Neutrophils and Asthma
Abstract
:1. Introduction
2. Definition of Neutrophilic Asthma
3. Association of Eosinophils and Neutrophils
4. Pathogenesis of Asthma
4.1. TSLP
4.2. IL-17
4.3. Bacterial Colonization and Microbiome in the Airway in Neutrophilic Asthma
4.4. Obesity
4.5. NETs and NETosis
4.6. NLRP3 Inflammasome and Asthma
4.7. S100A8/A9, HMGB-1, RAGE, and TLR4
4.8. House Dust Mites and Neutrophilic Asthma
4.9. Electric, Heat-Not-Burn Cigarettes, and Combustible Cigarettes
4.10. Air Pollution
4.11. Gastroesophageal Reflux Disease
5. Biomarkers of Neutrophilic Asthma
5.1. YKL40
5.2. Hydrogen Sulfide
5.3. Myeloperoxidase
5.4. Blood Neutrophil Count
5.5. MicroRNA
6. Airway Remodeling in Neutrophilic Asthma
6.1. Leukotriene B4
6.2. IL-8
6.3. TNF-α
6.4. IL-17A
6.5. Other Inflammatory Mediators and Cytokines
7. Treatment
7.1. Non-Pharmacological Approach
7.2. Nonspecific Treatment for Neutrophilic Inflammation
7.2.1. Macrolides
7.2.2. Phosphodiesterase Inhibitors
7.2.3. Anticholinergics
7.3. Specific Therapy for Neutrophils and Neutrophil Mediators
7.3.1. CXCR2 Antagonists
7.3.2. 5-Lipoxygenase-Activating Protein Inhibitors and 5-Lipoxygenase Inhibitors
7.4. Biologics
7.4.1. Targeting TSLP
7.4.2. Targeting TNF-α
7.4.3. Targeting IL-17
7.4.4. Targeting IL-23
7.4.5. Targeting IL-6
7.5. Other Potential Therapy for Neutrophilic Asthma
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Fukutomi, Y.; Nakamura, H.; Kobayashi, F.; Taniguchi, M.; Konno, S.; Nishimura, M.; Kawagishi, Y.; Watanabe, J.; Komase, Y.; Akamatsu, Y.; et al. Nationwide cross-sectional population-based study on the prevalences of asthma and asthma symptoms among Japanese adults. Int. Arch. Allergy Immunol. 2010, 153, 280–287. [Google Scholar] [CrossRef]
- Nakamura, Y.; Tamaoki, J.; Nagase, H.; Yamaguchi, M.; Horiguchi, T.; Hozawa, S.; Ichinose, M.; Iwanaga, T.; Kondo, R.; Nagata, M.; et al. Japanese guidelines for adult asthma 2020. Allergol. Int. 2020, 69, 519–548. [Google Scholar] [CrossRef]
- Lambrecht, B.N.; Hammad, H. The immunology of asthma. Nat. Immunol. 2015, 16, 45–56. [Google Scholar] [CrossRef]
- Robinson, D.; Humbert, M.; Buhl, R.; Cruz, A.A.; Inoue, H.; Korom, S.; Hanania, N.A.; Nair, P. Revisiting Type 2-high and Type 2-low airway inflammation in asthma: Current knowledge and therapeutic implications. Clin. Exp. Allergy 2017, 47, 161–175. [Google Scholar] [CrossRef]
- Hinks, T.S.C.; Hoyle, R.D.; Gelfand, E.W. CD8+ Tc2 cells: Underappreciated contributors to severe asthma. Eur. Respir. Rev. 2019, 28, 190092. [Google Scholar] [CrossRef] [Green Version]
- Bel, E.H. Clinical phenotypes of asthma. Curr. Opin. Pulm. Med. 2004, 10, 44–50. [Google Scholar] [CrossRef]
- Wenzel, S.E. Asthma phenotypes: The evolution from clinical to molecular approaches. Nat. Med. 2012, 18, 716–725. [Google Scholar] [CrossRef]
- Haldar, P.; Pavord, I.D.; Shaw, D.E.; Berry, M.A.; Thomas, M.; Brightling, C.E.; Wardlaw, A.J.; Green, R.H. Cluster analysis and clinical asthma phenotypes. Am. J. Respir. Crit. Care Med. 2008, 178, 218–224. [Google Scholar] [CrossRef] [Green Version]
- Grobal Initiative for Asthma. Global Strategy for Asthma Management and Prevention 2021. Available online: www.ginasthma.org (accessed on 21 September 2021).
- Moore, W.C.; Meyers, D.A.; Wenzel, S.E.; Teague, W.G.; Li, H.; Li, X.; D’Agostino, R., Jr.; Castro, M.; Curran-Everett, D.; Fitzpatrick, A.M.; et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am. J. Respir. Crit. Care Med. 2010, 181, 315–323. [Google Scholar] [CrossRef]
- Kuo, C.S.; Pavlidis, S.; Loza, M.; Baribaud, F.; Rowe, A.; Pandis, I.; Sousa, A.; Corfield, J.; Djukanovic, R.; Lutter, R.; et al. T-helper cell type 2 (Th2) and non-Th2 molecular phenotypes of asthma using sputum transcriptomics in U-BIOPRED. Eur. Respir. J. 2017, 49, 1602135. [Google Scholar] [CrossRef]
- Hogg, J.C. Neutrophil kinetics and lung injury. Physiol. Rev. 1987, 67, 1249–1295. [Google Scholar] [CrossRef]
- Nair, P.; Surette, M.G.; Virchow, J.C. Neutrophilic asthma: Misconception or misnomer? Lancet Respir. Med. 2021, 9, 441–443. [Google Scholar] [CrossRef]
- Nair, P.; Prabhavalkar, K.S. Neutrophilic Asthma and Potentially Related Target Therapies. Curr. Drug Targets 2020, 21, 374–388. [Google Scholar] [CrossRef]
- Nabe, T. Steroid-Resistant Asthma and Neutrophils. Biol. Pharm. Bull. 2020, 43, 31–35. [Google Scholar] [CrossRef] [Green Version]
- Crisford, H.; Sapey, E.; Rogers, G.B.; Taylor, S.; Nagakumar, P.; Lokwani, R.; Simpson, J.L. Neutrophils in asthma: The good, the bad and the bacteria. Thorax 2021, 76, 835–844. [Google Scholar] [CrossRef]
- Strickland, I.; Kisich, K.; Hauk, P.J.; Vottero, A.; Chrousos, G.P.; Klemm, D.J.; Leung, D.Y. High constitutive glucocorticoid receptor beta in human neutrophils enables them to reduce their spontaneous rate of cell death in response to corticosteroids. J. Exp. Med. 2001, 193, 585–593. [Google Scholar] [CrossRef] [Green Version]
- Saffar, A.S.; Ashdown, H.; Gounni, A.S. The molecular mechanisms of glucocorticoids-mediated neutrophil survival. Curr. Drug Targets 2011, 12, 556–562. [Google Scholar] [CrossRef] [Green Version]
- Shimoda, T.; Obase, Y.; Nagasaka, Y.; Nakano, H.; Kishikawa, R.; Iwanaga, T. Airway inflammation phenotype prediction in asthma patients using lung sound analysis with fractional exhaled nitric oxide. Allergol. Int. 2017, 66, 581–585. [Google Scholar] [CrossRef]
- Berry, M.; Morgan, A.; Shaw, D.E.; Parker, D.; Green, R.; Brightling, C.; Bradding, P.; Wardlaw, A.J.; Pavord, I.D. Pathological features and inhaled corticosteroid response of eosinophilic and non-eosinophilic asthma. Thorax 2007, 62, 1043–1049. [Google Scholar] [CrossRef] [Green Version]
- Toyran, M.; Bakirtas, A.; Dogruman-Al, F.; Turktas, I. Airway inflammation and bronchial hyperreactivity in steroid naive children with intermittent and mild persistent asthma. Pediatr. Pulmonol. 2014, 49, 140–147. [Google Scholar] [CrossRef]
- Louis, R.; Sele, J.; Henket, M.; Cataldo, D.; Bettiol, J.; Seiden, L.; Bartsch, P. Sputum eosinophil count in a large population of patients with mild to moderate steroid-naive asthma: Distribution and relationship with methacholine bronchial hyperresponsiveness. Allergy 2002, 57, 907–912. [Google Scholar] [CrossRef] [Green Version]
- Jatakanon, A.; Uasuf, C.; Maziak, W.; Lim, S.; Chung, K.F.; Barnes, P.J. Neutrophilic inflammation in severe persistent asthma. Am. J. Respir. Crit. Care Med. 1999, 160, 1532–1539. [Google Scholar] [CrossRef] [Green Version]
- Gibson, P.G.; Simpson, J.L.; Saltos, N. Heterogeneity of airway inflammation in persistent asthma: Evidence of neutrophilic inflammation and increased sputum interleukin-8. Chest 2001, 119, 1329–1336. [Google Scholar] [CrossRef] [Green Version]
- Moore, W.C.; Hastie, A.T.; Li, X.; Li, H.; Busse, W.W.; Jarjour, N.N.; Wenzel, S.E.; Peters, S.P.; Meyers, D.A.; Bleecker, E.R.; et al. Sputum neutrophil counts are associated with more severe asthma phenotypes using cluster analysis. J. Allergy Clin. Immunol. 2014, 133, 1557–1563.e5. [Google Scholar] [CrossRef] [Green Version]
- Mincham, K.T.; Bruno, N.; Singanayagam, A.; Snelgrove, R.J. Our evolving view of neutrophils in defining the pathology of chronic lung disease. Immunology 2021, 164, 701–721. [Google Scholar] [CrossRef]
- Alashkar Alhamwe, B.; Potaczek, D.P.; Miethe, S.; Alhamdan, F.; Hintz, L.; Magomedov, A.; Garn, H. Extracellular Vesicles and Asthma-More Than Just a Co-Existence. Int. J. Mol. Sci. 2021, 22, 4984. [Google Scholar] [CrossRef]
- Belda, J.; Leigh, R.; Parameswaran, K.; O’Byrne, P.M.; Sears, M.R.; Hargreave, F.E. Induced sputum cell counts in healthy adults. Am. J. Respir. Crit. Care Med. 2000, 161, 475–478. [Google Scholar] [CrossRef] [Green Version]
- Chung, K.F. Asthma phenotyping: A necessity for improved therapeutic precision and new targeted therapies. J. Intern. Med. 2016, 279, 192–204. [Google Scholar] [CrossRef]
- Schleich, F.; Brusselle, G.; Louis, R.; Vandenplas, O.; Michils, A.; Pilette, C.; Peche, R.; Manise, M.; Joos, G. Heterogeneity of phenotypes in severe asthmatics. The Belgian Severe Asthma Registry (BSAR). Respir. Med. 2014, 108, 1723–1732. [Google Scholar] [CrossRef] [Green Version]
- Grunwell, J.R.; Stephenson, S.T.; Tirouvanziam, R.; Brown, L.A.S.; Brown, M.R.; Fitzpatrick, A.M. Children with Neutrophil-Predominant Severe Asthma Have Proinflammatory Neutrophils With Enhanced Survival and Impaired Clearance. J. Allergy Clin. Immunol. Pract. 2019, 7, 516–525.e6. [Google Scholar] [CrossRef]
- Stemmy, E.J.; Benton, A.S.; Lerner, J.; Alcala, S.; Constant, S.L.; Freishtat, R.J. Extracellular cyclophilin levels associate with parameters of asthma in phenotypic clusters. J. Asthma 2011, 48, 986–993. [Google Scholar] [CrossRef] [Green Version]
- Kikuchi, S.; Nagata, M.; Kikuchi, I.; Hagiwara, K.; Kanazawa, M. Association between neutrophilic and eosinophilic inflammation in patients with severe persistent asthma. Int. Arch. Allergy Immunol. 2005, 137 (Suppl. S1), 7–11. [Google Scholar] [CrossRef]
- Jarjour, N.N.; Erzurum, S.C.; Bleecker, E.R.; Calhoun, W.J.; Castro, M.; Comhair, S.A.; Chung, K.F.; Curran-Everett, D.; Dweik, R.A.; Fain, S.B.; et al. Severe asthma: Lessons learned from the National Heart, Lung, and Blood Institute Severe Asthma Research Program. Am. J. Respir. Crit. Care Med. 2012, 185, 356–362. [Google Scholar] [CrossRef] [Green Version]
- Hastie, A.T.; Mauger, D.T.; Denlinger, L.C.; Coverstone, A.; Castro, M.; Erzurum, S.; Jarjour, N.; Levy, B.D.; Meyers, D.A.; Moore, W.C.; et al. Baseline sputum eosinophil + neutrophil subgroups’ clinical characteristics and longitudinal trajectories for NHLBI Severe Asthma Research Program (SARP 3) cohort. J. Allergy Clin. Immunol. 2020, 146, 222–226. [Google Scholar] [CrossRef] [Green Version]
- Marc-Malovrh, M.; Camlek, L.; Skrgat, S.; Kern, I.; Flezar, M.; Dezman, M.; Korosec, P. Elevated eosinophils, IL5 and IL8 in induced sputum in asthma patients with accelerated FEV1 decline. Respir. Med. 2020, 162, 105875. [Google Scholar] [CrossRef]
- Katz, B.; Sofonio, M.; Lyden, P.D.; Mitchell, M.D. Prostaglandin concentrations in cerebrospinal fluid of rabbits under normal and ischemic conditions. Stroke 1988, 19, 349–351. [Google Scholar] [CrossRef] [Green Version]
- Kikuchi, I.; Kikuchi, S.; Kobayashi, T.; Hagiwara, K.; Sakamoto, Y.; Kanazawa, M.; Nagata, M. Eosinophil trans-basement membrane migration induced by interleukin-8 and neutrophils. Am. J. Respir. Cell Mol. Biol. 2006, 34, 760–765. [Google Scholar] [CrossRef]
- Nishihara, F.; Nakagome, K.; Kobayashi, T.; Noguchi, T.; Araki, R.; Uchida, Y.; Soma, T.; Nagata, M. Trans-basement membrane migration of eosinophils induced by LPS-stimulated neutrophils from human peripheral blood in vitro. ERJ Open Res. 2015, 1, 00003-2015. [Google Scholar] [CrossRef] [Green Version]
- Kikuchi, I.; Kikuchi, S.; Kobayashi, T.; Takaku, Y.; Hagiwara, K.; Kanazawa, M.; Nagata, M. Theophylline attenuates the neutrophil-dependent augmentation of eosinophil trans-basement membrane migration. Int. Arch. Allergy Immunol. 2007, 143 (Suppl. S1), 44–49. [Google Scholar] [CrossRef]
- Lavinskiene, S.; Bajoriuniene, I.; Malakauskas, K.; Jeroch, J.; Sakalauskas, R. Sputum neutrophil count after bronchial allergen challenge is related to peripheral blood neutrophil chemotaxis in asthma patients. Inflamm. Res. 2014, 63, 951–959. [Google Scholar] [CrossRef]
- Gauvreau, G.M.; Sehmi, R.; Ambrose, C.S.; Griffiths, J.M. Thymic stromal lymphopoietin: Its role and potential as a therapeutic target in asthma. Expert Opin. Ther. Targets 2020, 24, 777–792. [Google Scholar] [CrossRef]
- Menzies-Gow, A.; Wechsler, M.E.; Brightling, C.E. Unmet need in severe, uncontrolled asthma: Can anti-TSLP therapy with tezepelumab provide a valuable new treatment option? Respir. Res. 2020, 21, 268. [Google Scholar] [CrossRef]
- Takai, T. TSLP expression: Cellular sources, triggers, and regulatory mechanisms. Allergol. Int. 2012, 61, 3–17. [Google Scholar] [CrossRef] [Green Version]
- Gour, N.; Lajoie, S. Epithelial Cell Regulation of Allergic Diseases. Curr. Allergy Asthma Rep. 2016, 16, 65. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, J.; Watanabe, N.; Kido, M.; Saga, K.; Akamatsu, T.; Nishio, A.; Chiba, T. Human TSLP and TLR3 ligands promote differentiation of Th17 cells with a central memory phenotype under Th2-polarizing conditions. Clin. Exp. Allergy 2009, 39, 89–100. [Google Scholar] [CrossRef]
- Gao, H.; Ying, S.; Dai, Y. Pathological Roles of Neutrophil-Mediated Inflammation in Asthma and Its Potential for Therapy as a Target. J. Immunol. Res. 2017, 2017, 3743048. [Google Scholar] [CrossRef] [Green Version]
- Moorehead, A.; Hanna, R.; Heroux, D.; Neighbour, H.; Sandford, A.; Gauvreau, G.M.; Sommer, D.D.; Denburg, J.A.; Akhabir, L. A thymic stromal lymphopoietin polymorphism may provide protection from asthma by altering gene expression. Clin. Exp. Allergy 2020, 50, 471–478. [Google Scholar] [CrossRef]
- Vazquez-Tello, A.; Semlali, A.; Chakir, J.; Martin, J.G.; Leung, D.Y.; Eidelman, D.H.; Hamid, Q. Induction of glucocorticoid receptor-beta expression in epithelial cells of asthmatic airways by T-helper type 17 cytokines. Clin. Exp. Allergy 2010, 40, 1312–1322. [Google Scholar] [CrossRef]
- Rahman, M.S.; Yamasaki, A.; Yang, J.; Shan, L.; Halayko, A.J.; Gounni, A.S. IL-17A induces eotaxin-1/CC chemokine ligand 11 expression in human airway smooth muscle cells: Role of MAPK (Erk1/2, JNK, and p38) pathways. J. Immunol. 2006, 177, 4064–4071. [Google Scholar] [CrossRef] [Green Version]
- Al-Ramli, W.; Prefontaine, D.; Chouiali, F.; Martin, J.G.; Olivenstein, R.; Lemiere, C.; Hamid, Q. T(H)17-associated cytokines (IL-17A and IL-17F) in severe asthma. J. Allergy Clin. Immunol. 2009, 123, 1185–1187. [Google Scholar] [CrossRef]
- Bullens, D.M.; Truyen, E.; Coteur, L.; Dilissen, E.; Hellings, P.W.; Dupont, L.J.; Ceuppens, J.L. IL-17 mRNA in sputum of asthmatic patients: Linking T cell driven inflammation and granulocytic influx? Respir. Res. 2006, 7, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oikawa, T.; Shimamura, M.; Ashino-Fuse, H.; Iwaguchi, T.; Ishizuka, M.; Takeuchi, T. Inhibition of angiogenesis by 15-deoxyspergualin. J. Antibiot. 1991, 44, 1033–1035. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Tan, Y.; Bajinka, O.; Wang, L.; Tang, Z. Th17/IL-17 Axis Regulated by Airway Microbes Get Involved in the Development of Asthma. Curr. Allergy Asthma Rep. 2020, 20, 11. [Google Scholar] [CrossRef] [PubMed]
- Noval Rivas, M.; Crother, T.R.; Arditi, M. The microbiome in asthma. Curr. Opin. Pediatr. 2016, 28, 764–771. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Liu, S.; Verma, M.; Zafar, I.; Good, J.T.; Rollins, D.; Groshong, S.; Gorska, M.M.; Martin, R.J.; Alam, R. Mechanism of TH2/TH17-predominant and neutrophilic TH2/TH17-low subtypes of asthma. J. Allergy Clin. Immunol. 2017, 139, 1548–1558.e4. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Aziz, M.I.; Brinkman, P.; Vijverberg, S.J.H.; Neerincx, A.H.; Riley, J.H.; Bates, S.; Hashimoto, S.; Kermani, N.Z.; Chung, K.F.; Djukanovic, R.; et al. Sputum microbiome profiles identify severe asthma phenotypes of relative stability at 12 to 18 months. J. Allergy Clin. Immunol. 2021, 147, 123–134. [Google Scholar] [CrossRef]
- Yang, X.; Li, H.; Ma, Q.; Zhang, Q.; Wang, C. Neutrophilic Asthma Is Associated with Increased Airway Bacterial Burden and Disordered Community Composition. Biomed. Res. Int. 2018, 2018, 9230234. [Google Scholar] [CrossRef] [Green Version]
- Green, B.J.; Wiriyachaiporn, S.; Grainge, C.; Rogers, G.B.; Kehagia, V.; Lau, L.; Carroll, M.P.; Bruce, K.D.; Howarth, P.H. Potentially pathogenic airway bacteria and neutrophilic inflammation in treatment resistant severe asthma. PLoS ONE 2014, 9, e100645. [Google Scholar] [CrossRef]
- Essilfie, A.T.; Simpson, J.L.; Horvat, J.C.; Preston, J.A.; Dunkley, M.L.; Foster, P.S.; Gibson, P.G.; Hansbro, P.M. Haemophilus influenzae infection drives IL-17-mediated neutrophilic allergic airways disease. PLoS Pathog. 2011, 7, e1002244. [Google Scholar] [CrossRef]
- Yang, B.; Liu, R.; Yang, T.; Jiang, X.; Zhang, L.; Wang, L.; Wang, Q.; Luo, Z.; Liu, E.; Fu, Z. Neonatal Streptococcus pneumoniae infection may aggravate adulthood allergic airways disease in association with IL-17A. PLoS ONE 2015, 10, e0123010. [Google Scholar] [CrossRef]
- Kozik, A.J.; Huang, Y.J. The microbiome in asthma: Role in pathogenesis, phenotype, and response to treatment. Ann. Allergy Asthma Immunol. 2019, 122, 270–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilliland, F.D.; Berhane, K.; Islam, T.; McConnell, R.; Gauderman, W.J.; Gilliland, S.S.; Avol, E.; Peters, J.M. Obesity and the risk of newly diagnosed asthma in school-age children. Am. J. Epidemiol. 2003, 158, 406–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mamun, A.A.; Lawlor, D.A.; Alati, R.; O’Callaghan, M.J.; Williams, G.M.; Najman, J.M. Increasing body mass index from age 5 to 14 years predicts asthma among adolescents: Evidence from a birth cohort study. Int. J. Obes. 2007, 31, 578–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weinmayr, G.; Forastiere, F.; Buchele, G.; Jaensch, A.; Strachan, D.P.; Nagel, G.; Group, I.P.T.S. Overweight/obesity and respiratory and allergic disease in children: International study of asthma and allergies in childhood (ISAAC) phase two. PLoS ONE 2014, 9, e113996. [Google Scholar] [CrossRef]
- Ho, W.C.; Lin, Y.S.; Caffrey, J.L.; Lin, M.H.; Hsu, H.T.; Myers, L.; Chen, P.C.; Lin, R.S. Higher body mass index may induce asthma among adolescents with pre-asthmatic symptoms: A prospective cohort study. BMC Public Health 2011, 11, 542. [Google Scholar] [CrossRef] [Green Version]
- Schatz, M.; Hsu, J.W.; Zeiger, R.S.; Chen, W.; Dorenbaum, A.; Chipps, B.E.; Haselkorn, T. Phenotypes determined by cluster analysis in severe or difficult-to-treat asthma. J. Allergy Clin. Immunol. 2014, 133, 1549–1556. [Google Scholar] [CrossRef]
- Holguin, F.; Bleecker, E.R.; Busse, W.W.; Calhoun, W.J.; Castro, M.; Erzurum, S.C.; Fitzpatrick, A.M.; Gaston, B.; Israel, E.; Jarjour, N.N.; et al. Obesity and asthma: An association modified by age of asthma onset. J. Allergy Clin. Immunol. 2011, 127, 1486–1493.e2. [Google Scholar] [CrossRef]
- Peters-Golden, M.; Swern, A.; Bird, S.S.; Hustad, C.M.; Grant, E.; Edelman, J.M. Influence of body mass index on the response to asthma controller agents. Eur. Respir. J. 2006, 27, 495–503. [Google Scholar] [CrossRef] [Green Version]
- Boulet, L.P.; Franssen, E. Influence of obesity on response to fluticasone with or without salmeterol in moderate asthma. Respir. Med. 2007, 101, 2240–2247. [Google Scholar] [CrossRef] [Green Version]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef]
- Peters, U.; Dixon, A.E.; Forno, E. Obesity and asthma. J. Allergy Clin. Immunol. 2018, 141, 1169–1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loffreda, S.; Yang, S.Q.; Lin, H.Z.; Karp, C.L.; Brengman, M.L.; Wang, D.J.; Klein, A.S.; Bulkley, G.B.; Bao, C.; Noble, P.W.; et al. Leptin regulates proinflammatory immune responses. FASEB J. 1998, 12, 57–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komakula, S.; Khatri, S.; Mermis, J.; Savill, S.; Haque, S.; Rojas, M.; Brown, L.; Teague, G.W.; Holguin, F. Body mass index is associated with reduced exhaled nitric oxide and higher exhaled 8-isoprostanes in asthmatics. Respir. Res. 2007, 8, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miethe, S.; Guarino, M.; Alhamdan, F.; Simon, H.U.; Renz, H.; Dufour, J.F.; Potaczek, D.P.; Garn, H. Effects of obesity on asthma: Immunometabolic links. Pol. Arch. Intern. Med. 2018, 128, 469–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.Y.; Lee, H.J.; Chang, Y.J.; Pichavant, M.; Shore, S.A.; Fitzgerald, K.A.; Iwakura, Y.; Israel, E.; Bolger, K.; Faul, J.; et al. Interleukin-17-producing innate lymphoid cells and the NLRP3 inflammasome facilitate obesity-associated airway hyperreactivity. Nat. Med. 2014, 20, 54–61. [Google Scholar] [CrossRef] [Green Version]
- Honda, K.; Wada, H.; Nakamura, M.; Nakamoto, K.; Inui, T.; Sada, M.; Koide, T.; Takata, S.; Yokoyama, T.; Saraya, T.; et al. IL-17A synergistically stimulates TNF-alpha-induced IL-8 production in human airway epithelial cells: A potential role in amplifying airway inflammation. Exp. Lung Res. 2016, 42, 205–216. [Google Scholar] [CrossRef]
- Prause, O.; Laan, M.; Lotvall, J.; Linden, A. Pharmacological modulation of interleukin-17-induced GCP-2-, GRO-alpha- and interleukin-8 release in human bronchial epithelial cells. Eur. J. Pharmacol. 2003, 462, 193–198. [Google Scholar] [CrossRef]
- Lee, K.H.; Lee, C.H.; Woo, J.; Jeong, J.; Jang, A.H.; Yoo, C.G. Cigarette Smoke Extract Enhances IL-17A-Induced IL-8 Production via Up-Regulation of IL-17R in Human Bronchial Epithelial Cells. Mol. Cells 2018, 41, 282–289. [Google Scholar] [CrossRef]
- Siew, L.Q.C.; Wu, S.Y.; Ying, S.; Corrigan, C.J. Cigarette smoking increases bronchial mucosal IL-17A expression in asthmatics, which acts in concert with environmental aeroallergens to engender neutrophilic inflammation. Clin. Exp. Allergy 2017, 47, 740–750. [Google Scholar] [CrossRef] [Green Version]
- Linden, A. Role of interleukin-17 and the neutrophil in asthma. Int. Arch. Allergy Immunol. 2001, 126, 179–184. [Google Scholar] [CrossRef]
- Al Heialy, S.; Gaudet, M.; Ramakrishnan, R.K.; Mogas, A.; Salameh, L.; Mahboub, B.; Hamid, Q. Contribution of IL-17 in Steroid Hyporesponsiveness in Obese Asthmatics Through Dysregulation of Glucocorticoid Receptors alpha and beta. Front. Immunol. 2020, 11, 1724. [Google Scholar] [CrossRef] [PubMed]
- Zijlstra, G.J.; Ten Hacken, N.H.; Hoffmann, R.F.; van Oosterhout, A.J.; Heijink, I.H. Interleukin-17A induces glucocorticoid insensitivity in human bronchial epithelial cells. Eur. Respir. J. 2012, 39, 439–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, Y.H.; Oh, E.Y.; Han, H.; Yang, M.; Park, H.J.; Park, K.H.; Lee, J.H.; Park, J.W. Insulin resistance mediates high-fat diet-induced pulmonary fibrosis and airway hyperresponsiveness through the TGF-beta1 pathway. Exp. Mol. Med. 2019, 51, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Cardet, J.C.; Ash, S.; Kusa, T.; Camargo, C.A., Jr.; Israel, E. Insulin resistance modifies the association between obesity and current asthma in adults. Eur. Respir. J. 2016, 48, 403–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez Jimenez, J.; Herrero Espinet, F.J.; Mengibar Garrido, J.M.; Roca Antonio, J.; Penos Mayor, S.; Penas Boira Mdel, M.; Roca Comas, A.; Ballester Martinez, A. Asthma and insulin resistance in obese children and adolescents. Pediatr. Allergy Immunol. 2014, 25, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Chung, S.I.; Park, H.J.; Oh, E.Y.; Kim, S.R.; Park, K.H.; Lee, J.H.; Park, J.W. Obesity-induced Vitamin D Deficiency Contributes to Lung Fibrosis and Airway Hyperresponsiveness. Am. J. Respir. Cell Mol. Biol. 2021, 64, 357–367. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Cheng, O.Z.; Palaniyar, N. NET balancing: A problem in inflammatory lung diseases. Front. Immunol. 2013, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.L.; Tangsombatvisit, S.; Rosenberg, J.M.; Mandelbaum, G.; Gillespie, E.C.; Gozani, O.P.; Alizadeh, A.A.; Utz, P.J. Specific post-translational histone modifications of neutrophil extracellular traps as immunogens and potential targets of lupus autoantibodies. Arthritis Res. Ther. 2012, 14, R25. [Google Scholar] [CrossRef] [Green Version]
- Doring, Y.; Manthey, H.D.; Drechsler, M.; Lievens, D.; Megens, R.T.; Soehnlein, O.; Busch, M.; Manca, M.; Koenen, R.R.; Pelisek, J.; et al. Auto-antigenic protein-DNA complexes stimulate plasmacytoid dendritic cells to promote atherosclerosis. Circulation 2012, 125, 1673–1683. [Google Scholar] [CrossRef] [Green Version]
- Krishnamoorthy, N.; Douda, D.N.; Bruggemann, T.R.; Ricklefs, I.; Duvall, M.G.; Abdulnour, R.E.; Martinod, K.; Tavares, L.; Wang, X.; Cernadas, M.; et al. Neutrophil cytoplasts induce TH17 differentiation and skew inflammation toward neutrophilia in severe asthma. Sci. Immunol. 2018, 3, eaao4747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, T.K.; Gibson, P.G.; Simpson, J.L.; McDonald, V.M.; Wood, L.G.; Baines, K.J. Neutrophil extracellular traps are associated with inflammation in chronic airway disease. Respirology 2016, 21, 467–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lachowicz-Scroggins, M.E.; Dunican, E.M.; Charbit, A.R.; Raymond, W.; Looney, M.R.; Peters, M.C.; Gordon, E.D.; Woodruff, P.G.; Lefrancais, E.; Phillips, B.R.; et al. Extracellular DNA, Neutrophil Extracellular Traps, and Inflammasome Activation in Severe Asthma. Am. J. Respir. Crit. Care Med. 2019, 199, 1076–1085. [Google Scholar] [CrossRef] [PubMed]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Y.; Hara, H.; Nunez, G. Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem. Sci. 2016, 41, 1012–1021. [Google Scholar] [CrossRef] [Green Version]
- Heppner, F.L.; Ransohoff, R.M.; Becher, B. Immune attack: The role of inflammation in Alzheimer disease. Nat. Rev. Neurosci. 2015, 16, 358–372. [Google Scholar] [CrossRef]
- Wang, S.; Yuan, Y.H.; Chen, N.H.; Wang, H.B. The mechanisms of NLRP3 inflammasome/pyroptosis activation and their role in Parkinson’s disease. Int. Immunopharmacol. 2019, 67, 458–464. [Google Scholar] [CrossRef]
- Strowig, T.; Henao-Mejia, J.; Elinav, E.; Flavell, R. Inflammasomes in health and disease. Nature 2012, 481, 278–286. [Google Scholar] [CrossRef]
- Lasithiotaki, I.; Giannarakis, I.; Tsitoura, E.; Samara, K.D.; Margaritopoulos, G.A.; Choulaki, C.; Vasarmidi, E.; Tzanakis, N.; Voloudaki, A.; Sidiropoulos, P.; et al. NLRP3 inflammasome expression in idiopathic pulmonary fibrosis and rheumatoid lung. Eur. Respir. J. 2016, 47, 910–918. [Google Scholar] [CrossRef] [Green Version]
- Jager, B.; Seeliger, B.; Terwolbeck, O.; Warnecke, G.; Welte, T.; Muller, M.; Bode, C.; Prasse, A. The NLRP3-Inflammasome-Caspase-1 Pathway Is Upregulated in Idiopathic Pulmonary Fibrosis and Acute Exacerbations and Is Inducible by Apoptotic A549 Cells. Front. Immunol. 2021, 12, 642855. [Google Scholar] [CrossRef]
- Huppertz, C.; Jager, B.; Wieczorek, G.; Engelhard, P.; Oliver, S.J.; Bauernfeind, F.G.; Littlewood-Evans, A.; Welte, T.; Hornung, V.; Prasse, A. The NLRP3 inflammasome pathway is activated in sarcoidosis and involved in granuloma formation. Eur. Respir. J. 2020, 55, 1900119. [Google Scholar] [CrossRef] [PubMed]
- Dostert, C.; Petrilli, V.; Van Bruggen, R.; Steele, C.; Mossman, B.T.; Tschopp, J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 2008, 320, 674–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinkerton, J.W.; Kim, R.Y.; Robertson, A.A.B.; Hirota, J.A.; Wood, L.G.; Knight, D.A.; Cooper, M.A.; O’Neill, L.A.J.; Horvat, J.C.; Hansbro, P.M. Inflammasomes in the lung. Mol. Immunol. 2017, 86, 44–55. [Google Scholar] [CrossRef]
- Lamkanfi, M. Emerging inflammasome effector mechanisms. Nat. Rev. Immunol. 2011, 11, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.Y.; Pinkerton, J.W.; Essilfie, A.T.; Robertson, A.A.B.; Baines, K.J.; Brown, A.C.; Mayall, J.R.; Ali, M.K.; Starkey, M.R.; Hansbro, N.G.; et al. Role for NLRP3 Inflammasome-mediated, IL-1beta-Dependent Responses in Severe, Steroid-Resistant Asthma. Am. J. Respir. Crit. Care Med. 2017, 196, 283–297. [Google Scholar] [CrossRef]
- Rossios, C.; Pavlidis, S.; Hoda, U.; Kuo, C.H.; Wiegman, C.; Russell, K.; Sun, K.; Loza, M.J.; Baribaud, F.; Durham, A.L.; et al. Sputum transcriptomics reveal upregulation of IL-1 receptor family members in patients with severe asthma. J. Allergy Clin. Immunol. 2018, 141, 560–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wood, L.G.; Li, Q.; Scott, H.A.; Rutting, S.; Berthon, B.S.; Gibson, P.G.; Hansbro, P.M.; Williams, E.; Horvat, J.; Simpson, J.L.; et al. Saturated fatty acids, obesity, and the nucleotide oligomerization domain-like receptor protein 3 (NLRP3) inflammasome in asthmatic patients. J. Allergy Clin. Immunol. 2019, 143, 305–315. [Google Scholar] [CrossRef] [Green Version]
- Ritter, M.; Straubinger, K.; Schmidt, S.; Busch, D.H.; Hagner, S.; Garn, H.; Prazeres da Costa, C.; Layland, L.E. Functional relevance of NLRP3 inflammasome-mediated interleukin (IL)-1beta during acute allergic airway inflammation. Clin. Exp. Immunol. 2014, 178, 212–223. [Google Scholar] [CrossRef]
- Li, H.; Willingham, S.B.; Ting, J.P.; Re, F. Cutting edge: Inflammasome activation by alum and alum’s adjuvant effect are mediated by NLRP3. J. Immunol. 2008, 181, 17–21. [Google Scholar] [CrossRef] [Green Version]
- Sebag, S.C.; Koval, O.M.; Paschke, J.D.; Winters, C.J.; Jaffer, O.A.; Dworski, R.; Sutterwala, F.S.; Anderson, M.E.; Grumbach, I.M. Mitochondrial CaMKII inhibition in airway epithelium protects against allergic asthma. JCI Insight 2017, 2, e88297. [Google Scholar] [CrossRef]
- Edgeworth, J.; Gorman, M.; Bennett, R.; Freemont, P.; Hogg, N. Identification of p8,14 as a highly abundant heterodimeric calcium binding protein complex of myeloid cells. J. Biol. Chem. 1991, 266, 7706–7713. [Google Scholar] [CrossRef]
- Bhardwaj, R.S.; Zotz, C.; Zwadlo-Klarwasser, G.; Roth, J.; Goebeler, M.; Mahnke, K.; Falk, M.; Meinardus-Hager, G.; Sorg, C. The calcium-binding proteins MRP8 and MRP14 form a membrane-associated heterodimer in a subset of monocytes/macrophages present in acute but absent in chronic inflammatory lesions. Eur. J. Immunol. 1992, 22, 1891–1897. [Google Scholar] [CrossRef] [PubMed]
- Hamada, N.; Maeyama, T.; Kawaguchi, T.; Yoshimi, M.; Fukumoto, J.; Yamada, M.; Yamada, S.; Kuwano, K.; Nakanishi, Y. The role of high mobility group box1 in pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2008, 39, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Lotze, M.T.; Tracey, K.J. High-mobility group box 1 protein (HMGB1): Nuclear weapon in the immune arsenal. Nat. Rev. Immunol. 2005, 5, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Ellerman, J.E.; Brown, C.K.; de Vera, M.; Zeh, H.J.; Billiar, T.; Rubartelli, A.; Lotze, M.T. Masquerader: High mobility group box-1 and cancer. Clin. Cancer Res. 2007, 13, 2836–2848. [Google Scholar] [CrossRef] [Green Version]
- Halayko, A.J.; Ghavami, S. S100A8/A9: A mediator of severe asthma pathogenesis and morbidity? Can. J. Physiol. Pharmacol. 2009, 87, 743–755. [Google Scholar] [CrossRef]
- Ogawa, E.N.; Ishizaka, A.; Tasaka, S.; Koh, H.; Ueno, H.; Amaya, F.; Ebina, M.; Yamada, S.; Funakoshi, Y.; Soejima, J.; et al. Contribution of high-mobility group box-1 to the development of ventilator-induced lung injury. Am. J. Respir. Crit. Care Med. 2006, 174, 400–407. [Google Scholar] [CrossRef]
- Liu, S.; Stolz, D.B.; Sappington, P.L.; Macias, C.A.; Killeen, M.E.; Tenhunen, J.J.; Delude, R.L.; Fink, M.P. HMGB1 is secreted by immunostimulated enterocytes and contributes to cytomix-induced hyperpermeability of Caco-2 monolayers. Am. J. Physiol. Cell Physiol. 2006, 290, C990–C999. [Google Scholar] [CrossRef] [Green Version]
- Buckley, S.T.; Ehrhardt, C. The receptor for advanced glycation end products (RAGE) and the lung. J. Biomed. Biotechnol. 2010, 2010, 917108. [Google Scholar] [CrossRef] [Green Version]
- Brett, J.; Schmidt, A.M.; Yan, S.D.; Zou, Y.S.; Weidman, E.; Pinsky, D.; Nowygrod, R.; Neeper, M.; Przysiecki, C.; Shaw, A.; et al. Survey of the distribution of a newly characterized receptor for advanced glycation end products in tissues. Am. J. Pathol. 1993, 143, 1699–1712. [Google Scholar]
- Akirav, E.M.; Preston-Hurlburt, P.; Garyu, J.; Henegariu, O.; Clynes, R.; Schmidt, A.M.; Herold, K.C. RAGE expression in human T cells: A link between environmental factors and adaptive immune responses. PLoS ONE 2012, 7, e34698. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Akirav, E.M.; Chen, W.; Henegariu, O.; Moser, B.; Desai, D.; Shen, J.M.; Webster, J.C.; Andrews, R.C.; Mjalli, A.M.; et al. RAGE ligation affects T cell activation and controls T cell differentiation. J. Immunol. 2008, 181, 4272–4278. [Google Scholar] [CrossRef] [Green Version]
- Manfredi, A.A.; Capobianco, A.; Esposito, A.; De Cobelli, F.; Canu, T.; Monno, A.; Raucci, A.; Sanvito, F.; Doglioni, C.; Nawroth, P.P.; et al. Maturing dendritic cells depend on RAGE for in vivo homing to lymph nodes. J. Immunol. 2008, 180, 2270–2275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moser, B.; Desai, D.D.; Downie, M.P.; Chen, Y.; Yan, S.F.; Herold, K.; Schmidt, A.M.; Clynes, R. Receptor for advanced glycation end products expression on T cells contributes to antigen-specific cellular expansion in vivo. J. Immunol. 2007, 179, 8051–8058. [Google Scholar] [CrossRef] [PubMed]
- Narumi, K.; Miyakawa, R.; Ueda, R.; Hashimoto, H.; Yamamoto, Y.; Yoshida, T.; Aoki, K. Proinflammatory Proteins S100A8/S100A9 Activate NK Cells via Interaction with RAGE. J. Immunol. 2015, 194, 5539–5548. [Google Scholar] [CrossRef] [Green Version]
- Perkins, T.N.; Oczypok, E.A.; Dutz, R.E.; Donnell, M.L.; Myerburg, M.M.; Oury, T.D. The receptor for advanced glycation end products is a critical mediator of type 2 cytokine signaling in the lungs. J. Allergy Clin. Immunol. 2019, 144, 796–808.e12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oczypok, E.A.; Milutinovic, P.S.; Alcorn, J.F.; Khare, A.; Crum, L.T.; Manni, M.L.; Epperly, M.W.; Pawluk, A.M.; Ray, A.; Oury, T.D. Pulmonary receptor for advanced glycation end-products promotes asthma pathogenesis through IL-33 and accumulation of group 2 innate lymphoid cells. J. Allergy Clin. Immunol. 2015, 136, 747–756.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hay, A.N.; Potter, A.; Kasmark, L.; Zhu, J.; Leeth, C.M. Rapid Communication: TLR4 expressed but with reduced functionality on equine B lymphocytes. J. Anim. Sci. 2019, 97, 2175–2180. [Google Scholar] [CrossRef]
- Zhao, S.; Sun, M.; Meng, H.; Ji, H.; Liu, Y.; Zhang, M.; Li, H.; Li, P.; Zhang, Y.; Zhang, Q. TLR4 expression correlated with PD-L1 expression indicates a poor prognosis in patients with peripheral T-cell lymphomas. Cancer Manag. Res. 2019, 11, 4743–4756. [Google Scholar] [CrossRef] [Green Version]
- Rossol, M.; Heine, H.; Meusch, U.; Quandt, D.; Klein, C.; Sweet, M.J.; Hauschildt, S. LPS-induced cytokine production in human monocytes and macrophages. Crit. Rev. Immunol. 2011, 31, 379–446. [Google Scholar] [CrossRef]
- Alves-Filho, J.C.; Tavares-Murta, B.M.; Barja-Fidalgo, C.; Benjamim, C.F.; Basile-Filho, A.; Arraes, S.M.; Cunha, F.Q. Neutrophil function in severe sepsis. Endocr. Metab. Immune Disord. Drug Targets 2006, 6, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.H.; Lee, Y.; Paik, M.J.; Yee, S.T. Inhibitions of HMGB1 and TLR4 alleviate DINP-induced asthma in mice. Toxicol. Res. 2019, 8, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Wang, L.; Shi, X.; Wang, N.; Zhao, L.; Wang, J.; Liu, C. HMGB1 was negatively regulated by HSF1 and mediated the TLR4/MyD88/NF-kappaB signal pathway in asthma. Life Sci. 2020, 241, 117120. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Asai, K.; Fujimoto, H.; Tanaka, H.; Kanazawa, H.; Hirata, K. Increased levels of HMGB-1 and endogenous secretory RAGE in induced sputum from asthmatic patients. Respir. Med. 2011, 105, 519–525. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Jiang, Y.Q.; Wang, W.X.; Zhou, Z.X.; Wang, Y.G.; Yang, L.; Ji, Y.L. HMGB1 and RAGE levels in induced sputum correlate with asthma severity and neutrophil percentage. Hum. Immunol. 2012, 73, 1171–1174. [Google Scholar] [CrossRef]
- Yang, H.; Hreggvidsdottir, H.S.; Palmblad, K.; Wang, H.; Ochani, M.; Li, J.; Lu, B.; Chavan, S.; Rosas-Ballina, M.; Al-Abed, Y.; et al. A critical cysteine is required for HMGB1 binding to Toll-like receptor 4 and activation of macrophage cytokine release. Proc. Natl. Acad. Sci. USA 2010, 107, 11942–11947. [Google Scholar] [CrossRef] [Green Version]
- Andersson, U.; Wang, H.; Palmblad, K.; Aveberger, A.C.; Bloom, O.; Erlandsson-Harris, H.; Janson, A.; Kokkola, R.; Zhang, M.; Yang, H.; et al. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J. Exp. Med. 2000, 192, 565–570. [Google Scholar] [CrossRef]
- Zhang, F.; Su, X.; Huang, G.; Xin, X.F.; Cao, E.H.; Shi, Y.; Song, Y. sRAGE alleviates neutrophilic asthma by blocking HMGB1/RAGE signalling in airway dendritic cells. Sci. Rep. 2017, 7, 14268. [Google Scholar] [CrossRef] [Green Version]
- Lyu, Y.; Zhao, H.; Ye, Y.; Liu, L.; Zhu, S.; Xia, Y.; Zou, F.; Cai, S. Decreased soluble RAGE in neutrophilic asthma is correlated with disease severity and RAGE G82S variants. Mol. Med. Rep. 2018, 17, 4131–4137. [Google Scholar] [CrossRef] [Green Version]
- Patregnani, J.T.; Brooks, B.A.; Chorvinsky, E.; Pillai, D.K. High BAL sRAGE is Associated with Low Serum Eosinophils and IgE in Children with Asthma. Children 2020, 7, 110. [Google Scholar] [CrossRef]
- Allam, V.; Faiz, A.; Lam, M.; Rathnayake, S.N.H.; Ditz, B.; Pouwels, S.D.; Brandsma, C.A.; Timens, W.; Hiemstra, P.S.; Tew, G.W.; et al. RAGE and TLR4 differentially regulate airway hyperresponsiveness: Implications for COPD. Allergy 2021, 76, 1123–1135. [Google Scholar] [CrossRef] [PubMed]
- Menson, K.E.; Mank, M.M.; Reed, L.F.; Walton, C.J.; Van Der Vliet, K.E.; Ather, J.L.; Chapman, D.G.; Smith, B.J.; Rincon, M.; Poynter, M.E. Therapeutic efficacy of IL-17A neutralization with corticosteroid treatment in a model of antigen-driven mixed-granulocytic asthma. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 319, L693–L709. [Google Scholar] [CrossRef] [PubMed]
- Mack, S.; Shin, J.; Ahn, Y.; Castaneda, A.R.; Peake, J.; Fulgar, C.; Zhang, J.; Cho, Y.H.; Pinkerton, K.E. Age-dependent pulmonary reactivity to house dust mite allergen: A model of adult-onset asthma? Am. J. Physiol. Lung Cell. Mol. Physiol. 2019, 316, L757–L763. [Google Scholar] [CrossRef] [PubMed]
- Sadamatsu, H.; Takahashi, K.; Tashiro, H.; Kurihara, Y.; Kato, G.; Uchida, M.; Noguchi, Y.; Kurata, K.; Omura, S.; Sunazuka, T.; et al. The Nonantibiotic Macrolide EM900 Attenuates House Dust Mite-Induced Airway Inflammation in a Mouse Model of Obesity-Associated Asthma. Int. Arch. Allergy Immunol. 2020, 181, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Huang, N.; Li, J.; Liu, X.; Xiong, Q.; Hu, C.; Chen, D.; Guan, L.; Chang, K.; Li, D.; et al. Cross-reactive antibodies against dust mite-derived enolase induce neutrophilic airway inflammation. Eur. Respir. J. 2021, 57, 1902375. [Google Scholar] [CrossRef] [PubMed]
- Kwak, D.W.; Park, D.; Kim, J.H. Leukotriene B4 receptors play critical roles in house dust mites-induced neutrophilic airway inflammation and IL-17 production. Biochem. Biophys. Res. Commun. 2021, 534, 646–652. [Google Scholar] [CrossRef]
- Mahmutovic Persson, I.; Menzel, M.; Ramu, S.; Cerps, S.; Akbarshahi, H.; Uller, L. IL-1beta mediates lung neutrophilia and IL-33 expression in a mouse model of viral-induced asthma exacerbation. Respir. Res. 2018, 19, 16. [Google Scholar] [CrossRef] [Green Version]
- Patel, D.F.; Peiro, T.; Bruno, N.; Vuononvirta, J.; Akthar, S.; Puttur, F.; Pyle, C.J.; Suveizdyte, K.; Walker, S.A.; Singanayagam, A.; et al. Neutrophils restrain allergic airway inflammation by limiting ILC2 function and monocyte-dendritic cell antigen presentation. Sci. Immunol. 2019, 4, eaax7006. [Google Scholar] [CrossRef]
- Chalmers, G.W.; Macleod, K.J.; Little, S.A.; Thomson, L.J.; McSharry, C.P.; Thomson, N.C. Influence of cigarette smoking on inhaled corticosteroid treatment in mild asthma. Thorax 2002, 57, 226–230. [Google Scholar] [CrossRef] [Green Version]
- Chaudhuri, R.; Livingston, E.; McMahon, A.D.; Lafferty, J.; Fraser, I.; Spears, M.; McSharry, C.P.; Thomson, N.C. Effects of smoking cessation on lung function and airway inflammation in smokers with asthma. Am. J. Respir. Crit. Care Med. 2006, 174, 127–133. [Google Scholar] [CrossRef]
- Shimoda, T.; Obase, Y.; Kishikawa, R.; Iwanaga, T. Influence of cigarette smoking on airway inflammation and inhaled corticosteroid treatment in patients with asthma. Allergy Asthma Proc. 2016, 37, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, A.; Hanaki, K.; Tomita, K.; Watanabe, M.; Hasagawa, Y.; Okazaki, R.; Igishi, T.; Horimukai, K.; Fukutani, K.; Sugimoto, Y.; et al. Environmental tobacco smoke and its effect on the symptoms and medication in children with asthma. Int. J. Environ. Health Res. 2009, 19, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Coakley, R.D.; Ghio, A.J.; Muhlebach, M.S.; Esther, C.R., Jr.; Alexis, N.E.; Tarran, R. Chronic E-Cigarette Use Increases Neutrophil Elastase and Matrix Metalloprotease Levels in the Lung. Am. J. Respir. Crit. Care Med. 2019, 200, 1392–1401. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, R.J.; Wills, T.A.; Tam, E.; Pagano, I.; Choi, K. E-cigarette use and asthma in a multiethnic sample of adolescents. Prev. Med. 2017, 105, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.; Bernat, D. E-Cigarette Use Among Florida Youth With and Without Asthma. Am. J. Prev. Med. 2016, 51, 446–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Q.; Jiang, D.; Minor, M.; Chu, H.W. Electronic cigarette liquid increases inflammation and virus infection in primary human airway epithelial cells. PLoS ONE 2014, 9, e108342. [Google Scholar] [CrossRef] [Green Version]
- Lerner, C.A.; Sundar, I.K.; Yao, H.; Gerloff, J.; Ossip, D.J.; McIntosh, S.; Robinson, R.; Rahman, I. Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS ONE 2015, 10, e0116732. [Google Scholar] [CrossRef]
- Kioi, Y.; Tabuchi, T. Electronic, heat-not-burn, and combustible cigarette use among chronic disease patients in Japan: A cross-sectional study. Tob. Induc. Dis. 2018, 16, 41. [Google Scholar] [CrossRef]
- Schaller, J.P.; Keller, D.; Poget, L.; Pratte, P.; Kaelin, E.; McHugh, D.; Cudazzo, G.; Smart, D.; Tricker, A.R.; Gautier, L.; et al. Evaluation of the Tobacco Heating System 2.2. Part 2: Chemical composition, genotoxicity, cytotoxicity, and physical properties of the aerosol. Regul. Toxicol. Pharmacol. 2016, 81 (Suppl. S2), S27–S47. [Google Scholar] [CrossRef] [Green Version]
- Smith, M.R.; Clark, B.; Ludicke, F.; Schaller, J.P.; Vanscheeuwijck, P.; Hoeng, J.; Peitsch, M.C. Evaluation of the Tobacco Heating System 2.2. Part 1: Description of the system and the scientific assessment program. Regul. Toxicol. Pharmacol. 2016, 81 (Suppl. S2), S17–S26. [Google Scholar] [CrossRef] [Green Version]
- Protano, C.; Manigrasso, M.; Cammalleri, V.; Biondi Zoccai, G.; Frati, G.; Avino, P.; Vitali, M. Impact of Electronic Alternatives to Tobacco Cigarettes on Indoor Air Particular Matter Levels. Int. J. Environ. Res. Public Health 2020, 17, 2947. [Google Scholar] [CrossRef] [PubMed]
- Belvisi, M.G.; Baker, K.; Malloy, N.; Raemdonck, K.; Dekkak, B.; Pieper, M.; Nials, A.T.; Birrell, M.A. Modelling the asthma phenotype: Impact of cigarette smoke exposure. Respir. Res. 2018, 19, 89. [Google Scholar] [CrossRef] [PubMed]
- Perret, J.L.; Bonevski, B.; McDonald, C.F.; Abramson, M.J. Smoking cessation strategies for patients with asthma: Improving patient outcomes. J. Asthma Allergy 2016, 9, 117–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masaki, K.; Tateno, H.; Kameyama, N.; Morino, E.; Watanabe, R.; Sekine, K.; Ono, T.; Satake, K.; Suzuki, S.; Nomura, A.; et al. Impact of a Novel Smartphone App (CureApp Smoking Cessation) on Nicotine Dependence: Prospective Single-Arm Interventional Pilot Study. JMIR Mhealth Uhealth 2019, 7, e12694. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, M.; Balmes, J.R. Outdoor air pollution and asthma. Lancet 2014, 383, 1581–1592. [Google Scholar] [CrossRef] [Green Version]
- Tiotiu, A.I.; Novakova, P.; Nedeva, D.; Chong-Neto, H.J.; Novakova, S.; Steiropoulos, P.; Kowal, K. Impact of Air Pollution on Asthma Outcomes. Int. J. Environ. Res. Public Health 2020, 17, 6212. [Google Scholar] [CrossRef] [PubMed]
- Gowers, A.M.; Cullinan, P.; Ayres, J.G.; Anderson, H.R.; Strachan, D.P.; Holgate, S.T.; Mills, I.C.; Maynard, R.L. Does outdoor air pollution induce new cases of asthma? Biological plausibility and evidence; a review. Respirology 2012, 17, 887–898. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.Z.; Ge, D.D.; Zhou, L.F.; Hou, L.Y.; Zhou, Y.; Li, Q.Y. Effects of particulate matter on allergic respiratory diseases. Chronic Dis. Transl. Med. 2018, 4, 95–102. [Google Scholar] [CrossRef]
- Wang, P.; Thevenot, P.; Saravia, J.; Ahlert, T.; Cormier, S.A. Radical-containing particles activate dendritic cells and enhance Th17 inflammation in a mouse model of asthma. Am. J. Respir. Cell Mol. Biol. 2011, 45, 977–983. [Google Scholar] [CrossRef] [Green Version]
- van Voorhis, M.; Knopp, S.; Julliard, W.; Fechner, J.H.; Zhang, X.; Schauer, J.J.; Mezrich, J.D. Exposure to atmospheric particulate matter enhances Th17 polarization through the aryl hydrocarbon receptor. PLoS ONE 2013, 8, e82545. [Google Scholar] [CrossRef] [Green Version]
- Brandt, E.B.; Kovacic, M.B.; Lee, G.B.; Gibson, A.M.; Acciani, T.H.; Le Cras, T.D.; Ryan, P.H.; Budelsky, A.L.; Khurana Hershey, G.K. Diesel exhaust particle induction of IL-17A contributes to severe asthma. J. Allergy Clin. Immunol. 2013, 132, 1194–1204.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, M.M.; Wu, R.; Plopper, C.G.; Hyde, D.M. IL-8 is one of the major chemokines produced by monkey airway epithelium after ozone-induced injury. Am. J. Physiol. 1998, 275, L524–L532. [Google Scholar] [CrossRef] [PubMed]
- Hiltermann, T.J.; Stolk, J.; Hiemstra, P.S.; Fokkens, P.H.; Rombout, P.J.; Sont, J.K.; Sterk, P.J.; Dijkman, J.H. Effect of ozone exposure on maximal airway narrowing in non-asthmatic and asthmatic subjects. Clin. Sci. 1995, 89, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Havemann, B.D.; Henderson, C.A.; El-Serag, H.B. The association between gastro-oesophageal reflux disease and asthma: A systematic review. Gut 2007, 56, 1654–1664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Teague, W.G.; Erzurum, S.; Fitzpatrick, A.; Mantri, S.; Dweik, R.A.; Bleecker, E.R.; Meyers, D.; Busse, W.W.; Calhoun, W.J.; et al. Determinants of exhaled breath condensate pH in a large population with asthma. Chest 2011, 139, 328–336. [Google Scholar] [CrossRef] [Green Version]
- Paoletti, G.; Melone, G.; Ferri, S.; Puggioni, F.; Baiardini, I.; Racca, F.; Canonica, G.W.; Heffler, E.; Malipiero, G. Gastroesophageal reflux and asthma: When, how, and why. Curr. Opin. Allergy Clin. Immunol. 2021, 21, 52–58. [Google Scholar] [CrossRef]
- Simpson, J.L.; Baines, K.J.; Ryan, N.; Gibson, P.G. Neutrophilic asthma is characterised by increased rhinosinusitis with sleep disturbance and GERD. Asian Pac. J. Allergy Immunol. 2014, 32, 66–74. [Google Scholar] [CrossRef]
- Icitovic, N.; Onyebeke, L.C.; Wallenstein, S.; Dasaro, C.R.; Harrison, D.; Jiang, J.; Kaplan, J.R.; Lucchini, R.G.; Luft, B.J.; Moline, J.M.; et al. The association between body mass index and gastroesophageal reflux disease in the World Trade Center Health Program General Responder Cohort. Am. J. Ind. Med. 2016, 59, 761–766. [Google Scholar] [CrossRef]
- Gupta, S.; Lodha, R.; Kabra, S.K. Asthma, GERD and Obesity: Triangle of Inflammation. Indian J. Pediatr. 2018, 85, 887–892. [Google Scholar] [CrossRef]
- Chupp, G.L.; Lee, C.G.; Jarjour, N.; Shim, Y.M.; Holm, C.T.; He, S.; Dziura, J.D.; Reed, J.; Coyle, A.J.; Kiener, P.; et al. A chitinase-like protein in the lung and circulation of patients with severe asthma. N. Engl. J. Med. 2007, 357, 2016–2027. [Google Scholar] [CrossRef]
- James, A.J.; Reinius, L.E.; Verhoek, M.; Gomes, A.; Kupczyk, M.; Hammar, U.; Ono, J.; Ohta, S.; Izuhara, K.; Bel, E.; et al. Increased YKL-40 and Chitotriosidase in Asthma and Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2016, 193, 131–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Zhang, X.; Liu, Y.; Zhang, L.; Zheng, J.; Wang, J.; Hansbro, P.M.; Wang, L.; Wang, G.; Hsu, A.C. Chitinase-like protein YKL-40 correlates with inflammatory phenotypes, anti-asthma responsiveness and future exacerbations. Respir. Res. 2019, 20, 95. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Saito, J.; Munakata, M.; Shibata, Y. Hydrogen sulfide as a novel biomarker of asthma and chronic obstructive pulmonary disease. Allergol. Int. 2021, 70, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Saito, J.; Zhang, Q.; Hui, C.; Macedo, P.; Gibeon, D.; Menzies-Gow, A.; Bhavsar, P.K.; Chung, K.F. Sputum hydrogen sulfide as a novel biomarker of obstructive neutrophilic asthma. J. Allergy Clin. Immunol. 2013, 131, 232–234.e3. [Google Scholar] [CrossRef]
- Suzuki, Y.; Saito, J.; Kikuchi, M.; Uematsu, M.; Fukuhara, A.; Sato, S.; Munakata, M. Sputum-to-serum hydrogen sulphide ratio as a novel biomarker of predicting future risks of asthma exacerbation. Clin. Exp. Allergy 2018, 48, 1155–1163. [Google Scholar] [CrossRef] [Green Version]
- Hinks, T.S.C.; Brown, T.; Lau, L.C.K.; Rupani, H.; Barber, C.; Elliott, S.; Ward, J.A.; Ono, J.; Ohta, S.; Izuhara, K.; et al. Multidimensional endotyping in patients with severe asthma reveals inflammatory heterogeneity in matrix metalloproteinases and chitinase 3-like protein 1. J. Allergy Clin. Immunol. 2016, 138, 61–75. [Google Scholar] [CrossRef] [Green Version]
- Backman, H.; Lindberg, A.; Hedman, L.; Stridsman, C.; Jansson, S.A.; Sandstrom, T.; Lundback, B.; Ronmark, E. FEV1 decline in relation to blood eosinophils and neutrophils in a population-based asthma cohort. World Allergy Organ. J. 2020, 13, 100110. [Google Scholar] [CrossRef]
- Backman, H.; Jansson, S.A.; Stridsman, C.; Muellerova, H.; Wurst, K.; Hedman, L.; Lindberg, A.; Ronmark, E. Chronic airway obstruction in a population-based adult asthma cohort: Prevalence, incidence and prognostic factors. Respir. Med. 2018, 138, 115–122. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, S.; Fang, X.; Qin, L.; Fan, Y.; Ding, D.; Liu, X.; Xie, M. Plasma miR-199a-5p is increased in neutrophilic phenotype asthma patients and negatively correlated with pulmonary function. PLoS ONE 2018, 13, e0193502. [Google Scholar] [CrossRef]
- Maes, T.; Cobos, F.A.; Schleich, F.; Sorbello, V.; Henket, M.; De Preter, K.; Bracke, K.R.; Conickx, G.; Mesnil, C.; Vandesompele, J.; et al. Asthma inflammatory phenotypes show differential microRNA expression in sputum. J. Allergy Clin. Immunol. 2016, 137, 1433–1446. [Google Scholar] [CrossRef] [Green Version]
- Jafari-Nakhjavani, M.R.; Ghorbanihaghjo, A.; Bagherzadeh-Nobari, B.; Malek-Mahdavi, A.; Rashtchizadeh, N. Serum YKL-40 levels and disease characteristics in patients with rheumatoid arthritis. Casp. J. Intern. Med. 2019, 10, 92–97. [Google Scholar] [CrossRef]
- Malmestrom, C.; Axelsson, M.; Lycke, J.; Zetterberg, H.; Blennow, K.; Olsson, B. CSF levels of YKL-40 are increased in MS and replaces with immunosuppressive treatment. J. Neuroimmunol. 2014, 269, 87–89. [Google Scholar] [CrossRef] [PubMed]
- Przysucha, N.; Gorska, K.; Krenke, R. Chitinases and Chitinase-Like Proteins in Obstructive Lung Diseases—Current Concepts and Potential Applications. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 885–899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, X.; Wang, D.; Liu, S.; Ma, Y.; Li, Z.; Tian, P.; Fan, H. The YKL-40 protein is a potential biomarker for COPD: A meta-analysis and systematic review. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Olsson, B.; Lautner, R.; Andreasson, U.; Ohrfelt, A.; Portelius, E.; Bjerke, M.; Holtta, M.; Rosen, C.; Olsson, C.; Strobel, G.; et al. CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: A systematic review and meta-analysis. Lancet Neurol. 2016, 15, 673–684. [Google Scholar] [CrossRef]
- Harrison, L.I.; Schuppan, D.; Rohlfing, S.R.; Hansen, A.R.; Hansen, C.S.; Funk, M.L.; Collins, S.H.; Ober, R.E. Determination of flumequine and a hydroxy metabolite in biological fluids by high-pressure liquid chromatographic, fluorometric, and microbiological methods. Antimicrob. Agents Chemother. 1984, 25, 301–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harnan, S.E.; Essat, M.; Gomersall, T.; Tappenden, P.; Pavord, I.; Everard, M.; Lawson, R. Exhaled nitric oxide in the diagnosis of asthma in adults: A systematic review. Clin. Exp. Allergy 2017, 47, 410–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Yao, X.; Yu, R.; Bai, J.; Sun, Y.; Huang, M.; Adcock, I.M.; Barnes, P.J. Exhaled carbon monoxide in asthmatics: A meta-analysis. Respir. Res. 2010, 11, 50. [Google Scholar] [CrossRef]
- Gao, J.; Iwamoto, H.; Koskela, J.; Alenius, H.; Hattori, N.; Kohno, N.; Laitinen, T.; Mazur, W.; Pulkkinen, V. Characterization of sputum biomarkers for asthma-COPD overlap syndrome. Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 2457–2465. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.Y.; Simpson, J.L.; Powell, H.; Yang, I.A.; Upham, J.W.; Reynolds, P.N.; Hodge, S.; James, A.L.; Jenkins, C.; Peters, M.J.; et al. Full blood count parameters for the detection of asthma inflammatory phenotypes. Clin. Exp. Allergy 2014, 44, 1137–1145. [Google Scholar] [CrossRef] [Green Version]
- Hastie, A.T.; Moore, W.C.; Li, H.; Rector, B.M.; Ortega, V.E.; Pascual, R.M.; Peters, S.P.; Meyers, D.A.; Bleecker, E.R.; National Heart, L.; et al. Biomarker surrogates do not accurately predict sputum eosinophil and neutrophil percentages in asthmatic subjects. J. Allergy Clin. Immunol. 2013, 132, 72–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartjes, F.J.; Vonk, J.M.; Faiz, A.; Hiemstra, P.S.; Lapperre, T.S.; Kerstjens, H.A.M.; Postma, D.S.; van den Berge, M.; Groningen and Leiden Universities Corticosteroids in Obstructive Lung Disease (GLUCOLD) Study Group. Predictive value of eosinophils and neutrophils on clinical effects of ICS in COPD. Respirology 2018, 23, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Panganiban, R.P.; Pinkerton, M.H.; Maru, S.Y.; Jefferson, S.J.; Roff, A.N.; Ishmael, F.T. Differential microRNA epression in asthma and the role of miR-1248 in regulation of IL-5. Am. J. Clin. Exp. Immunol. 2012, 1, 154–165. [Google Scholar]
- Milger, K.; Gotschke, J.; Krause, L.; Nathan, P.; Alessandrini, F.; Tufman, A.; Fischer, R.; Bartel, S.; Theis, F.J.; Behr, J.; et al. Identification of a plasma miRNA biomarker signature for allergic asthma: A translational approach. Allergy 2017, 72, 1962–1971. [Google Scholar] [CrossRef] [PubMed]
- Gomez, J.L.; Chen, A.; Diaz, M.P.; Zirn, N.; Gupta, A.; Britto, C.; Sauler, M.; Yan, X.; Stewart, E.; Santerian, K.; et al. A Network of Sputum MicroRNAs Is Associated with Neutrophilic Airway Inflammation in Asthma. Am. J. Respir. Crit. Care Med. 2020, 202, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Canas, J.A.; Rodrigo-Munoz, J.M.; Sastre, B.; Gil-Martinez, M.; Redondo, N.; Del Pozo, V. MicroRNAs as Potential Regulators of Immune Response Networks in Asthma and Chronic Obstructive Pulmonary Disease. Front. Immunol. 2020, 11, 608666. [Google Scholar] [CrossRef]
- Specjalski, K.; Niedoszytko, M. MicroRNAs: Future biomarkers and targets of therapy in asthma? Curr. Opin. Pulm. Med. 2020, 26, 285–292. [Google Scholar] [CrossRef]
- Gelfand, E.W. Importance of the leukotriene B4-BLT1 and LTB4-BLT2 pathways in asthma. Semin. Immunol. 2017, 33, 44–51. [Google Scholar] [CrossRef]
- Ford-Hutchinson, A.W.; Bray, M.A.; Doig, M.V.; Shipley, M.E.; Smith, M.J. Leukotriene B, a potent chemokinetic and aggregating substance released from polymorphonuclear leukocytes. Nature 1980, 286, 264–265. [Google Scholar] [CrossRef]
- Teixeira, M.M.; Lindsay, M.A.; Giembycz, M.A.; Hellewell, P.G. Role of arachidonic acid in leukotriene B(4)-induced guinea-pig eosinophil homotypic aggregation. Eur. J. Pharmacol. 1999, 384, 183–190. [Google Scholar] [CrossRef]
- Ng, C.F.; Sun, F.F.; Taylor, B.M.; Wolin, M.S.; Wong, P.Y. Functional properties of guinea pig eosinophil leukotriene B4 receptor. J. Immunol. 1991, 147, 3096–3103. [Google Scholar] [PubMed]
- Watanabe, S.; Yamasaki, A.; Hashimoto, K.; Shigeoka, Y.; Chikumi, H.; Hasegawa, Y.; Sumikawa, T.; Takata, M.; Okazaki, R.; Watanabe, M.; et al. Expression of functional leukotriene B4 receptors on human airway smooth muscle cells. J. Allergy Clin. Immunol. 2009, 124, 59–65.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamblin, C.; Gosset, P.; Tillie-Leblond, I.; Saulnier, F.; Marquette, C.H.; Wallaert, B.; Tonnel, A.B. Bronchial neutrophilia in patients with noninfectious status asthmaticus. Am. J. Respir. Crit. Care Med. 1998, 157, 394–402. [Google Scholar] [CrossRef]
- Wood, L.G.; Baines, K.J.; Fu, J.; Scott, H.A.; Gibson, P.G. The neutrophilic inflammatory phenotype is associated with systemic inflammation in asthma. Chest 2012, 142, 86–93. [Google Scholar] [CrossRef]
- Hosoki, K.; Ying, S.; Corrigan, C.; Qi, H.; Kurosky, A.; Jennings, K.; Sun, Q.; Boldogh, I.; Sur, S. Analysis of a Panel of 48 Cytokines in BAL Fluids Specifically Identifies IL-8 Levels as the Only Cytokine that Distinguishes Controlled Asthma from Uncontrolled Asthma, and Correlates Inversely with FEV1. PLoS ONE 2015, 10, e0126035. [Google Scholar] [CrossRef]
- Kuo, P.L.; Hsu, Y.L.; Huang, M.S.; Chiang, S.L.; Ko, Y.C. Bronchial epithelium-derived IL-8 and RANTES increased bronchial smooth muscle cell migration and proliferation by Kruppel-like factor 5 in areca nut-mediated airway remodeling. Toxicol. Sci. 2011, 121, 177–190. [Google Scholar] [CrossRef] [Green Version]
- Govindaraju, V.; Michoud, M.C.; Al-Chalabi, M.; Ferraro, P.; Powell, W.S.; Martin, J.G. Interleukin-8: Novel roles in human airway smooth muscle cell contraction and migration. Am. J. Physiol. Cell Physiol. 2006, 291, C957–C965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halwani, R.; Al-Abri, J.; Beland, M.; Al-Jahdali, H.; Halayko, A.J.; Lee, T.H.; Al-Muhsen, S.; Hamid, Q. CC and CXC chemokines induce airway smooth muscle proliferation and survival. J. Immunol. 2011, 186, 4156–4163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamaoki, J.; Nakata, J.; Tagaya, E.; Konno, K. Effects of roxithromycin and erythromycin on interleukin 8-induced neutrophil recruitment and goblet cell secretion in guinea pig tracheas. Antimicrob. Agents Chemother. 1996, 40, 1726–1728. [Google Scholar] [CrossRef] [Green Version]
- Smirnova, M.G.; Birchall, J.P.; Pearson, J.P. In vitro study of IL-8 and goblet cells: Possible role of IL-8 in the aetiology of otitis media with effusion. Acta Oto-Laryngol. 2002, 122, 146–152. [Google Scholar] [CrossRef]
- Tang, H.; Sun, Y.; Shi, Z.; Huang, H.; Fang, Z.; Chen, J.; Xiu, Q.; Li, B. YKL-40 induces IL-8 expression from bronchial epithelium via MAPK (JNK and ERK) and NF-kappaB pathways, causing bronchial smooth muscle proliferation and migration. J. Immunol. 2013, 190, 438–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Zou, F.; Lu, Y.; Fan, X.; Wu, Y.; Feng, X.; Sun, X.; Liu, Y. Notch1 contributes to TNF-alpha-induced proliferation and migration of airway smooth muscle cells through regulation of the Hes1/PTEN axis. Int. Immunopharmacol. 2020, 88, 106911. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Huang, Y.; Li, F.; Li, H.; Zhang, B.; Jin, L. MicroRNA-874 inhibits TNF-alpha-induced remodeling in human fetal airway smooth muscle cells by targeting STAT3. Respir. Physiol. Neurobiol. 2018, 251, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.Y.; Pham, A.; Rosenthal, P.; Miller, M.; Doherty, T.; Broide, D.H. Chronic OVA allergen challenged TNF p55/p75 receptor deficient mice have reduced airway remodeling. Int. Immunopharmacol. 2011, 11, 1038–1044. [Google Scholar] [CrossRef] [Green Version]
- Dejager, L.; Dendoncker, K.; Eggermont, M.; Souffriau, J.; Van Hauwermeiren, F.; Willart, M.; Van Wonterghem, E.; Naessens, T.; Ballegeer, M.; Vandevyver, S.; et al. Neutralizing TNFalpha restores glucocorticoid sensitivity in a mouse model of neutrophilic airway inflammation. Mucosal Immunol. 2015, 8, 1212–1225. [Google Scholar] [CrossRef] [Green Version]
- Agache, I.; Ciobanu, C.; Agache, C.; Anghel, M. Increased serum IL-17 is an independent risk factor for severe asthma. Respir. Med. 2010, 104, 1131–1137. [Google Scholar] [CrossRef] [Green Version]
- Vittal, R.; Fan, L.; Greenspan, D.S.; Mickler, E.A.; Gopalakrishnan, B.; Gu, H.; Benson, H.L.; Zhang, C.; Burlingham, W.; Cummings, O.W.; et al. IL-17 induces type V collagen overexpression and EMT via TGF-beta-dependent pathways in obliterative bronchiolitis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013, 304, L401–L414. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Thai, P.; Zhao, Y.H.; Ho, Y.S.; DeSouza, M.M.; Wu, R. Stimulation of airway mucin gene expression by interleukin (IL)-17 through IL-6 paracrine/autocrine loop. J. Biol. Chem. 2003, 278, 17036–17043. [Google Scholar] [CrossRef] [Green Version]
- Fujisawa, T.; Chang, M.M.; Velichko, S.; Thai, P.; Hung, L.Y.; Huang, F.; Phuong, N.; Chen, Y.; Wu, R. NF-kappaB mediates IL-1beta- and IL-17A-induced MUC5B expression in airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 2011, 45, 246–252. [Google Scholar] [CrossRef] [Green Version]
- Fujisawa, T.; Velichko, S.; Thai, P.; Hung, L.Y.; Huang, F.; Wu, R. Regulation of airway MUC5AC expression by IL-1beta and IL-17A; the NF-kappaB paradigm. J. Immunol. 2009, 183, 6236–6243. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Liu, Y.; Zou, J.F.; Cheng, Z.S. Interleukin-17 induces human alveolar epithelial to mesenchymal cell transition via the TGF-beta1 mediated Smad2/3 and ERK1/2 activation. PLoS ONE 2017, 12, e0183972. [Google Scholar] [CrossRef]
- Ogawa, H.; Azuma, M.; Tsunematsu, T.; Morimoto, Y.; Kondo, M.; Tezuka, T.; Nishioka, Y.; Tsuneyama, K. Neutrophils induce smooth muscle hyperplasia via neutrophil elastase-induced FGF-2 in a mouse model of asthma with mixed inflammation. Clin. Exp. Allergy 2018, 48, 1715–1725. [Google Scholar] [CrossRef] [PubMed]
- Camargo, L.D.N.; Dos Santos, T.M.; de Andrade, F.C.P.; Fukuzaki, S.; Dos Santos Lopes, F.; de Arruda Martins, M.; Prado, C.M.; Leick, E.A.; Righetti, R.F.; Tiberio, I. Bronchial Vascular Remodeling Is Attenuated by Anti-IL-17 in Asthmatic Responses Exacerbated by LPS. Front. Pharmacol. 2020, 11, 1269. [Google Scholar] [CrossRef]
- Camargo, L.D.N.; Righetti, R.F.; Aristoteles, L.; Dos Santos, T.M.; de Souza, F.C.R.; Fukuzaki, S.; Cruz, M.M.; Alonso-Vale, M.I.C.; Saraiva-Romanholo, B.M.; Prado, C.M.; et al. Effects of Anti-IL-17 on Inflammation, Remodeling, and Oxidative Stress in an Experimental Model of Asthma Exacerbated by LPS. Front. Immunol. 2017, 8, 1835. [Google Scholar] [CrossRef]
- Ramakrishnan, R.K.; Al Heialy, S.; Hamid, Q. Role of IL-17 in asthma pathogenesis and its implications for the clinic. Expert Rev. Respir. Med. 2019, 13, 1057–1068. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Xiao, H.T.; Zhang, Y.; Tong, R.S.; Zhang, L.J.; Bian, Y.; He, X. IL-1beta: A key modulator in asthmatic airway smooth muscle hyper-reactivity. Expert Rev. Respir. Med. 2015, 9, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Lappalainen, U.; Whitsett, J.A.; Wert, S.E.; Tichelaar, J.W.; Bry, K. Interleukin-1beta causes pulmonary inflammation, emphysema, and airway remodeling in the adult murine lung. Am. J. Respir. Cell Mol. Biol. 2005, 32, 311–318. [Google Scholar] [CrossRef]
- Mehta, A.K.; Doherty, T.; Broide, D.; Croft, M. Tumor necrosis factor family member LIGHT acts with IL-1beta and TGF-beta to promote airway remodeling during rhinovirus infection. Allergy 2018, 73, 1415–1424. [Google Scholar] [CrossRef]
- Pothoven, K.L.; Norton, J.E.; Hulse, K.E.; Suh, L.A.; Carter, R.G.; Rocci, E.; Harris, K.E.; Shintani-Smith, S.; Conley, D.B.; Chandra, R.K.; et al. Oncostatin M promotes mucosal epithelial barrier dysfunction, and its expression is increased in patients with eosinophilic mucosal disease. J. Allergy Clin. Immunol. 2015, 136, 737–746.e4. [Google Scholar] [CrossRef] [Green Version]
- Pothoven, K.L.; Norton, J.E.; Suh, L.A.; Carter, R.G.; Harris, K.E.; Biyasheva, A.; Welch, K.; Shintani-Smith, S.; Conley, D.B.; Liu, M.C.; et al. Neutrophils are a major source of the epithelial barrier disrupting cytokine oncostatin M in patients with mucosal airways disease. J. Allergy Clin. Immunol. 2017, 139, 1966–1978.e9. [Google Scholar] [CrossRef]
- Simpson, J.L.; Baines, K.J.; Boyle, M.J.; Scott, R.J.; Gibson, P.G. Oncostatin M (OSM) is increased in asthma with incompletely reversible airflow obstruction. Exp. Lung Res. 2009, 35, 781–794. [Google Scholar] [CrossRef] [PubMed]
- Ventura, I.; Vega, A.; Chacon, P.; Chamorro, C.; Aroca, R.; Gomez, E.; Bellido, V.; Puente, Y.; Blanca, M.; Monteseirin, J. Neutrophils from allergic asthmatic patients produce and release metalloproteinase-9 upon direct exposure to allergens. Allergy 2014, 69, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Cundall, M.; Sun, Y.; Miranda, C.; Trudeau, J.B.; Barnes, S.; Wenzel, S.E. Neutrophil-derived matrix metalloproteinase-9 is increased in severe asthma and poorly inhibited by glucocorticoids. J. Allergy Clin. Immunol. 2003, 112, 1064–1071. [Google Scholar] [CrossRef] [PubMed]
- Nadel, J.A. Role of enzymes from inflammatory cells on airway submucosal gland secretion. Respiration 1991, 58 (Suppl. S1), 3–5. [Google Scholar] [CrossRef]
- McGrath, K.W.; Icitovic, N.; Boushey, H.A.; Lazarus, S.C.; Sutherland, E.R.; Chinchilli, V.M.; Fahy, J.V.; Asthma Clinical Research Network of the National Heart, Lung, and Blood Institute. A large subgroup of mild-to-moderate asthma is persistently noneosinophilic. Am. J. Respir. Crit. Care Med. 2012, 185, 612–619. [Google Scholar] [CrossRef]
- Barnes, P.J. Therapeutic approaches to asthma-chronic obstructive pulmonary disease overlap syndromes. J. Allergy Clin. Immunol. 2015, 136, 531–545. [Google Scholar] [CrossRef]
- Westergaard, C.G.; Porsbjerg, C.; Backer, V. The effect of smoking cessation on airway inflammation in young asthma patients. Clin. Exp. Allergy 2014, 44, 353–361. [Google Scholar] [CrossRef]
- Pakhale, S.; Baron, J.; Dent, R.; Vandemheen, K.; Aaron, S.D. Effects of weight loss on airway responsiveness in obese adults with asthma: Does weight loss lead to reversibility of asthma? Chest 2015, 147, 1582–1590. [Google Scholar] [CrossRef]
- Simpson, J.L.; Powell, H.; Boyle, M.J.; Scott, R.J.; Gibson, P.G. Clarithromycin targets neutrophilic airway inflammation in refractory asthma. Am. J. Respir. Crit. Care Med. 2008, 177, 148–155. [Google Scholar] [CrossRef] [Green Version]
- Bardin, P.; Kanniess, F.; Gauvreau, G.; Bredenbroker, D.; Rabe, K.F. Roflumilast for asthma: Efficacy findings in mechanism of action studies. Pulm. Pharmacol. Ther. 2015, 35, S4–S10. [Google Scholar] [CrossRef]
- Casale, T.B.; Aalbers, R.; Bleecker, E.R.; Meltzer, E.O.; Zaremba-Pechmann, L.; de la Hoz, A.; Kerstjens, H.A.M. Tiotropium Respimat(R) add-on therapy to inhaled corticosteroids in patients with symptomatic asthma improves clinical outcomes regardless of baseline characteristics. Respir. Med. 2019, 158, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Szefler, S.J.; Vogelberg, C.; Bernstein, J.A.; Goldstein, S.; Mansfield, L.; Zaremba-Pechmann, L.; Engel, M.; Hamelmann, E. Tiotropium Is Efficacious in 6- to 17-Year-Olds with Asthma, Independent of T2 Phenotype. J. Allergy Clin. Immunol. Pract. 2019, 7, 2286–2295.e4. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.; Gaga, M.; Zervas, E.; Alagha, K.; Hargreave, F.E.; O’Byrne, P.M.; Stryszak, P.; Gann, L.; Sadeh, J.; Chanez, P.; et al. Safety and efficacy of a CXCR2 antagonist in patients with severe asthma and sputum neutrophils: A randomized, placebo-controlled clinical trial. Clin. Exp. Allergy 2012, 42, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Follows, R.M.; Snowise, N.G.; Ho, S.Y.; Ambery, C.L.; Smart, K.; McQuade, B.A. Efficacy, safety and tolerability of GSK2190915, a 5-lipoxygenase activating protein inhibitor, in adults and adolescents with persistent asthma: A randomised dose-ranging study. Respir. Res. 2013, 14, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Connor, B.J.; Lofdahl, C.G.; Balter, M.; Szczeklik, A.; Boulet, L.P.; Cairns, C.B. Zileuton added to low-dose inhaled beclomethasone for the treatment of moderate to severe persistent asthma. Respir. Med. 2007, 101, 1088–1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menzies-Gow, A.; Corren, J.; Bourdin, A.; Chupp, G.; Israel, E.; Wechsler, M.E.; Brightling, C.E.; Griffiths, J.M.; Hellqvist, A.; Bowen, K.; et al. Tezepelumab in Adults and Adolescents with Severe, Uncontrolled Asthma. N. Engl. J. Med. 2021, 384, 1800–1809. [Google Scholar] [CrossRef]
- Wenzel, S.E.; Barnes, P.J.; Bleecker, E.R.; Bousquet, J.; Busse, W.; Dahlen, S.E.; Holgate, S.T.; Meyers, D.A.; Rabe, K.F.; Antczak, A.; et al. A randomized, double-blind, placebo-controlled study of tumor necrosis factor-alpha blockade in severe persistent asthma. Am. J. Respir. Crit. Care Med. 2009, 179, 549–558. [Google Scholar] [CrossRef]
- Holgate, S.T.; Noonan, M.; Chanez, P.; Busse, W.; Dupont, L.; Pavord, I.; Hakulinen, A.; Paolozzi, L.; Wajdula, J.; Zang, C.; et al. Efficacy and safety of etanercept in moderate-to-severe asthma: A randomised, controlled trial. Eur. Respir. J. 2011, 37, 1352–1359. [Google Scholar] [CrossRef]
- Busse, W.W.; Holgate, S.; Kerwin, E.; Chon, Y.; Feng, J.; Lin, J.; Lin, S.L. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am. J. Respir. Crit. Care Med. 2013, 188, 1294–1302. [Google Scholar] [CrossRef]
- Brightling, C.E.; Nair, P.; Louis, R.; Singh, D. Risankizumab in severe asthma: A Phase IIa, placebo-controlled study. Eur. Respir. J. 2020, 56, 3699. [Google Scholar]
- Revez, J.A.; Bain, L.M.; Watson, R.M.; Towers, M.; Collins, T.; Killian, K.J.; O’Byrne, P.M.; Gauvreau, G.M.; Upham, J.W.; Ferreira, M.A. Effects of interleukin-6 receptor blockade on allergen-induced airway responses in mild asthmatics. Clin. Transl. Immunol. 2019, 8, e1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, H.A.; Gibson, P.G.; Garg, M.L.; Pretto, J.J.; Morgan, P.J.; Callister, R.; Wood, L.G. Dietary restriction and exercise improve airway inflammation and clinical outcomes in overweight and obese asthma: A randomized trial. Clin. Exp. Allergy 2013, 43, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Boulet, L.P.; Turcotte, H.; Martin, J.; Poirier, P. Effect of bariatric surgery on airway response and lung function in obese subjects with asthma. Respir. Med. 2012, 106, 651–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freitas, P.D.; Ferreira, P.G.; Silva, A.G.; Stelmach, R.; Carvalho-Pinto, R.M.; Fernandes, F.L.; Mancini, M.C.; Sato, M.N.; Martins, M.A.; Carvalho, C.R. The Role of Exercise in a Weight-Loss Program on Clinical Control in Obese Adults with Asthma. A Randomized Controlled Trial. Am. J. Respir. Crit. Care Med. 2017, 195, 32–42. [Google Scholar] [CrossRef]
- da Silva, P.L.; de Mello, M.T.; Cheik, N.C.; Sanches, P.L.; Correia, F.A.; de Piano, A.; Corgosinho, F.C.; Campos, R.M.; do Nascimento, C.M.; Oyama, L.M.; et al. Interdisciplinary therapy improves biomarkers profile and lung function in asthmatic obese adolescents. Pediatr. Pulmonol. 2012, 47, 8–17. [Google Scholar] [CrossRef]
- Crosbie, P.A.; Woodhead, M.A. Long-term macrolide therapy in chronic inflammatory airway diseases. Eur. Respir. J. 2009, 33, 171–181. [Google Scholar] [CrossRef]
- Kraft, M.; Cassell, G.H.; Pak, J.; Martin, R.J. Mycoplasma pneumoniae and Chlamydia pneumoniae in asthma: Effect of clarithromycin. Chest 2002, 121, 1782–1788. [Google Scholar] [CrossRef]
- Gibson, P.G.; Yang, I.A.; Upham, J.W.; Reynolds, P.N.; Hodge, S.; James, A.L.; Jenkins, C.; Peters, M.J.; Marks, G.B.; Baraket, M.; et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): A randomised, double-blind, placebo-controlled trial. Lancet 2017, 390, 659–668. [Google Scholar] [CrossRef] [Green Version]
- Taylor, S.L.; Ivey, K.L.; Gibson, P.G.; Simpson, J.L.; Rogers, G.B.; Group, A.S.R. Airway abundance of Haemophilus influenzae predicts response to azithromycin in adults with persistent uncontrolled asthma. Eur. Respir. J. 2020, 56, 2000194. [Google Scholar] [CrossRef]
- Niessen, N.M.; Gibson, P.G.; Baines, K.J.; Barker, D.; Yang, I.A.; Upham, J.W.; Reynolds, P.N.; Hodge, S.; James, A.L.; Jenkins, C.; et al. Sputum TNF markers are increased in neutrophilic and severe asthma and are reduced by azithromycin treatment. Allergy 2021, 76, 2090–2101. [Google Scholar] [CrossRef]
- Brusselle, G.G.; Vanderstichele, C.; Jordens, P.; Deman, R.; Slabbynck, H.; Ringoet, V.; Verleden, G.; Demedts, I.K.; Verhamme, K.; Delporte, A.; et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): A multicentre randomised double-blind placebo-controlled trial. Thorax 2013, 68, 322–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calverley, P.M.; Rabe, K.F.; Goehring, U.M.; Kristiansen, S.; Fabbri, L.M.; Martinez, F.J.; The M2-124 and M2-125 Study Groups. Roflumilast in symptomatic chronic obstructive pulmonary disease: Two randomised clinical trials. Lancet 2009, 374, 685–694. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y.; Fan, L.; Ye, J.; Fan, J.; Xu, X.; You, D.; Liu, S.; Chen, X.; Luo, P. Pharmacological mechanism of roflumilast in the treatment of asthma-COPD overlap. Drug Des. Dev. Ther. 2018, 12, 2371–2379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timmer, W.; Leclerc, V.; Birraux, G.; Neuhauser, M.; Hatzelmann, A.; Bethke, T.; Wurst, W. The new phosphodiesterase 4 inhibitor roflumilast is efficacious in exercise-induced asthma and leads to suppression of LPS-stimulated TNF-alpha ex vivo. J. Clin. Pharmacol. 2002, 42, 297–303. [Google Scholar] [CrossRef]
- Bousquet, J.; Aubier, M.; Sastre, J.; Izquierdo, J.L.; Adler, L.M.; Hofbauer, P.; Rost, K.D.; Harnest, U.; Kroemer, B.; Albrecht, A.; et al. Comparison of roflumilast, an oral anti-inflammatory, with beclomethasone dipropionate in the treatment of persistent asthma. Allergy 2006, 61, 72–78. [Google Scholar] [CrossRef]
- Bateman, E.D.; Goehring, U.M.; Richard, F.; Watz, H. Roflumilast combined with montelukast versus montelukast alone as add-on treatment in patients with moderate-to-severe asthma. J. Allergy Clin. Immunol. 2016, 138, 142–149.e8. [Google Scholar] [CrossRef] [Green Version]
- Gauvreau, G.M.; Boulet, L.P.; Schmid-Wirlitsch, C.; Cote, J.; Duong, M.; Killian, K.J.; Milot, J.; Deschesnes, F.; Strinich, T.; Watson, R.M.; et al. Roflumilast attenuates allergen-induced inflammation in mild asthmatic subjects. Respir. Res. 2011, 12, 140. [Google Scholar] [CrossRef]
- Phillips, J.E. Inhaled Phosphodiesterase 4 (PDE4) Inhibitors for Inflammatory Respiratory Diseases. Front. Pharmacol. 2020, 11, 259. [Google Scholar] [CrossRef] [Green Version]
- Singh, D.; Leaker, B.; Boyce, M.; Nandeuil, M.A.; Collarini, S.; Mariotti, F.; Santoro, D.; Barnes, P.J. A novel inhaled phosphodiesterase 4 inhibitor (CHF6001) reduces the allergen challenge response in asthmatic patients. Pulm. Pharmacol. Ther. 2016, 40, 1–6. [Google Scholar] [CrossRef]
- Franciosi, L.G.; Diamant, Z.; Banner, K.H.; Zuiker, R.; Morelli, N.; Kamerling, I.M.; de Kam, M.L.; Burggraaf, J.; Cohen, A.F.; Cazzola, M.; et al. Efficacy and safety of RPL554, a dual PDE3 and PDE4 inhibitor, in healthy volunteers and in patients with asthma or chronic obstructive pulmonary disease: Findings from four clinical trials. Lancet Respir. Med. 2013, 1, 714–727. [Google Scholar] [CrossRef]
- Luo, J.; Yang, L.; Yang, J.; Yang, D.; Liu, B.C.; Liu, D.; Liang, B.M.; Liu, C.T. Efficacy and safety of phosphodiesterase 4 inhibitors in patients with asthma: A systematic review and meta-analysis. Respirology 2018, 23, 467–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damera, G.; Jiang, M.; Zhao, H.; Fogle, H.W.; Jester, W.F.; Freire, J.; Panettieri, R.A., Jr. Aclidinium bromide abrogates allergen-induced hyperresponsiveness and reduces eosinophilia in murine model of airway inflammation. Eur. J. Pharmacol. 2010, 649, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Ohta, S.; Oda, N.; Yokoe, T.; Tanaka, A.; Yamamoto, Y.; Watanabe, Y.; Minoguchi, K.; Ohnishi, T.; Hirose, T.; Nagase, H.; et al. Effect of tiotropium bromide on airway inflammation and remodelling in a mouse model of asthma. Clin. Exp. Allergy 2010, 40, 1266–1275. [Google Scholar] [CrossRef] [PubMed]
- Toumpanakis, D.; Loverdos, K.; Tzouda, V.; Vassilakopoulou, V.; Litsiou, E.; Magkou, C.; Karavana, V.; Pieper, M.; Vassilakopoulos, T. Tiotropium bromide exerts anti-inflammatory effects during resistive breathing, an experimental model of severe airway obstruction. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 2207–2220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anzalone, G.; Gagliardo, R.; Bucchieri, F.; Albano, G.D.; Siena, L.; Montalbano, A.M.; Bonanno, A.; Riccobono, L.; Pieper, M.P.; Gjomarkaj, M.; et al. IL-17A induces chromatin remodeling promoting IL-8 release in bronchial epithelial cells: Effect of Tiotropium. Life Sci. 2016, 152, 107–116. [Google Scholar] [CrossRef]
- Suzaki, I.; Asano, K.; Shikama, Y.; Hamasaki, T.; Kanei, A.; Suzaki, H. Suppression of IL-8 production from airway cells by tiotropium bromide in vitro. Int. J. Chronic Obstr. Pulm. Dis. 2011, 6, 439–448. [Google Scholar] [CrossRef] [Green Version]
- Peters, S.P.; Kunselman, S.J.; Icitovic, N.; Moore, W.C.; Pascual, R.; Ameredes, B.T.; Boushey, H.A.; Calhoun, W.J.; Castro, M.; Cherniack, R.M.; et al. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N. Engl. J. Med. 2010, 363, 1715–1726. [Google Scholar] [CrossRef] [Green Version]
- Kerstjens, H.A.; Engel, M.; Dahl, R.; Paggiaro, P.; Beck, E.; Vandewalker, M.; Sigmund, R.; Seibold, W.; Moroni-Zentgraf, P.; Bateman, E.D. Tiotropium in asthma poorly controlled with standard combination therapy. N. Engl. J. Med. 2012, 367, 1198–1207. [Google Scholar] [CrossRef] [Green Version]
- Iwamoto, H.; Yokoyama, A.; Shiota, N.; Shoda, H.; Haruta, Y.; Hattori, N.; Kohno, N. Tiotropium bromide is effective for severe asthma with noneosinophilic phenotype. Eur. Respir. J. 2008, 31, 1379–1380. [Google Scholar] [CrossRef]
- Kerstjens, H.A.; Moroni-Zentgraf, P.; Tashkin, D.P.; Dahl, R.; Paggiaro, P.; Vandewalker, M.; Schmidt, H.; Engel, M.; Bateman, E.D. Tiotropium improves lung function, exacerbation rate, and asthma control, independent of baseline characteristics including age, degree of airway obstruction, and allergic status. Respir. Med. 2016, 117, 198–206. [Google Scholar] [CrossRef] [Green Version]
- Casale, T.B.; Bateman, E.D.; Vandewalker, M.; Virchow, J.C.; Schmidt, H.; Engel, M.; Moroni-Zentgraf, P.; Kerstjens, H.A.M. Tiotropium Respimat Add-on Is Efficacious in Symptomatic Asthma, Independent of T2 Phenotype. J. Allergy Clin. Immunol. Pract. 2018, 6, 923–935.e9. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, S.C.; Krishnan, J.A.; King, T.S.; Lang, J.E.; Blake, K.V.; Covar, R.; Lugogo, N.; Wenzel, S.; Chinchilli, V.M.; Mauger, D.T.; et al. Mometasone or Tiotropium in Mild Asthma with a Low Sputum Eosinophil Level. N. Engl. J. Med. 2019, 380, 2009–2019. [Google Scholar] [CrossRef] [PubMed]
- O’Byrne, P.M.; Metev, H.; Puu, M.; Richter, K.; Keen, C.; Uddin, M.; Larsson, B.; Cullberg, M.; Nair, P. Efficacy and safety of a CXCR2 antagonist, AZD5069, in patients with uncontrolled persistent asthma: A randomised, double-blind, placebo-controlled trial. Lancet Respir. Med. 2016, 4, 797–806. [Google Scholar] [CrossRef]
- Watz, H.; Uddin, M.; Pedersen, F.; Kirsten, A.; Goldmann, T.; Stellmacher, F.; Groth, E.; Larsson, B.; Bottcher, G.; Malmgren, A.; et al. Effects of the CXCR2 antagonist AZD5069 on lung neutrophil recruitment in asthma. Pulm. Pharmacol. Ther. 2017, 45, 121–123. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, R.; Norris, V.; Kelly, K.; Zhu, C.Q.; Ambery, C.; Lafferty, J.; Cameron, E.; Thomson, N.C. Effects of a FLAP inhibitor, GSK2190915, in asthmatics with high sputum neutrophils. Pulm. Pharmacol. Ther. 2014, 27, 62–69. [Google Scholar] [CrossRef]
- Snowise, N.G.; Clements, D.; Ho, S.Y.; Follows, R.M. Addition of a 5-lipoxygenase-activating protein inhibitor to an inhaled corticosteroid (ICS) or an ICS/long-acting beta-2-agonist combination in subjects with asthma. Curr. Med. Res. Opin. 2013, 29, 1663–1674. [Google Scholar] [CrossRef]
- Thalanayar Muthukrishnan, P.; Nouraie, M.; Parikh, A.; Holguin, F. Zileuton use and phenotypic features in asthma. Pulm. Pharmacol. Ther. 2020, 60, 101872. [Google Scholar] [CrossRef]
- Chiu, C.J.; Huang, M.T. Asthma in the Precision Medicine Era: Biologics and Probiotics. Int. J. Mol. Sci. 2021, 22, 4528. [Google Scholar] [CrossRef]
- Corren, J.; Parnes, J.R.; Wang, L.; Mo, M.; Roseti, S.L.; Griffiths, J.M.; van der Merwe, R. Tezepelumab in Adults with Uncontrolled Asthma. N. Engl. J. Med. 2017, 377, 936–946. [Google Scholar] [CrossRef]
- Venkataramani, S.; Low, S.; Weigle, B.; Dutcher, D.; Jerath, K.; Menzenski, M.; Frego, L.; Truncali, K.; Gupta, P.; Kroe-Barrett, R.; et al. Design and characterization of Zweimab and Doppelmab, high affinity dual antagonistic anti-TSLP/IL13 bispecific antibodies. Biochem. Biophys. Res. Commun. 2018, 504, 19–24. [Google Scholar] [CrossRef]
- Howarth, P.H.; Babu, K.S.; Arshad, H.S.; Lau, L.; Buckley, M.; McConnell, W.; Beckett, P.; Al Ali, M.; Chauhan, A.; Wilson, S.J.; et al. Tumour necrosis factor (TNFalpha) as a novel therapeutic target in symptomatic corticosteroid dependent asthma. Thorax 2005, 60, 1012–1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berry, M.A.; Hargadon, B.; Shelley, M.; Parker, D.; Shaw, D.E.; Green, R.H.; Bradding, P.; Brightling, C.E.; Wardlaw, A.J.; Pavord, I.D. Evidence of a role of tumor necrosis factor alpha in refractory asthma. N. Engl. J. Med. 2006, 354, 697–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morjaria, J.B.; Chauhan, A.J.; Babu, K.S.; Polosa, R.; Davies, D.E.; Holgate, S.T. The role of a soluble TNFalpha receptor fusion protein (etanercept) in corticosteroid refractory asthma: A double blind, randomised, placebo controlled trial. Thorax 2008, 63, 584–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, L.; Hur, J.; Kang, J.Y.; Rhee, C.K.; Kim, Y.K.; Lee, S.Y. Effect of the anti-IL-17 antibody on allergic inflammation in an obesity-related asthma model. Korean J. Intern. Med. 2018, 33, 1210–1223. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, T.M.; Righetti, R.F.; Camargo, L.D.N.; Saraiva-Romanholo, B.M.; Aristoteles, L.; de Souza, F.C.R.; Fukuzaki, S.; Alonso-Vale, M.I.C.; Cruz, M.M.; Prado, C.M.; et al. Effect of Anti-IL17 Antibody Treatment Alone and in Combination With Rho-Kinase Inhibitor in a Murine Model of Asthma. Front. Physiol. 2018, 9, 1183. [Google Scholar] [CrossRef]
- Khokhlovich, E.; Grant, S.; Kazani, S.; Strieter, R.; Thornton-Wells, T.; Laramie, J.; Morgan, T.; Kennedy, S. The biological pathways underlying response to anti-IL-17A (AIN457; secukinumab) therapy differ across severe asthmatic patients. Eur. Respir. J. 2017, 50, OA2897. [Google Scholar]
- Staton, T.L.; Peng, K.; Owen, R.; Choy, D.F.; Cabanski, C.R.; Fong, A.; Brunstein, F.; Alatsis, K.R.; Chen, H. A phase I, randomized, observer-blinded, single and multiple ascending-dose study to investigate the safety, pharmacokinetics, and immunogenicity of BITS7201A, a bispecific antibody targeting IL-13 and IL-17, in healthy volunteers. BMC Pulm. Med. 2019, 19, 5. [Google Scholar] [CrossRef]
- Langrish, C.L.; Chen, Y.; Blumenschein, W.M.; Mattson, J.; Basham, B.; Sedgwick, J.D.; McClanahan, T.; Kastelein, R.A.; Cua, D.J. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 2005, 201, 233–240. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Huang, Y.; He, J.; Li, C.; Deng, W.; Ran, X.; Wang, D. Rosiglitazone, a peroxisome proliferator-activated receptor-gamma agonist, attenuates airway inflammation by inhibiting the proliferation of effector T cells in a murine model of neutrophilic asthma. Immunol. Lett. 2014, 157, 9–15. [Google Scholar] [CrossRef]
- Han, W.; Li, J.; Tang, H.; Sun, L. Treatment of obese asthma in a mouse model by simvastatin is associated with improving dyslipidemia and decreasing leptin level. Biochem. Biophys. Res. Commun. 2017, 484, 396–402. [Google Scholar] [CrossRef]
- Lee, H.Y.; Lee, E.G.; Hur, J.; Rhee, C.K.; Kim, Y.K.; Lee, S.Y.; Kang, J.Y. Pravastatin alleviates allergic airway inflammation in obesity-related asthma mouse model. Exp. Lung Res. 2019, 45, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Norman, P. Evaluation of WO2013136076: Two crystalline forms of the phosphatidylinositol 3-kinase-delta inhibitor RV-1729. Expert Opin. Ther. Pat. 2014, 24, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Leaker, B.R.; Barnes, P.J.; O’Connor, B.J.; Ali, F.Y.; Tam, P.; Neville, J.; Mackenzie, L.F.; MacRury, T. The effects of the novel SHIP1 activator AQX-1125 on allergen-induced responses in mild-to-moderate asthma. Clin. Exp. Allergy 2014, 44, 1146–1153. [Google Scholar] [CrossRef] [PubMed]
- Cahn, A.; Hamblin, J.N.; Begg, M.; Wilson, R.; Dunsire, L.; Sriskantharajah, S.; Montembault, M.; Leemereise, C.N.; Galinanes-Garcia, L.; Watz, H.; et al. Safety, pharmacokinetics and dose-response characteristics of GSK2269557, an inhaled PI3Kdelta inhibitor under development for the treatment of COPD. Pulm. Pharmacol. Ther. 2017, 46, 69–77. [Google Scholar] [CrossRef]
- Winkler, D.G.; Faia, K.L.; DiNitto, J.P.; Ali, J.A.; White, K.F.; Brophy, E.E.; Pink, M.M.; Proctor, J.L.; Lussier, J.; Martin, C.M.; et al. PI3K-delta and PI3K-gamma inhibition by IPI-145 abrogates immune responses and suppresses activity in autoimmune and inflammatory disease models. Chem. Biol. 2013, 20, 1364–1374. [Google Scholar] [CrossRef] [Green Version]
- Kampe, M.; Lampinen, M.; Stolt, I.; Janson, C.; Stalenheim, G.; Carlson, M. PI3-kinase regulates eosinophil and neutrophil degranulation in patients with allergic rhinitis and allergic asthma irrespective of allergen challenge model. Inflammation 2012, 35, 230–239. [Google Scholar] [CrossRef]
- Toki, S.; Newcomb, D.C.; Printz, R.L.; Cahill, K.N.; Boyd, K.L.; Niswender, K.D.; Peebles, R.S., Jr. Glucagon-like peptide-1 receptor agonist inhibits aeroallergen-induced activation of ILC2 and neutrophilic airway inflammation in obese mice. Allergy 2021, 76, 3433–3445. [Google Scholar] [CrossRef]
- Foer, D.; Beeler, P.E.; Cui, J.; Karlson, E.W.; Bates, D.W.; Cahill, K.N. Asthma Exacerbations in Patients with Type 2 Diabetes and Asthma on Glucagon-like Peptide-1 Receptor Agonists. Am. J. Respir. Crit. Care Med. 2021, 203, 831–840. [Google Scholar] [CrossRef]

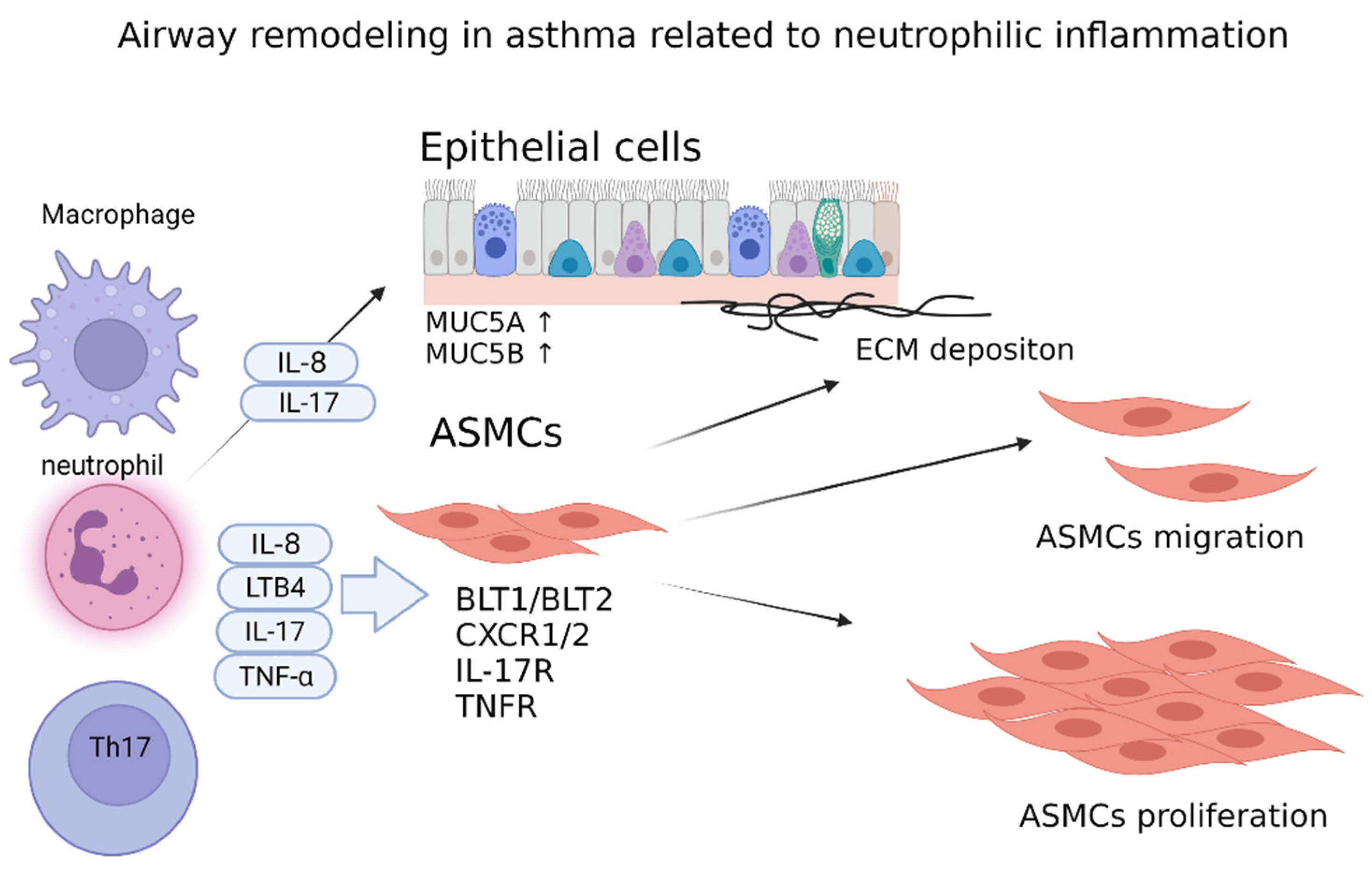
| Biomarker | Sample | Definition | Significance | Refs. |
|---|---|---|---|---|
| YKL-40 | Serum, sputum | Not established, but serum YKL-40 > 60.94 ng/mL showed impaired lung function and require corticosteroid | YKL-40 is released from neutrophil and epithelial cells, YKL-40 is released from neutrophils and epithelial cells Serum YKL-40 correlates with sputum neutrophil counts | [181,182,183] |
| Hydrogen sulfide (H2S) | Serum, exhaled breath, sputum | Not established | Sputum H2S correlates with the degree of airflow limitation Serum/sputum H2S predicts asthma exacerbation | [184,185,186] |
| MPO | Sputum | Not established | Sputum MPO correlates with sputum YKL-40 and neutrophils | [23,187] |
| Neutrophil | Serum, sputum | Sputum > 60% or 76% | Associated with chronic airway obstruction, annual decline of FEV1 | [188,189] |
| MicroRNA | Sputum, serum, and plasma | Not established | miR-199a-5p, miR142-3p, miR233-3p, and miR629-3p are increased in neutrophilic asthmamiR299a -5p is negatively correlated with FEV1 | [190,191] |
| Non-Pharmacological Approach | |||
|---|---|---|---|
| Approach | Patient Population | Outcomes | Ref. |
| Smoking cessation | Young patients with asthma (19–40 years old), steroid-free, 17% neutrophilic asthma | Improved asthma control and flung function | [248] |
| Weight loss | 18–75-year-old, obese patients with asthma (BMI > 35 kg/m2) | Improved asthma control, QOL, lung function, and AHR | [249] |
| Nonspecific treatment for neutrophilic asthma | |||
| Therapy | Patient population | Outcomes | Ref. |
| Macrolide (azithromycin, clarithromycin) | Non-eosinophilic or neutrophilic severe asthma (18–75-year-old patients) | Reduced asthma exacerbation, QOL, and lung function | [250] |
| PDE inhibitor | Patients 18–70 years of age, moderate-to-severe asthma | Improved lung function and asthma control | [251] |
| Tiotropium | Adult symptomatic patients with asthma despite treatment with medium-dose ICS | Improved lung function and asthma control, reduced risk of severe exacerbation, independent of type 2 inflammation | [252] |
| Tiotropium | 6–17-year-old patients, symptomatic severe asthma | Improved lung function and ACQ, reduced risk of exacerbation, independent of type 2 inflammation | [253] |
| Specific treatment for neutrophil and mediators | |||
| SCH527123/CXCR2 | Severe asthma and sputum neutrophil >40% | Fewer mild exacerbations and a trend towards improvement in the ACQ, but not statistically significant | [254] |
| GSK2090915/FLAP | Persistent asthma treated with SABA only | Improved symptom score and reduced SABA use | [255] |
| Zileuton/5-LO | Moderate-to-severe asthma treated with low dose ICS | Improved PEF and symptoms | [256] |
| Biologics | |||
| Tezepelumab/TSLP | Moderate-to-severe asthma | Reduced rate of exacerbation, improved lung function, ACQ, and AQLQ, regardless of type 2 inflammation | [257] |
| Golimumab/TNF-α | Uncontrolled asthma with high-dose ICS/LABA | No improvement in FEV1 and exacerbation | [258] |
| Etanercept/TNF-α | Moderate-to-severe persistent asthma | No improvement in FEV1 and ACQ, exacerbation, AHR, AQLQ | [259] |
| Brodalumab/IL-17 receptor | Inadequately controlled moderate-to-severe asthma treated with high-dose ICS ± LABA | No treatment differences were observed | [260] |
| Risankinumab/IL-23 | Adult patients with severe asthma | No improvement in asthma exacerbation | [261] |
| Tocilizumab/IL-6 | Mild asthma | No improvement in allergen-induced bronchoconstriction | [262] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamasaki, A.; Okazaki, R.; Harada, T. Neutrophils and Asthma. Diagnostics 2022, 12, 1175. https://doi.org/10.3390/diagnostics12051175
Yamasaki A, Okazaki R, Harada T. Neutrophils and Asthma. Diagnostics. 2022; 12(5):1175. https://doi.org/10.3390/diagnostics12051175
Chicago/Turabian StyleYamasaki, Akira, Ryota Okazaki, and Tomoya Harada. 2022. "Neutrophils and Asthma" Diagnostics 12, no. 5: 1175. https://doi.org/10.3390/diagnostics12051175
APA StyleYamasaki, A., Okazaki, R., & Harada, T. (2022). Neutrophils and Asthma. Diagnostics, 12(5), 1175. https://doi.org/10.3390/diagnostics12051175






