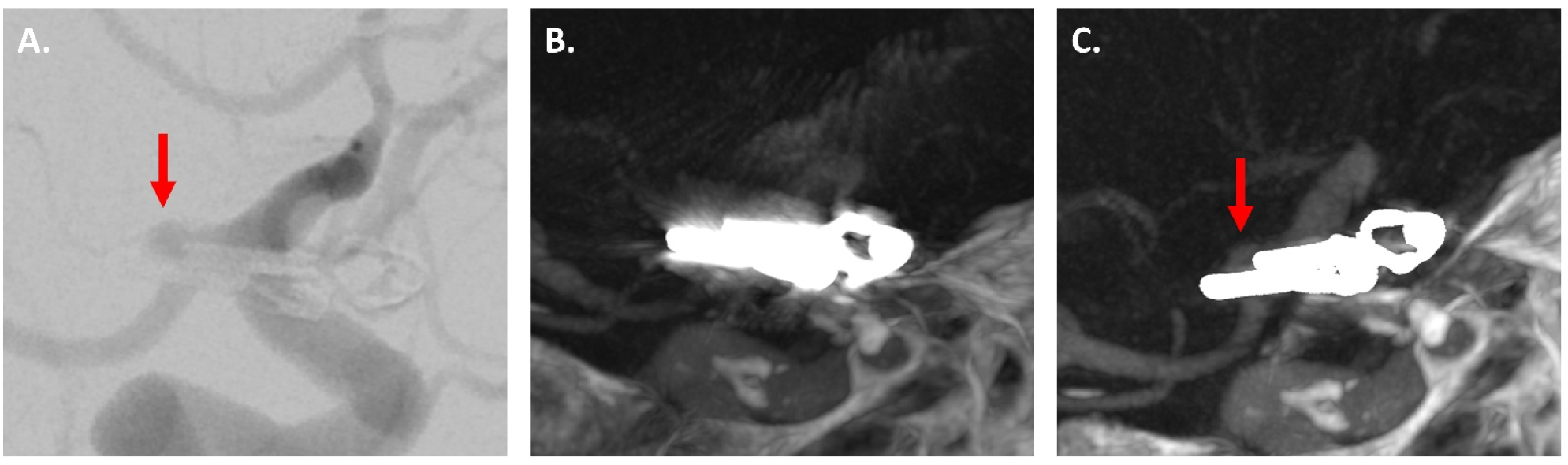
3.3.2. IAs Treated via Clipping
3.4. CPD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rinkel, G.J.; Djibuti, M.; Algra, A.; van Gijn, J. Prevalence and risk of rupture of intracranial aneurysms: A systematic review. Stroke 1998, 29, 251–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Study of Unruptured Intracranial Aneurysms Investigators. Unruptured intracranial aneurysms--risk of rupture and risks of surgical intervention. N. Engl. J. Med. 1998, 339, 1725–1733. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, R.L.; Schweizer, T.A. Spontaneous subarachnoid haemorrhage. Lancet 2017, 389, 655–666. [Google Scholar] [CrossRef]
- van Gijn, J.; Kerr, R.S.; Rinkel, G.J. Subarachnoid haemorrhage. Lancet 2007, 369, 306–318. [Google Scholar] [CrossRef]
- Steiner, T.; Juvela, S.; Unterberg, A.; Jung, C.; Forsting, M.; Rinkel, G. European Stroke Organization guidelines for the management of intracranial aneurysms and subarachnoid haemorrhage. Cerebrovasc. Dis. 2013, 35, 93–112. [Google Scholar] [CrossRef] [Green Version]
- Wagner, M.; Stenger, K. Unruptured intracranial aneurysms: Using evidence and outcomes to guide patient teaching. Crit. Care Nurs. Q. 2005, 28, 341–354. [Google Scholar] [CrossRef]
- Howard, B.M.; Hu, R.; Barrow, J.W.; Barrow, D.L. Comprehensive review of imaging of intracranial aneurysms and angiographically negative subarachnoid hemorrhage. Neurosurg. Focus 2019, 47, E20. [Google Scholar] [CrossRef] [Green Version]
- Doerfler, A.; Gölitz, P.; Engelhorn, T.; Kloska, S.; Struffert, T. Flat-Panel Computed Tomography (DYNA-CT) in Neuroradiology. From High-Resolution Imaging of Implants to One-Stop-Shopping for Acute Stroke. Clin. Neuroradiol. 2015, 25 (Suppl. 2), 291–297. [Google Scholar] [CrossRef]
- Turan, N.; Heider, R.A.; Roy, A.K.; Miller, B.A.; Mullins, M.E.; Barrow, D.L.; Grossberg, J.; Pradilla, G. Current Perspectives in Imaging Modalities for the Assessment of Unruptured Intracranial Aneurysms: A Comparative Analysis and Review. World Neurosurg. 2018, 113, 280–292. [Google Scholar] [CrossRef]
- Raymond, J.; Guilbert, F.; Weill, A.; Georganos, S.A.; Juravsky, L.; Lambert, A.; Lamoureux, J.; Chagnon, M.; Roy, D. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke 2003, 34, 1398–1403. [Google Scholar] [CrossRef] [Green Version]
- Tsutsumi, K.; Ueki, K.; Morita, A.; Usui, M.; Kirino, T. Risk of aneurysm recurrence in patients with clipped cerebral aneurysms: Results of long-term follow-up angiography. Stroke 2001, 32, 1191–1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prell, D.; Kyriakou, Y.; Struffert, T.; Dörfler, A.; Kalender, W.A. Metal artifact reduction for clipping and coiling in interventional C-arm CT. AJNR Am. J. Neuroradiol. 2010, 31, 634–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stidd, D.A.; Theessen, H.; Deng, Y.; Li, Y.; Scholz, B.; Rohkohl, C.; Jhaveri, M.D.; Moftakhar, R.; Chen, M.; Lopes, D.K. Evaluation of a metal artifacts reduction algorithm applied to postinterventional flat panel detector CT imaging. AJNR Am. J. Neuroradiol. 2014, 35, 2164–2169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mennecke, A.; Svergun, S.; Scholz, B.; Royalty, K.; Dörfler, A.; Struffert, T. Evaluation of a metal artifact reduction algorithm applied to post-interventional flat detector CT in comparison to pre-treatment CT in patients with acute subarachnoid haemorrhage. Eur. Radiol. 2017, 27, 88–96. [Google Scholar] [CrossRef]
- Pjontek, R.; Önenköprülü, B.; Scholz, B.; Kyriakou, Y.; Schubert, G.A.; Nikoubashman, O.; Othman, A.; Wiesmann, M.; Brockmann, M.A. Metal artifact reduction for flat panel detector intravenous CT angiography in patients with intracranial metallic implants after endovascular and surgical treatment. J. Neurointerv. Surg. 2016, 8, 824–829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Privalov, M.; Mohr, M.; Swartman, B.; Beisemann, N.; Keil, H.; Franke, J.; Grützner, P.A.; Vetter, S.Y. Evaluation of Software-Based Metal Artifact Reduction in Intraoperative 3D Imaging of the Spine Using a Mobile Cone Beam CT. J. Digit. Imaging 2020, 33, 1136–1143. [Google Scholar] [CrossRef]
- Troeltzsch, D.; Shnayien, S.; Heiland, M.; Kreutzer, K.; Raguse, J.D.; Hamm, B.; Niehues, S.M. Detectability of Head and Neck Cancer via New Computed Tomography Reconstruction Tools including Iterative Reconstruction and Metal Artifact Reduction. Diagnostics 2021, 11, 2154. [Google Scholar] [CrossRef]
- Mohammadinejad, P.; Khandelwal, A.; Inoue, A.; Takahashi, H.; Yalon, M.; Long, Z.; Halaweish, A.F.; Leng, S.; Yu, L.; Lee, Y.S.; et al. Utility of an automatic adaptive iterative metal artifact reduction AiMAR algorithm in improving CT imaging of patients with hip prostheses evaluated for suspected bladder malignancy. Abdom. Radiol. 2022, 1–10. [Google Scholar] [CrossRef]
- Brendlin, A.S.; Reinert, C.P.; Baumgartner, H.; Bongers, M.N.; Thomas, C.; Afat, S.; Springer, F.; Almansour, H. CT in Patients With External Fixation for Complex Lower Extremity Fractures: Impact of Iterative Metal Artifact Reduction Techniques on Metal Artifact Burden and Subjective Quality. AJR. Am. J. Roentgenol. 2022, 218, 300–309. [Google Scholar] [CrossRef]
- Yasuda, M.; Yoshikawa, K.; Kato, K.; Sai, S.; Sakiyama, K.; Kobayashi, Y.; Oosawa, M.; Sato, H.; Matsumoto, H.; Nakazawa, Y. Validation of a Metal Artifact Reduction Algorithm Using 1D Linear Interpolation for Cone Beam CT after Endovascular Coiling Therapy for Cerebral Aneurysms. Neuroradiol. J. 2014, 27, 742–754. [Google Scholar] [CrossRef] [Green Version]
- Meyer, E.; Raupach, R.; Lell, M.; Schmidt, B.; Kachelriess, M. Normalized metal artifact reduction (NMAR) in computed tomography. Med. Phys. 2010, 37, 5482–5493. [Google Scholar] [CrossRef] [PubMed]
- Meyer, E.; Raupach, R.; Lell, M.; Schmidt, B.; Kachelrieß, M. Frequency split metal artifact reduction (FSMAR) in computed tomography. Med. Phys. 2012, 39, 1904–1916. [Google Scholar] [CrossRef] [PubMed]
- Amelung, N.; Maus, V.; Behme, D.; Papageorgiou, I.E.; Leyhe, J.R.; Knauth, M.; Psychogios, M.N. Evaluation of an optimized metal artifact reduction algorithm for flat-detector angiography compared to DSA imaging in follow-up after neurovascular procedures. BMC Med. Imaging 2019, 19, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gölitz, P.; Struffert, T.; Knossalla, F.; Saake, M.; Ott, S.; Ganslandt, O.; Doerfler, A. Angiographic CT with intravenous contrast injection compared with conventional rotational angiography in the diagnostic work-up of cerebral aneurysms. AJNR Am. J. Neuroradiol. 2012, 33, 982–987. [Google Scholar] [CrossRef] [Green Version]
- Struffert, T.; Kloska, S.; Engelhorn, T.; Deuerling-Zheng, Y.; Ott, S.; Doelken, M.; Saake, M.; Köhrmann, M.; Doerfler, A. Optimized intravenous Flat Detector CT for non-invasive visualization of intracranial stents: First results. Eur. Radiol. 2011, 21, 411–418. [Google Scholar] [CrossRef]
- Mascitelli, J.R.; Moyle, H.; Oermann, E.K.; Polykarpou, M.F.; Patel, A.A.; Doshi, A.H.; Gologorsky, Y.; Bederson, J.B.; Patel, A.B. An update to the Raymond-Roy Occlusion Classification of intracranial aneurysms treated with coil embolization. J. Neurointerv. Surg. 2015, 7, 496–502. [Google Scholar] [CrossRef] [Green Version]
- Sindou, M.; Acevedo, J.C.; Turjman, F. Aneurysmal remnants after microsurgical clipping: Classification and results from a prospective angiographic study (in a consecutive series of 305 operated intracranial aneurysms). Acta Neurochir. 1998, 140, 1153–1159. [Google Scholar] [CrossRef]
- Katsura, M.; Sato, J.; Akahane, M.; Kunimatsu, A.; Abe, O. Current and Novel Techniques for Metal Artifact Reduction at CT: Practical Guide for Radiologists. Radiographics 2018, 38, 450–461. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Subhas, N.; Primak, A.N.; Nittka, M.; Liu, K. Metal artifact reduction: Standard and advanced magnetic resonance and computed tomography techniques. Radiol. Clin. N. Am. 2015, 53, 531–547. [Google Scholar] [CrossRef]
- Jungmann, P.M.; Agten, C.A.; Pfirrmann, C.W.; Sutter, R. Advances in MRI around metal. J. Magn. Reson. Imaging 2017, 46, 972–991. [Google Scholar] [CrossRef]
- Morawitz, J.; Martin, O.; Boos, J.; Sawicki, L.M.; Wingendorf, K.; Sedlmair, M.; Mamlins, E.; Antke, C.; Antoch, G.; Schaarschmidt, B.M. Impact of Different Metal Artifact Reduction Techniques on Attenuation Correction of Normal Organs in 18F-FDG-PET/CT. Diagnostics 2022, 12, 375. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, T.; Nishihori, M.; Izumi, T.; Uemura, T.; Sakai, T.; Nakano, M.; Kato, N.; Kanamori, F.; Tsukada, T.; Uda, K.; et al. Streak Metal Artifact Reduction Technique in Cone Beam Computed Tomography Images after Endovascular Neurosurgery. Neurol. Med. Chir. 2021, 61, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Wellenberg, R.H.H.; Hakvoort, E.T.; Slump, C.H.; Boomsma, M.F.; Maas, M.; Streekstra, G.J. Metal artifact reduction techniques in musculoskeletal CT-imaging. Eur. J. Radiol. 2018, 107, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Yang, M.; Jia, Y.; Zhang, L.; Sun, X.; Zhang, Y.; Nie, Z.; Wu, H.; Zhang, X.; Lei, Z.; et al. A Novel Subtraction Method to Reduce Metal Artifacts of Cerebral Aneurysm Embolism Coils. Clin. Neuroradiol. 2022, 30, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Nagayama, Y.; Tanoue, S.; Oda, S.; Sakabe, D.; Emoto, T.; Kidoh, M.; Uetani, H.; Sasao, A.; Nakaura, T.; Ikeda, O.; et al. Metal Artifact Reduction in Head CT Performed for Patients with Deep Brain Stimulation Devices: Effectiveness of a Single-Energy Metal Artifact Reduction Algorithm. AJNR Am. J. Neuroradiol. 2020, 41, 231–237. [Google Scholar] [CrossRef]
- Hakim, A.; Pastore-Wapp, M.; Vulcu, S.; Dobrocky, T.; Z’Graggen, W.J.; Wagner, F. Efficiency of Iterative Metal Artifact Reduction Algorithm (iMAR) Applied to Brain Volume Perfusion CT in the Follow-up of Patients after Coiling or Clipping of Ruptured Brain Aneurysms. Sci. Rep. 2019, 9, 19423. [Google Scholar] [CrossRef]
- Prell, D.; Kalender, W.A.; Kyriakou, Y. Development, implementation and evaluation of a dedicated metal artefact reduction method for interventional flat-detector CT. Br. J. Radiol. 2010, 83, 1052–1062. [Google Scholar] [CrossRef] [Green Version]
- Psychogios, M.N.; Scholz, B.; Rohkohl, C.; Kyriakou, Y.; Mohr, A.; Schramm, P.; Wachter, D.; Wasser, K.; Knauth, M. Impact of a new metal artefact reduction algorithm in the noninvasive follow-up of intracranial clips, coils, and stents with flat-panel angiographic CTA: Initial results. Neuroradiology 2013, 55, 813–818. [Google Scholar] [CrossRef]
- Enomoto, Y.; Yamauchi, K.; Asano, T.; Otani, K.; Iwama, T. Effect of metal artifact reduction software on image quality of C-arm cone-beam computed tomography during intracranial aneurysm treatment. Interv. Neuroradiol. 2018, 24, 303–308. [Google Scholar] [CrossRef]
- Murai, S.; Hiramatsu, M.; Takasugi, Y.; Takahashi, Y.; Kidani, N.; Nishihiro, S.; Shinji, Y.; Haruma, J.; Hishikawa, T.; Sugiu, K.; et al. Metal artifact reduction algorithm for image quality improvement of cone-beam CT images of medium or large cerebral aneurysms treated with stent-assisted coil embolization. Neuroradiology 2020, 62, 89–96. [Google Scholar] [CrossRef]
- Chintalapani, G.; Chinnadurai, P.; Srinivasan, V.; Chen, S.R.; Shaltoni, H.; Morsi, H.; Mawad, M.E.; Kan, P. Evaluation of C-arm CT metal artifact reduction algorithm during intra-aneurysmal coil embolization: Assessment of brain parenchyma, stents and flow-diverters. Eur. J. Radiol. 2016, 85, 1312–1321. [Google Scholar] [CrossRef] [PubMed]



| MRROC | |
|---|---|
| 0 | not assessable |
| 1 | no reperfusion |
| 2 | reperfusion at the aneurysm base |
| 3 | reperfusion at the center of the aneurysm |
| 4 | reperfusion along the aneurysm wall |
| SC | |
|---|---|
| 0 | not assessable |
| 1 | reperfusion of <50% of the aneurysm neck |
| 2 | reperfusion of >50% of the aneurysm neck |
| 3 | residual lobe of a multilobulated aneurysm sac |
| 4 | residual portion < 50% of the initial aneurysm sac |
| 5 | residual portion > 50% of the initial aneurysm sac |
| 6 | no residuum |
| Treatment Method | n | |
|---|---|---|
| coiling | 24 | |
| clipping | 16 | |
| aneurysm location | n | |
| coiling | clipping | |
| anterior communicating artery | 11 | 2 |
| middle cerebral artery | 4 | 9 |
| basilar artery | 4 | / |
| posterior communicating artery | 3 | 3 |
| internal carotid artery | 1 | 2 |
| posterior inferior cerebellar artery | 1 | / |
| IQ Regarding Diagnostic Value | |||
|---|---|---|---|
| iMAR− | iMAR+ | p-Value | |
| coiling | 1.25 ± 0.53 | 3.04 ± 0.55 | <0.0001 |
| clipping | 1.00 ± 0.0 | 2.81 ± 0.40 | <0.0001 |
| IQ regarding metal-artifacts burden | |||
| iMAR− | iMAR+ | p-Value | |
| coiling | 3.46 ± 0.66 | 0.88 ± 0.74 | <0.0001 |
| clipping | 3.81 ± 0.54 | 1.56 ± 0.51 | <0.0001 |
| MRROC 0 | MRROC 1 | MRROC 2 | MRROC 3 | MRROC 4 | Reperfusion Detection Rate | |
|---|---|---|---|---|---|---|
| iMAR− | 22 | 1 | 0 | 0 | 1 | 1 of 22 (4.5%) |
| iMAR+ | 0 | 4 | 7 | 1 | 12 | 20 of 22 (90.1%) |
| DSA | 0 | 2 | 6 | 0 | 16 | 22 (100%) |
| SC 0 | SC 1 | SC 2 | SC 3 | SC 4 | SC 5 | SC 6 | Residua Detection Rate | |
|---|---|---|---|---|---|---|---|---|
| iMAR− | 14 | 0 | 0 | 0 | 0 | 0 | 2 | 0 of 4 (0%) |
| iMAR+ | 0 | 1 | 1 | 0 | 0 | 0 | 14 | 2 of 4 (50%) |
| DSA | 0 | 3 | 1 | 0 | 0 | 0 | 12 | 4 (100%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eisenhut, F.; Schmidt, M.A.; Kalik, A.; Struffert, T.; Feulner, J.; Schlaffer, S.-M.; Manhart, M.; Doerfler, A.; Lang, S. Clinical Evaluation of an Innovative Metal-Artifact-Reduction Algorithm in FD-CT Angiography in Cerebral Aneurysms Treated by Endovascular Coiling or Surgical Clipping. Diagnostics 2022, 12, 1140. https://doi.org/10.3390/diagnostics12051140
Eisenhut F, Schmidt MA, Kalik A, Struffert T, Feulner J, Schlaffer S-M, Manhart M, Doerfler A, Lang S. Clinical Evaluation of an Innovative Metal-Artifact-Reduction Algorithm in FD-CT Angiography in Cerebral Aneurysms Treated by Endovascular Coiling or Surgical Clipping. Diagnostics. 2022; 12(5):1140. https://doi.org/10.3390/diagnostics12051140
Chicago/Turabian StyleEisenhut, Felix, Manuel Alexander Schmidt, Alexander Kalik, Tobias Struffert, Julian Feulner, Sven-Martin Schlaffer, Michael Manhart, Arnd Doerfler, and Stefan Lang. 2022. "Clinical Evaluation of an Innovative Metal-Artifact-Reduction Algorithm in FD-CT Angiography in Cerebral Aneurysms Treated by Endovascular Coiling or Surgical Clipping" Diagnostics 12, no. 5: 1140. https://doi.org/10.3390/diagnostics12051140






