Abstract
Abnormal uterine bleeding (AUB) is a frequent symptom in perimenopausal women. It is defined as uterine bleeding in which the duration, frequency, or amount of bleeding is considered excessive and negatively affects the woman’s quality of life (QoL) and psychological well-being. In cases of structural uterine pathology, hysterectomy (usually performed via a minimally invasive approach) offers definitive symptom relief and is associated with long-lasting improvement of QoL and sexuality. However, over the past 30 years, uterus-preserving treatments have been introduced as alternatives to hysterectomy. Hysteroscopic polypectomy, myomectomy, or endometrial resection/endometrial ablation are minimally invasive techniques that can be used as an alternative to hysterectomy to treat AUB due to benign conditions. Although associated with high patient satisfaction and short-term improvement in their QoL, hysteroscopic treatments do not eliminate the risk of AUB recurrence or the need for further intervention. Therefore, considering the impact of different treatment options on QoL and sexuality during preoperative shared decision making could help identify the most appropriate and personalized treatment options for perimenopausal women suffering from AUB.
1. Abnormal Uterine Bleeding (AUB) in Perimenopausal Women
Perimenopause is the period between the first symptoms of diminished ovarian function, usually beginning in the early forties, lasting up to two years after the Final Menstrual Period (FMP). The variety and inconsistency of perimenopause definitions imply that a sharp distinction between “premenopausal”, “perimenopausal”, and “postmenopausal” AUB is difficult. In 1996, the World Health Organization defined perimenopause as “the period immediately prior to menopause (when the endocrinological, biological and clinical features of menopause begin) and the first year after menopause” [1]. In contrast, the term menopausal transition should be reserved for “that period of time before FMP when variability in the menstrual cycle is usually increased” [1]. Still, some studies arbitrarily set lower and upper limits of perimenopause, e.g., between 40 and 54 [2] or 42 and 52 [3] years of age or from four years before to 12 months after FMP [4].
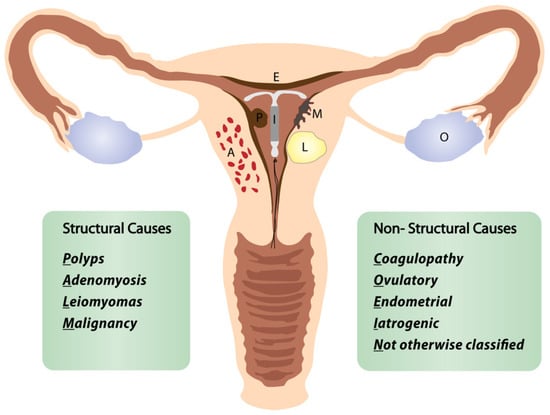
In this review, we define the “perimenopausal AUB” as any abnormal menstrual bleeding during menopausal transition or within the first year after menopause (except for cyclic bleeding in women using hormonal replacement therapy) [1,3,5]. AUB is the leading cause of approximately one-third of all outpatient gynecological visits, particularly in the perimenopausal period [3,4,5]. More than 90% of women experience at least one episode of AUB, and 78% of them at least three episodes of AUB during their transition to menopause [3]. The popular classification of nongestational causes of AUB was introduced by FIGO (International Federation of Gynecology and Obstetrics) in 2011 and revised in 2018 (Figure 1) [6,7,8]. The causes of nongestational AUB have been classified into nine categories arranged according to the acronym PALM-COEIN, including the structural causes (“PALM”): polyp, adenomyosis, leiomyoma, malignancy/hyperplasia, and non-structural causes (“COEIN”): coagulopathy, ovulatory dysfunction, endometrial, iatrogenic, and “not otherwise classified” [6,7]. The contribution of individual PALM-COEIN categories to the spectrum of AUB causes changes with age; nevertheless, endometrial polyps and fibroids remain the most common structural causes of AUB in perimenopause [9,10,11]. In the fourth decade of life, the impact of myomas and adenomyosis as AUB causes increases. Likewise, the highest incidence of endometrial polyps is reported in women aged 40–44 years [12]. Notably, the rate of structural pathologies (fibroids, polyps, adenomyosis) coexisting with each other or with uterine malignancies also increases with age [13,14]. Additionally, various local or systemic conditions, and hormonal or non-hormonal medications (tamoxifen, oral anticoagulants), can trigger uterine bleeding. Hematologic dysfunctions are also a frequent cause of AUB in perimenopausal women, being reported as the cardinal symptom in 32–100% of women with von Willebrand factor deficiency (relevant to 0.5–1% of the general population), in 5–98% of women with platelet dysfunction, and 35–70% of women with rare factor deficiencies [15]. Although the annual probability of spontaneous conception is about 10% by age 40–44, falling to 3% by age 45–49, the percentage of women fulfilling their reproductive goals in their fourth decade is continuously rising [16]. However, 84% of pregnancies in women over 48 years end in first-trimester miscarriage, and the rate of ectopics in women over 44 years rises to 7% [17]. Therefore, up to 12 months after FMP, excluding pregnancy is a mandatory part of the AUB diagnostic workup.
2. Initial Diagnostic Evaluation of AUB
The self-reported perception of AUB by the woman is the first step to determine its impact on QoL. It is well known that the patient’s subjective estimation does not always correlate with the objective amount of blood loss [18]. According to Munro et al. [6], menstrual bleeding exceeding 80 mL, as well as any intermenstrual and postcoital bleeding, should be considered abnormal. Nevertheless, about 14% of patients with mild to moderate blood loss consider their bleeding as heavy, and 40% of women with excessive blood loss consider their bleeding as standard [19]. In those cases, Pictorial Blood Assessment Charts can be helpful for the semiquantitative determination of AUB [20].
A detailed medical history (including hereditary disposition for uterine malignancies), the vaginal speculum exam, and transvaginal ultrasound (TVS) are essential parts of evaluating patients complaining of AUB [7,14,21]. However, standard diagnostic criteria and reproducibility of TVS are not consistent [14], and the accuracy of TVS in diagnosing benign uterine conditions is suboptimal [22,23]. TVS is helpful to exclude endometrial cancer (EC) in postmenopausal women if the endometrial echo is less than or equal to 4 mm, providing a negative predictive value of >99% [24]. The advantage of TVS is the holistic assessment of the uterus and its surrounding structures [7,24]. Saline infusion sonohysterography (SIS) offers a superior detection rate of benign lesions compared to TVS, but costs, convenience, and tolerability are the limiting factors [25,26]. Laboratory tests, e.g., hemoglobin and human chorionic gonadotropin determination, supplement the physical examination and sonography. Further laboratory tests may be indicated to uncover hereditary bleeding disorders or hormonal alterations depending on the history and the developed clinical suspicion.
3. Role of Hysteroscopy in the Management of Women with AUB
Hysteroscopy is considered the gold standard technique for diagnosing and managing pathological conditions affecting the uterine cavity [27,28]. In turn, AUB is the most common indication to perform hysteroscopy in perimenopausal women [29]. The hysteroscopic “see-and-treat” approach allows exploration of the uterine cavity, targeted endometrial and endocervical biopsies, and—if indicated—immediate treatment of endocervical, endometrial, or submucosal pathologies (polyps, myomas) [24,30,31,32,33,34]. It is essential to highlight that hysteroscopy is unsuitable for evaluating and treating deep myometrial pathologies (such as adenomyosis or myomas FIGO-Grade ≥ 3). Most hysteroscopic procedures can be performed in an office setting depending on the patient’s preferences, available infrastructure (staffing, equipment), surgeon’s experience, and comfort level [24,28,31,32,33,34]. More complex and prolonged procedures, such as hysteroscopic myomectomy and extensive lysis of intrauterine adhesions, are typically performed in the operating room, thus providing the patient with general anesthesia and the ability of the surgeon to perform more extensive surgery. Moreover, hysteroscopy can be complemented with laparoscopy if necessary [24,28,35,36]. Regardless of the setting in which the hysteroscopic procedure is completed, it is helpful to distinguish diagnostic and operative hysteroscopy.
3.1. Diagnostic Hysteroscopy
Diagnostic hysteroscopy aims to diagnose lesions within the endometrial cavity and, if necessary, to obtain targeted biopsies [24,28,37]. Diagnostic hysteroscopy can be a single intervention or may immediately precede hysteroscopic surgery. The feasibility of diagnostic hysteroscopy decreases in patients with previous surgeries, pelvic infections, IUD use, and postmenopausal status. Cobellis et al. [38] developed a predictive score for office hysteroscopy failure using different predictors, the most significant of which were history of procedures on the cervix, cesarean section, recurrent vaginitis, retroflexed uterus, and menopause. Depending on the previously indicated factors, office hysteroscopy could not be completed in 6 to 76% of the interventions [38].
AUB is the presenting sign in >90% of postmenopausal women with EC [39]. In turn, the prevalence of EC or atypical hyperplasia in postmenopausal women with AUB is 21%, rising to 29% when AUB is accompanied by an endometrium thickness of ≥4 mm on TVS [39]. The systematic review by Clark et al. [40] confirmed high diagnostic accuracy of hysteroscopy with regard to EC, but only moderate for other types of endometrial disease. When comparing studies on the diagnostic accuracy of hysteroscopy, it is helpful to distinguish between studies reporting results based only on the hysteroscopic view and those obtained after a hysteroscopically-guided biopsy. For example, Elfayomy et al. [41] found “hysteroscopy” (meaning hysteroscopic image of the pathology) insufficient to exclude endometrial hyperplasia and cancer in women with AUB, based on 0.57/0.50 sensitivity and 0.92/0.94 specificity for endometrial hyperplasia and cancer, respectively (Figure 2).
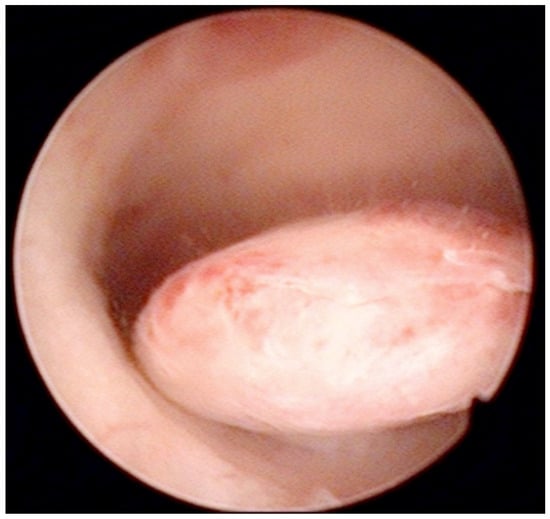
Figure 2.
Pedunculate low-risk endometrial polyp in the posterior uterine wall during diagnostic hysteroscopy (intraoperative picture by F.B. and S.F.).
Similarly, Garuti et al. [42] used the terms “hysteroscopy” and “hysteroscopic view” interchangeably, reporting a low sensitivity (0.64 and 0.61) and specificity (0.92 and 0.95) for the diagnosis of endometrial hyperplasia. In the study of De Franciscis et al. [43], the concordance between the final histopathological result and the hysteroscopic impression was 86% for benign disease or normal endometrium, but only 58% for endometrial hyperplasia. In addition, the lowest agreement (52%) was noted for postmenopausal endometrial hyperplasia. When restricted to women with postmenopausal AUB, the sensitivity and specificity of in-office hysteroscopy for endometrial hyperplasia (with histopathology as reference) was 87% and 43%, respectively [43]. In contrast, Tinelli et al. [44] not only confirmed the diagnostic superiority of hysteroscopy with an eye-directed biopsy for detecting endometrial pathologies compared to TVS (and thus recommended hysteroscopy for all postmenopausal women with AUB and endometrial thickness > 4 mm) but also demonstrated the unique efficiency of hysteroscopy for diagnosing focal abnormalities (including EC) in the atrophic endometrium that would otherwise likely be missed by TVS. The authors advocate hysteroscopic evaluation even in patients with AUB with endometrium on TVS of <4 mm, aiming to decrease the chance of failing to diagnose carcinomas that develop focally in the atrophic endometrium (Figure 3) [44].
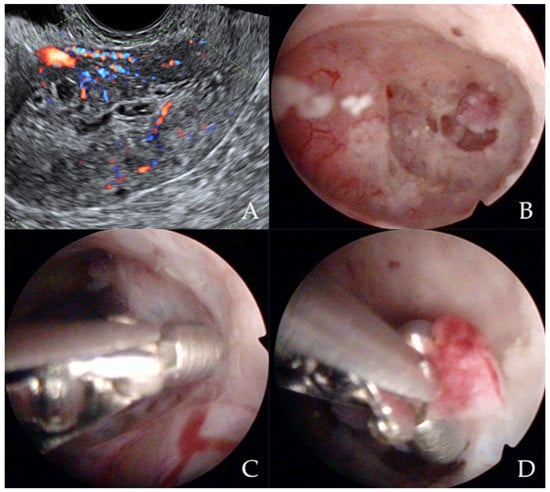
Figure 3.
Ultrasonographic appearance of uterine lesion suspected for early-stage endometrial cancer (A). The diagnostic hysteroscopic allows for visualizing the suspected uterine area (B) and for obtaining the histologic biopsy (C,D). Intraoperative photographs by F.B. and S.F.
Less threatening but a more common finding in patients with AUB (20–31% of cases) are endometrial polyps [9,10,11,25,33]. The sensitivity of TVS for the diagnosis of polyps is particularly low (0.51) [25]. In contrast, in women with postmenopausal AUB, the sensitivity and specificity of hysteroscopy for the diagnosis of polyps are reported at 0.81–0.92 and 0.85–0.98, respectively [11,26]. The study by de Godoy Borges et al. [23] confirmed the superiority of diagnostic hysteroscopy (96.4% sensitivity, 74.6% specificity) in comparison to TVS (88.7% sensitivity, 25.4% specificity) for detecting endometrial polyps in women aged 41 to 82 presenting with AUB. Saline Infusion Sonohysterogram (SIS) provides a similar sensitivity compared with hysteroscopy but lower specificity (0.93 and 0.83 compared to 0.95 and 0.90, respectively). Finally, a recent meta-analysis reported sensitivity and specificity of 0.87 and 0.86, 0.62 and 0.73, and 0.92 and 0.85 for SIS, TVS, and hysteroscopy, respectively, for detecting endometrial polyps in women with AUB [11]. Similarly, hysteroscopy offers the highest diagnostic accuracy (>90%) for detecting submucous myomas as compared to TVS or SIS [45]. Further developments in the field of sonography, based on the three-dimensional virtual image synthesis (so called “virtual sonographic hysteroscopy”), will show to what extent diagnostic hysteroscopy could be replaced by next-generation, three-dimensional imaging modalities for detecting intracavitary uterine pathologies [46].
3.2. Operative Hysteroscopy
Operative hysteroscopy uses mechanical, electrosurgical, and laser instruments to treat intracavitary pathologies. The introduction of the small-diameter coaxial bipolar electrode (Versapoint, Gynecare, Ethicon, NJ, USA) in 1999 was a milestone for outpatient operative hysteroscopy (Figure 4) [47]. The development of miniaturized mechanical instruments with small diameter scopes and working channels with continuous flow systems enabled the “see-and-treat” approach without general anesthesia [32,34,48,49,50]. Wortman et al. [51] confirmed that major operative hysteroscopic surgery could be performed in an office-based setting resulting in a 98.8% rate of “satisfied” or “very satisfied” patients. Both outpatient (73%) and inpatient (80%) hysteroscopic polypectomy offer comparable success rates, as determined by the patients’ subjective bleeding and QoL assessment after six months [52]. However, procedure failure is higher (19% vs. 7%), and acceptability is lower (83% vs. 92%) with outpatient compared to inpatient polypectomy [52]. An essential aspect of hysteroscopic surgery is a very high level of physician satisfaction (e.g., 95% reported in [53]) associated with this approach. Factors such as incomplete resection and recurrence of the pathology decrease the acceptability of hysteroscopic surgery [38,52].
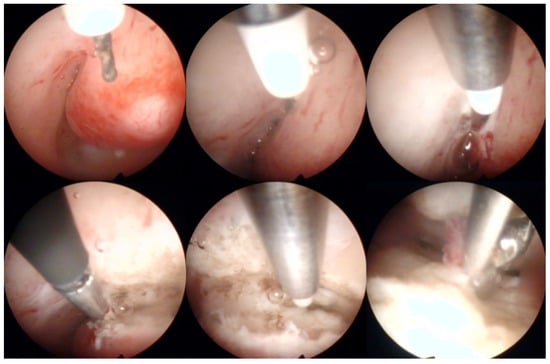
Figure 4.
Operative hysteroscopic removal of an endometrial polyp (mean diameter 13 mm) by Versapoint system (Gynecare, Ethicon Inc., Raritan, NJ, USA). Intraoperative photographs by F.B. and S.F.
As with any other surgical procedure, hysteroscopic operations can be associated with complications. Every second complication of operative hysteroscopy is mechanical (52%), including cervical lacerations, uterine perforations, and injuries to the adjacent organs such as the bowel or bladder, sometimes associated with internal bleeding and conversion to laparoscopy or laparotomy [54,55]. Further short-term complications, such as excess fluid absorption, pulmonary edema, critical electrolyte disbalance, or genital tract burns, and long-term consequences (intrauterine adhesions) should be mentioned [54,55,56]. Venous air embolism during endometrial resection/endometrial ablation (ER/EA) or hysteroscopic myomectomy, although exceedingly rare (1:1140 surgeries), is a potentially fatal complication [57,58]. Myomectomies are hysteroscopic procedures with the highest complication rate (up to 14%) and the highest risk of distension medium-related complications (more than seven times more common compared to polypectomy) [54]. The application of monopolar energy is associated with more frequent local and systemic adverse events (perforations, burns, hyponatremia) as compared to bipolar energy [59,60]. Many of these complications can be prevented by strict adherence to basic surgical principles, e.g., limited use of monopolar devices [59,60]. Furthermore, some complications of intracavitary resections, e.g., intrauterine adhesions, can be successfully reduced by the use of antiadhesive barriers [61].
4. The Place of Hysteroscopy in the Treatment of the Perimenopausal Patient with AUB
The goal of AUB treatment in perimenopausal women—after the exclusion of an oncological cause—is to normalize menstrual blood loss and improve QoL [18,62]. The presence and severity of subsequent anemia influence the subjective experience of AUB and the therapeutic goals [62]. Irrespective of the underlying condition and planned surgical intervention, the treatment of anemia should be started as soon as it is recognized [18]. Clinical practice guidelines recommend early initiation of non-surgical methods such as tranexamic acid, oral or intramuscular progestins, gonadotropin-releasing hormone agonists, non-steroidal antiinflammatory drugs, or levonorgestrel-releasing intrauterine systems (LNG-IUS) [24]. From the QoL perspective, a patient’s preferences should be considered when selecting treatments. Before 1990, hysterectomy was considered the only available definitive treatment of AUB for women without a desire for future fertility [63]. Hysterectomy is associated with the resolution of the AUB symptoms and high satisfaction rates. Although minimally invasive approaches have significantly reduced perioperative morbidity, hysterectomy remains a major surgical procedure associated with severe complications [64,65]. Therefore, uterine sparing procedures should also be considered. Safe and cost-effective removal of myomas, polyps, and ER/EA for AUB treatment became possible via hysteroscopy and due to the evolution of mechanical or energy-based instruments [66,67,68]. However, uterine sparing procedures may require subsequent reinterventions [66,69].
5. Hysteroscopy for Perimenopausal AUB: Impact on QoL and Sexuality
When considering the mutual relation between hysteroscopy, AUB, QoL, and sexuality, it is necessary to address the interplay of the following factors: bleeding (with/without anemia), underlying conditions (e.g., menopausal status, cancer), treatments (effectiveness, discomfort caused during the procedure), over- or undertreatment (need to perform surgical procedures), age and menopausal status (regardless of AUB or its treatments), and the controversy about the impact of hysterectomy (regarding uterus-preserving techniques) on QoL and sexuality.
Furthermore, the impact of AUB on individual QoL and sexuality should be seen in the broader context of the menopausal transition, a phase marked by hormonal, physical, mental, sexual, biological, and social changes and an increasing prevalence of non-gynecological diseases. All of these changes can potentially impact women’s QoL and sexuality negatively. More than 80% of perimenopausal women experience complaints affecting QoL [70], and more than 60% of them suffer from three or more symptoms [71]. Additionally, of women aged 40–45 years, 32% experience heavy menstrual bleeding. These women have significantly worse QoL [72]. Perimenopausal symptoms critically impairing QoL include sleep disturbances, fatigue, and anxiety [71]. AUB can further compromise the overall health status and the health-related QoL (HRQOL), its sequelae (e.g., iron deficiency anemia, chronic fatigue), or associated comorbidities (e.g., pelvic pain) [62,72,73,74,75,76]. The treatment of endometriosis, myomas, and polyps leads to the best HRQoL scores, while treating nonspecific pelvic pain and AUB is less frequently associated with HRQoL improvement [75]. Post-AUB anemia has a detrimental impact on a woman’s QoL by interfering with physical activity, vitality, cognition, work performance, and social and emotional life [62,73,77]. In addition, age alone is one of the strongest independent factors compromising the QoL in women with AUB.
Lastly, it is essential to highlight that HRQoL is substantially reduced in patients experiencing the combination of AUB and pain [73]. AUB associated with uterine fibroids is responsible for a significantly lower HRQoL than women without fibroids [78]. In premenopausal patients with menorrhagia, concomitant pain, mood changes/fatigue, and general malaise are the most bothersome concomitant symptoms in 27.4%, 17.4%, and 10.8% of patients, respectively [74].
As well as physical complaints, fear of cancer is also common among women with AUB. Timmermanns et al. [79] reported that 100% of postmenopausal women presenting with bleeding wanted to be sure that cancer is not the cause. Only 5% of women were willing to accept more than a 5% risk of cancer and not having intervention. Moreover, if the risk of recurrent bleeding due to benign disease exceeded 25%, most women would prefer immediate treatment of benign lesions instead of surveillance [79]. Since age is an independent risk factor for malignancy, several international boards (e.g., British, American, Canadian) recommend endometrial biopsies in women over 40 or 45 who have failed conservative treatments [18].
Once pregnancy, neoplasia, or hyperplasia are excluded, AUB treatment should always aim to improve the individual’s QoL [76,80]. For a reliable assessment of QoL, psychological well-being, or sexual function, the use of validated psychometric tools such as SF36 [73,80,81], WBQ-12 [82], or FSFI [70] is recommended. Vitale et al. [80] proposed a three-step multidisciplinary approach to AUB in perimenopausal women, considering at each stage the impact of the disease itself, the diagnostic approach, and specific treatment options. Table 1 provides an overview of this approach.
6. Procedure-Specific Factors Influencing QoL
Hysteroscopy is generally well accepted by patients; however, fear of the procedure and anticipated pain are the two most important factors. Sorrentino et al. [83] reported that 27% of women undergoing outpatient hysteroscopy had mild pain, 33% moderate pain, and 40% severe pain during the procedure. In 43% of those patients, analgesia was required. Preprocedural anxiety and perceived stress levels negatively affect the pain experienced during and soon after office hysteroscopy [83,84]. Among the non-modifiable factors, patient age and menopausal status positively correlate with hysteroscopy-related pain [85]. Of the modifiable factors, the length of waiting time before the procedure negatively influences pain perception [84,85]. The longer the time, the worse the pain reported by the patients during the procedure. Therefore, reducing waiting times, providing detailed information about the method, and reassuring the patient that the operation will be aborted at her request are effective measures to reduce anxiety and alleviate the pain during hysteroscopy [80,84,86]. The use of multimedia has improved patient comprehension, reduced fear about the procedure, and increased patient satisfaction. Before office hysteroscopy, a video-based multimedia informative session was the preferred method for lowering preprocedural anxiety and improving patients’ satisfaction [87].
The individualized approach to AUB should always prioritize patients’ preferences. ER/EA improves HRQoL and high satisfaction rates in women with AUB [88]. The complete resolution of AUB symptoms after hysteroscopic polypectomy ranges from 73 to 100% at follow-up intervals between 2 and 52 months [52,89]. Similarly, in women suffering from AUB due to intrauterine polyps or myomas, hysteroscopic removal using the MyoSure® device in an outpatient setting provided significant and sustained HRQoL improvements up to 12 months after the procedure [90]. As reported by Laughlin-Tommaso et al. [91], hysteroscopic myomectomy enables fast return to daily activities, substantial improvements in short-term HRQoL, and lowering of the severity of the symptoms comparable to the outcomes of the laparoscopic or abdominal approaches. Nevertheless, the reliability of the cited analysis is questionable because the mean number and diameter of the myomas removed hysteroscopically were lower than those removed with laparoscopic or abdominal procedures. Following hysteroscopic myomectomy, the proportion of women with persistent symptoms dropped from 92% to 51%, and the overall burden was reduced by half [91].
6.1. AUB, Hysteroscopy, and Sexuality
The impact of AUB on sexual function during perimenopause is barely addressed in the literature. It is known that sexuality in perimenopause is influenced by changes in partnership, body image, general health, hormonal factors (estrogen and testosterone), increased prevalence of depressed mood, sleep disturbances, and vaginal dryness [92]. In the study of Trento et al. [92], 64% of women aged 40 to 65 years were at risk of sexual dysfunction, with lower scores in the domains of sexual desire and interest, comfort, orgasm, and satisfaction. Similarly, 70% of Polish perimenopausal women reported sexual dysfunction when assessed by FSFI [70]. However, impaired sexual function is less distressing for menopausal than for premenopausal women [93]. The additional contribution of AUB to sexual health is underreported. In women in late reproductive age suffering from AUB, the scores for sexual function reached only 55–69 out of 100 [94]. Marnach et al. [95] demonstrated that female sexual function improved and personal distress decreased after ER/EA for AUB. In contrast, Zhang et al. [96] compared the impact of laparoscopic and hysteroscopic myomectomy on sexual desire, sexual arousal, vaginal lubrication, orgasm, sexual satisfaction, and sexual intercourse pain in women at 3 and 6 months after the operation and did not observe any change before and after treatment independently of surgical modality or patients age. Notably, similar levels of sexual activity and sexual satisfaction were reported in women who underwent hysterectomy and hysteroscopic techniques [97,98,99].
6.2. Hysterectomy versus ER/EA
The role of QoL and the importance of an individualized approach to AUB is essential when offering uterine-preserving treatment for AUB versus hysterectomy. Hysterectomy is not the only treatment for AUB due to benign pathology. Therefore, the patient’s preferences and concerns, short- and long-term complications, and benefits of each treatment, including AUB recurrence, anemia, or progression from endometrial hyperplasia to carcinoma, and the implications for quality of life and sexuality, should be discussed during patient’s counseling and decision making. ER/EA is a minimally invasive alternative to hysterectomy for patients wishing to preserve their uterus [67]. However, according to the latest Cochrane Review, ER/EA results in lower resolution rates of symptoms, less patient satisfaction, and similar rates of serious adverse events compared to both open and minimally invasive hysterectomy. In addition, EA/ER results in poorer QoL and up to a 7.5-fold increased risk of further surgical intervention due to treatment failure compared to hysterectomy [66]. In an English cohort study of 114,910 women undergoing ER/EA, 16.7% had at least one subsequent procedure within five years [69]. Nonetheless, ER/EA shortens the return time to normal activities compared to open hysterectomy [66].
Regarding hysterectomy, postoperative complications (Clavien-Dindo grade ≥ III) and reoperations associated with hysterectomy are currently reported at 4% and 2.1%, respectively [65]. A complication usually related to sexual intercourse is vaginal cuff dehiscence, observed after 0.6–1.35% of TLHs and 1.6% of robotic hysterectomies [64]. The higher costs of hysterectomy and the longer duration of hospital stays should be outweighed against long-term costs related to AUB (anemia, fatigue, repeated treatments, etc.). Both hysteroscopic surgery and hysterectomy significantly reduce anxiety and depression without differences in women’s mental health at 12 months after surgery [97]. Additionally, psychological and social outcomes improve substantially one year after endometrial resection and hysterectomy without a significant difference between the treatment groups [100].
A recently published 10-year follow-up study confirmed that hysterectomy produces the most significant improvement in decreasing stress, discomfort and symptoms, and sexual activity, both in the short- and long-term [101]. Hysterectomy performed for benign conditions has beneficial effects on sexual function and overall well-being, regardless of the surgical technique [67,102,103,104], although 10–20% of women may experience deterioration in sexual function, such as dyspareunia or an altered orgasmic response [102]. In a survey performed 12 months after hysterectomy, over 96% of women did not regret having had the hysterectomy [105]. Radosa et al. [103] prospectively examined sexual function in patients undergoing vaginal, laparoscopic supracervical (LSH), and total laparoscopic hysterectomy (TLH). They found that all procedures led to an improvement in postoperative sexuality without significant differences between the procedures. Improvement in sexual functioning after LSH and TLH were observed by Berlit et al. [104]. The beneficial effects of hysterectomy on QoL and sexual health are likely attributed to the absence of vaginal bleeding, coital pain, and contraception-related issues [102].
6.3. Role of Hysteroscopy in Individualized Treatment of Uterine Malignancies
Lastly, the hysteroscopic treatments in cases of atypical endometrial hyperplasia [106], EC [107], or rare uterine tumors [108] have been reported. If uterus-sparing treatment of atypical endometrial hyperplasia or EC is considered, hysteroscopy constitutes both the primary surgical modality and, in combination with serial endometrial biopsies, the cornerstone of follow-up [109,110]. To date, uterus preservation in the presence of malignancy is controversial and cannot be recommended. However, the upcoming molecular classification of EC is expected to support the selection of patients eventually suitable for fertility-sparing treatments of early, low-grade malignancies [111].
7. Conclusions
AUB is a common symptom in perimenopausal women. Accurate diagnosis is essential to rule out pre-malignant or malignant conditions and provide the most appropriate treatment, taking into account the impact of both the disease and treatment on QoL and sexuality. Hysteroscopy offers a minimally invasive way to diagnose and treat intrauterine pathologies, and is well-tolerated, but it carries the potential risk of symptom recurrence and repeated surgeries. In contrast, hysterectomy enables definitive treatment of AUB and underlying conditions and long-standing improvement in QoL and sexuality but is more invasive than hysteroscopic treatments. In order to make the best treatment choice for the individual patients, short-term and long-term goals, including patient safety, QoL, and sexual well-being should always be considered.
Author Contributions
Conceptualization, S.G.V. and R.W.; writing—original draft preparation, R.W. and S.G.V.; writing—review and editing, S.G.V., R.W., F.B., M.N.D., J.C., T.S., I.K., E.R.-M., L.-T.L., B.U., S.F. and S.A.; visualization, F.B. and S.F.; supervision, S.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors have no relevant financial or non-financial interests to disclose.
References
- World Health Organization. Research on the Menopause in the 1990s: Report of a WHO Scientific Group; WHO Scientific Group on Research on the Menopause in the 1990s, Ed.; WHO Technical Report Series; World Health Organization: Geneva, Switzerland, 1996; ISBN 978-92-4-120866-6. [Google Scholar]
- Shapley, M.; Blagojevic-Bucknall, M.; Jordan, K.; Croft, P. The epidemiology of self-reported intermenstrual and postcoital bleeding in the perimenopausal years. BJOG Int. J. Obstet. Gynaecol. 2013, 120, 1348–1355. [Google Scholar] [CrossRef] [PubMed]
- Paramsothy, P.; Harlow, S.D.; Greendale, G.A.; Gold, E.B.; Crawford, S.L.; Elliott, M.R.; Lisabeth, L.D.; Randolph, J.F. Bleeding patterns during the menopausal transition in the multi-ethnic Study of Women’s Health Across the Nation (SWAN): A prospective cohort study. BJOG Int. J. Obstet. Gynaecol. 2014, 121, 1564–1573. [Google Scholar] [CrossRef] [PubMed]
- Carugno, J. Clinical management of vaginal bleeding in postmenopausal women. Climacteric 2020, 23, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, S.R. Appropriate evaluation of postmenopausal bleeding. Menopause 2018, 25, 1476–1478. [Google Scholar] [CrossRef]
- Munro, M.G.; Critchley, H.O.; Broder, M.S.; Fraser, I.S. FIGO Working Group on Menstrual Disorders FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int. J. Gynecol. Obstet. 2011, 113, 3–13. [Google Scholar] [CrossRef]
- Munro, M.G.; Critchley, H.O.; Fraser, I.S.; The FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int. J. Gynecol. Obstet. 2018, 143, 393–408. [Google Scholar] [CrossRef]
- Chodankar, R.; Critchley, H.O.D. Biomarkers in abnormal uterine bleeding. Biol. Reprod. 2018, 101, 1155–1166. [Google Scholar] [CrossRef]
- Soja, M.; Masternak, M.; Piwowarczyk, I.; Janas, Ł.; Szyłło, K.; Nowak, M. Analysis of the results of invasive diagnostic procedures in patients referred to gynecologic department due to abnormal uterine bleeding. Menopausal Rev. 2020, 19, 155–159. [Google Scholar] [CrossRef]
- Kim, M.K.; Jung, Y.S.; Chon, S.J.; Yun, B.H.; Cho, S.; Choi, Y.S.; Lee, B.S.; Seo, S.K. Common Causes of Postmenopausal Bleeding in Korean Women: 10-Year Outcomes from a Single Medical Center. J. Korean Med. Sci. 2017, 32, 830–834. [Google Scholar] [CrossRef]
- Kaveh, M.; Sadegi, K.; Salarzaei, M.; Parooei, F. Comparison of diagnostic accuracy of saline infusion sonohysterography, transvaginal sonography, and hysteroscopy in evaluating the endometrial polyps in women with abnormal uterine bleeding: A systematic review and meta-analysis. Videosurgery Other Miniinvasive Tech. 2020, 15, 403–415. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Y.; Mao, L.; Wen, J.; Bai, W. Prevalence of abnormal uterine bleeding according to new International Federation of Gynecology and Obstetrics classification in Chinese women of reproductive age. Medicine 2018, 97, e11457. [Google Scholar] [CrossRef] [PubMed]
- Johnatty, S.E.; Stewart, C.J.R.; Smith, D.; Nguyen, A.; Dwyer, J.O.; O’Mara, T.A.; Webb, P.M.; Spurdle, A.B. Co-existence of leiomyomas, adenomyosis and endometriosis in women with endometrial cancer. Sci. Rep. 2020, 10, 362. [Google Scholar] [CrossRef] [PubMed]
- Bosch, T.V.D.; Verbakel, J.Y.; Valentin, L.; Wynants, L.; De Cock, B.; Pascual, M.A.; Leone, F.P.G.; Sladkevicius, P.; Alcazar, J.L.; Votino, A.; et al. Typical ultrasound features of various endometrial pathologies described using International Endometrial Tumor Analysis (IETA) terminology in women with abnormal uterine bleeding. Ultrasound Obstet. Gynecol. 2021, 57, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Von Mackensen, S. Quality of life in women with bleeding disorders. Haemophilia 2011, 17, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Tower, C. Pregnancy in peri- and postmenopausal women: Challenges in management. Menopause Int. 2009, 15 (Suppl. 1), 165–168. [Google Scholar] [CrossRef]
- Andersen, A.-M.N.; Wohlfahrt, J.; Christens, P.; Olsen, J.; Melbye, M. Maternal age and fetal loss: Population based register linkage study. BMJ 2000, 320, 1708–1712. [Google Scholar] [CrossRef]
- Levy-Zauberman, Y.; Pourcelot, A.-G.; Capmas, P.; Fernandez, H. Update on the management of abnormal uterine bleeding. J. Gynecol. Obstet. Hum. Reprod. 2017, 46, 613–622. [Google Scholar] [CrossRef]
- Weisberg, E.; McGeehan, K.; Fraser, I.S. Effect of perceptions of menstrual blood loss and menstrual pain on women’s quality of life. Eur. J. Contracept. Reprod. Health Care 2016, 21, 431–435. [Google Scholar] [CrossRef]
- Magnay, J.L.; O’Brien, S.; Gerlinger, C.; Seitz, C. Pictorial methods to assess heavy menstrual bleeding in research and clinical practice: A systematic literature review. BMC Women’s Health 2020, 20, 24. [Google Scholar] [CrossRef]
- Kostov, S.; Watrowski, R.; Kornovski, Y.; Dzhenkov, D.; Slavchev, S.; Ivanova, Y.; Yordanov, A. Hereditary Gynecologic Cancer Syndromes—A Narrative Review. Onco Targets Ther. 2022, 15, 381–405. [Google Scholar] [CrossRef]
- Vitale, S.G.; Riemma, G.; Pacheco, L.A.; Carugno, J.; Haimovich, S.; Tesarik, J.; De Angelis, M.C.; Sardo, A.D.S.; De Franciscis, P. Hysteroscopic endometrial biopsy: From indications to instrumentation and techniques. A call to action. Minim. Invasive Ther. Allied Technol. 2021, 30, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Borges, P.C.D.G.; Dias, R.; Machado, R.B.; Borges, J.B.R.; Spadoto-Dias, D. Transvaginal Ultrasonography and Hysteroscopy as Predictors of Endometrial Polyps in Postmenopause. Women’s Health 2015, 11, 29–33. [Google Scholar] [CrossRef]
- Hill, M.J.; Levens, E.D.; Decherney, A.H. Committee on Practice Bulletins—Gynecology Practice Bulletin No. 128. Obstet. Gynecol. 2012, 120, 197–206. [Google Scholar] [CrossRef]
- Maheux-Lacroix, S.; Li, F.; Laberge, P.Y.; Abbott, J. Imaging for Polyps and Leiomyomas in Women with Abnormal Uterine Bleeding. Obstet. Gynecol. 2016, 128, 1425–1436. [Google Scholar] [CrossRef]
- Vroom, A.J.; Timmermans, A.; Bongers, M.Y.; Heuvel, E.R.V.D.; Geomini, P.M.A.J.; Van Hanegem, N. Diagnostic accuracy of saline contrast sonohysterography in detecting endometrial polyps in women with postmenopausal bleeding: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2019, 54, 28–34. [Google Scholar] [CrossRef]
- The Use of Hysteroscopy for the Diagnosis and Treatment of Intrauterine Pathology. Obstet. Gynecol. 2020, 135, 754–756. [CrossRef]
- Carugno, J.; Grimbizis, G.; Franchini, M.; Alonso, L.; Bradley, L.; Campo, R.; Catena, U.; De Angelis, C.; Sardo, A.D.S.; Farrugia, M.; et al. International Consensus Statement for recommended terminology describing hysteroscopic procedures. Facts Views Vis. ObGyn 2021, 13, 287–294. [Google Scholar] [CrossRef]
- Nagele, F.; O’Connor, H.; Davies, A.; Badawy, A.; Mohamed, H.; Magos, A. 2500 Outpatient diagnostic hysteroscopies. Obstet. Gynecol. 1996, 88, 87–92. [Google Scholar] [CrossRef]
- Nappi, L.; Sorrentino, F.; Angioni, S.; Pontis, A.; Litta, P.; Greco, P. Feasibility of hysteroscopic endometrial polypectomy using a new dual wavelengths laser system (DWLS): Preliminary results of a pilot study. Arch. Gynecol. Obstet. 2017, 295, 3–7. [Google Scholar] [CrossRef]
- Sardo, A.D.S.; Bettocchi, S.; Spinelli, M.; Guida, M.; Nappi, L.; Angioni, S.; Fernandez, L.M.S.; Nappi, C. Review of New Office-Based Hysteroscopic Procedures 2003–2009. J. Minim. Invasive Gynecol. 2010, 17, 436–448. [Google Scholar] [CrossRef]
- Vitale, S.G.; Bruni, S.; Chiofalo, B.; Riemma, G.; Lasmar, R.B. Updates in office hysteroscopy: A practical decalogue to perform a correct procedure. Updat. Surg. 2020, 72, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Giampaolino, P.; Della Corte, L.; Di Filippo, C.; Mercorio, A.; Vitale, S.G.; Bifulco, G. Office hysteroscopy in the management of women with postmenopausal bleeding. Climacteric 2020, 23, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Vitale, S.G.; Haimovich, S.; Riemma, G.; Ludwin, A.; Zizolfi, B.; De Angelis, M.C.; Carugno, J. Innovations in hysteroscopic surgery: Expanding the meaning of “in-office”. Minim. Invasive Ther. Allied Technol. 2021, 30, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Fagioli, R.; Vitagliano, A.; Carugno, J.; Castellano, G.; De Angelis, M.C.; Sardo, A.D.S. Hysteroscopy in postmenopause: From diagnosis to the management of intrauterine pathologies. Climacteric 2020, 23, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Vitale, S.G.; Riemma, G.; Ciebiera, M.; Cianci, S. Hysteroscopic treatment of submucosal fibroids in perimenopausal women: When, why, and how? Climacteric 2020, 23, 355–359. [Google Scholar] [CrossRef]
- Vitale, S.G. The Biopsy Snake Grasper Sec. VITALE: A New Tool for Office Hysteroscopy. J. Minim. Invasive Gynecol. 2020, 27, 1414–1416. [Google Scholar] [CrossRef]
- Cobellis, L.; Castaldi, M.A.; Giordano, V.; De Franciscis, P.; Signoriello, G.; Colacurci, N. Is it possible to predict office hysteroscopy failure? Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 181, 328–333. [Google Scholar] [CrossRef]
- Saccardi, C.; Vitagliano, A.; Marchetti, M.; Turco, A.L.; Tosatto, S.; Palumbo, M.; De Lorenzo, L.S.; Vitale, S.G.; Scioscia, M.; Noventa, M. Endometrial Cancer Risk Prediction According to Indication of Diagnostic Hysteroscopy in Post-Menopausal Women. Diagnostics 2020, 10, 257. [Google Scholar] [CrossRef]
- Clark, T.J.; Voit, D.; Gupta, J.K.; Hyde, C.; Song, F.; Khan, K.S. Accuracy of Hysteroscopy in the Diagnosis of Endometrial Cancer and Hyperplasia. JAMA 2002, 288, 1610–1621. [Google Scholar] [CrossRef]
- Elfayomy, A.K.; Habib, F.A.; Alkabalawy, M.A. Role of hysteroscopy in the detection of endometrial pathologies in women presenting with postmenopausal bleeding and thickened endometrium. Arch. Gynecol. Obstet. 2012, 285, 839–843. [Google Scholar] [CrossRef]
- Garuti, G.; Cellani, F.; Garzia, D.; Colonnelli, M.; Luerti, M. Accuracy of hysteroscopic diagnosis of endometrial hyperplasia: A retrospective study of 323 patients. J. Minim. Invasive Gynecol. 2005, 12, 247–253. [Google Scholar] [CrossRef] [PubMed]
- De Franciscis, P.; Riemma, G.; Schiattarella, A.; Cobellis, L.; Guadagno, M.; Vitale, S.G.; Mosca, L.; Cianci, A.; Colacurci, N. Concordance between the Hysteroscopic Diagnosis of Endometrial Hyperplasia and Histopathological Examination. Diagnostics 2019, 9, 142. [Google Scholar] [CrossRef] [PubMed]
- Tinelli, R.; Tinelli, F.G.; Cicinelli, E.; Malvasi, A.; Tinelli, A. The role of hysteroscopy with eye-directed biopsy in postmenopausal women with uterine bleeding and endometrial atrophy. Menopause 2008, 15, 737–742. [Google Scholar] [CrossRef]
- Wanderley, M.D.S.; Álvares, M.M.; Vogt, M.D.F.B.; Sazaki, L.M.P. Accuracy of Transvaginal Ultrasonography, Hysteroscopy and Uterine Curettage in Evaluating Endometrial Pathologies. Rev. Bras. Ginecol. Obstet. 2016, 38, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Vitale, S.G.; Laganà, A.S.; Török, P.; Lasmar, R.B.; Carugno, J.; Palumbo, M.; Tesarik, J. Virtual sonographic hysteroscopy in assisted reproduction: A retrospective cost-effectiveness analysis. Int. J. Gynecol. Obstet. 2022, 156, 112–118. [Google Scholar] [CrossRef]
- Vilos, G.A. Correspondence. Fertil. Steril. 1999, 72, 740–743. [Google Scholar] [CrossRef]
- Sardo, A.D.S.; Mazzon, I.; Bramante, S.; Bettocchi, S.; Bifulco, G.; Guida, M.; Nappi, C. Hysteroscopic myomectomy: A comprehensive review of surgical techniques. Hum. Reprod. Updat. 2008, 14, 101–119. [Google Scholar] [CrossRef]
- Vitale, S.G.; Laganà, A.S.; Caruso, S.; Garzon, S.; Vecchio, G.M.; La Rosa, V.L.; Casarin, J.; Ghezzi, F. Comparison of three biopsy forceps for hysteroscopic endometrial biopsy in postmenopausal patients (HYGREB-1): A multicenter, single-blind randomized clinical trial. Int. J. Gynecol. Obstet. 2021, 155, 425–432. [Google Scholar] [CrossRef]
- Luerti, M.; Vitagliano, A.; Sardo, A.D.S.; Angioni, S.; Garuti, G.; De Angelis, C.; Del Zoppo, S.; Dealberti, D.; Nappi, L.; Perrini, G.; et al. Effectiveness of Hysteroscopic Techniques for Endometrial Polyp Removal: The Italian Multicenter Trial. J. Minim. Invasive Gynecol. 2019, 6, 1169–1176. [Google Scholar] [CrossRef]
- Wortman, M.; Daggett, A.; Ball, C. Operative Hysteroscopy in an Office-Based Surgical Setting: Review of Patient Safety and Satisfaction in 414 Cases. J. Minim. Invasive Gynecol. 2013, 20, 56–63. [Google Scholar] [CrossRef]
- Clark, T.J.; Middleton, L.J.; Cooper, N.A.; Diwakar, L.; Denny, E.; Smith, P.; Gennard, L.; Stobert, L.; E Roberts, T.; Cheed, V.; et al. A randomised controlled trial of Outpatient versus inpatient Polyp Treatment (OPT) for abnormal uterine bleeding. Health Technol. Assess. 2015, 19, 1–194. [Google Scholar] [CrossRef] [PubMed]
- Scheiber, M.D.; Chen, S.H. A Prospective Multicenter Registry of Patients Undergoing Hysteroscopic Morcellation of Uterine Polyps and Myomas. J. Gynecol. Surg. 2016, 32, 318–323. [Google Scholar] [CrossRef]
- Famada, A.V.; Plans, R.C.; Canals, L.C.; Torrijos, M.R.; Vicente, A.R.; Albadalejo, A.B. Outcomes of surgical hysteroscopy: 25 years of observational study. J. Obstet. Gynaecol. 2021, 1–5. [Google Scholar] [CrossRef] [PubMed]
- MacLean-Fraser, E.; Penava, D.; Vilos, G.A. Perioperative Complication Rates of Primary and Repeat Hysteroscopic Endometrial Ablations. J. Am. Assoc. Gynecol. Laparosc. 2002, 9, 175–177. [Google Scholar] [CrossRef]
- Jo, Y.Y.; Jeon, H.J.; Choi, E.; Choi, Y.-S. Extreme hyponatremia with moderate metabolic acidosis during hysteroscopic myomectomy -A case report-. Korean J. Anesthesiol. 2011, 60, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Van Dijck, C.; Timmerman, D.; Teunkens, A.; Rex, S.; Verguts, J.; Van De Velde, M. Venous Air Embolism during Hysteroscopic Myomectomy: An Analysis of 7 Cases. Gynecol. Obstet. Investig. 2017, 82, 569–574. [Google Scholar] [CrossRef]
- Vilos, G.A.; Hutson, J.R.; Singh, I.S.; Giannakopoulos, F.; Abu Rafea, B.; Vilos, A.G. Venous Gas Embolism during Hysteroscopic Endometrial Ablation: Report of 5 Cases and Review of the Literature. J. Minim. Invasive Gynecol. 2020, 27, 748–754. [Google Scholar] [CrossRef]
- Tammam, A.E. Comparative Study between Monopolar Electrodes and Bipolar Electrodes in Hysteroscopic Surgery. J. Clin. Diagn. Res. 2015, 9, QC11-1. [Google Scholar] [CrossRef]
- De Franciscis, P.; Grauso, F.; Cobellis, L.; Messalli, E.M.; Cucinella, G.; Perino, A.; Colacurci, N.; Torella, M. Outcomes of monopolar versus bipolar endometrial ablation on uterine bleeding and psychophysical wellbeing. Minerva Ginecol. 2017, 69, 328–335. [Google Scholar] [CrossRef]
- Vitale, S.G.; Riemma, G.; Carugno, J.; Perez-Medina, T.; Pacheco, L.A.; Haimovich, S.; Parry, J.P.; Sardo, A.D.S.; De Franciscis, P. Postsurgical barrier strategies to avoid the recurrence of intrauterine adhesion formation after hysteroscopic adhesiolysis: A network meta-analysis of randomized controlled trials. Am. J. Obstet. Gynecol. 2022, 226, 487–498.e8. [Google Scholar] [CrossRef]
- Peuranpää, P.; Heliövaara-Peippo, S.; Fraser, I.; Paavonen, J.; Hurskainen, R. Effects of anemia and iron deficiency on quality of life in women with heavy menstrual bleeding. Acta Obstet. Gynecol. Scand. 2014, 93, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Reich, H.; Ribeiro, S.C.; Vidali, A. Hysterectomy as treatment for dysfunctional uterine bleeding. Best Pr. Res. Clin. Obstet. Gynaecol. 1999, 13, 251–269. [Google Scholar] [CrossRef] [PubMed]
- Watrowski, R.; Kostov, S.; Alkatout, I. Complications in laparoscopic and robotic-assisted surgery: Definitions, classifications, incidence and risk factors—An up-to-date review. Videosurgery Other Miniinvasive Tech. 2021, 16, 501–525. [Google Scholar] [CrossRef] [PubMed]
- Alliende, R.I.; Carrasco, M.; Levancini, M.; Kovoor, E.; Guzmán-Rojas, R.A.; Miranda-Mendoza, I. 5,926 hysterectomies: Complications described by Clavien–Dindo classification. J. Obstet. Gynaecol. 2021, 41, 1102–1106. [Google Scholar] [CrossRef]
- Rodriguez, M.B.; Lethaby, A.; Fergusson, R.J. Endometrial resection and ablation versus hysterectomy for heavy menstrual bleeding. Cochrane Database Syst. Rev. 2021, 2021, CD000329. [Google Scholar] [CrossRef]
- Vitale, S.G.; Ferrero, S.; Ciebiera, M.; Barra, F.; Török, P.; Tesarik, J.; Vilos, G.A.; Cianci, A. Hysteroscopic endometrial resection vs. hysterectomy for abnormal uterine bleeding: Impact on quality of life and sexuality. Evidence from a systematic review of randomized controlled trials. Curr. Opin. Obstet. Gynecol. 2020, 32, 159–165. [Google Scholar] [CrossRef]
- Vitale, S.G.; Sapia, F.; Rapisarda, A.M.C.; Valenti, G.; Santangelo, F.; Rossetti, D.; Chiofalo, B.; Sarpietro, G.; La Rosa, V.L.; Triolo, O.; et al. Hysteroscopic Morcellation of Submucous Myomas: A Systematic Review. BioMed Res. Int. 2017, 2017, 6848250. [Google Scholar] [CrossRef]
- Bansi-Matharu, L.; Gurol-Urganci, I.; Mahmood, T.; Templeton, A.; van der Meulen, J.; Cromwell, D. Rates of subsequent surgery following endometrial ablation among English women with menorrhagia: Population-based cohort study. BJOG Int. J. Obstet. Gynaecol. 2013, 120, 1500–1507. [Google Scholar] [CrossRef]
- Dąbrowska-Galas, M.; Dąbrowska, J.; Michalski, B. Sexual Dysfunction in Menopausal Women. Sex. Med. 2019, 7, 472–479. [Google Scholar] [CrossRef]
- Greenblum, C.A.; Rowe, M.A.; Neff, D.F.; Greenblum, J.S. Midlife women. Menopause 2013, 20, 22–27. [Google Scholar] [CrossRef]
- Karlsson, T.S.; Marions, L.B.; Edlund, M.G. Heavy menstrual bleeding significantly affects quality of life. Acta Obstet. Gynecol. Scand. 2014, 93, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Shankar, M.; Chi, C.; Kadir, R.A. Review of quality of life: Menorrhagia in women with or without inherited bleeding disorders. Haemophilia 2007, 14, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Santer, M.; Wyke, S.; Warner, P. What aspects of periods are most bothersome for women reporting heavy menstrual bleeding? Community survey and qualitative study. BMC Women’s Health 2007, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Pynnä, K.; Räsänen, P.; Sintonen, H.; Roine, R.P.; Vuorela, P. The health-related quality of life of patients with a benign gynecological condition: A 2-year follow-up. J. Comp. Eff. Res. 2021, 10, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Doan, Q.V.; Blumenthal, P.; Dubois, R.W. A Systematic Review Evaluating Health-Related Quality of Life, Work Impairment, and Health-Care Costs and Utilization in Abnormal Uterine Bleeding. Value Health 2007, 10, 183–194. [Google Scholar] [CrossRef]
- Gokyildiz, S.; Aslan, E.; Beji, N.K.; Mecdi, M. The Effects of Menorrhagia on Women’s Quality of Life: A Case-Control Study. ISRN Obstet. Gynecol. 2013, 2013, 918179. [Google Scholar] [CrossRef][Green Version]
- Williams, V.S.; Jones, G.; Mauskopf, J.; Spalding, J.; DuChane, J. Uterine Fibroids: A Review of Health-Related Quality of Life Assessment. J. Women’s Health 2006, 15, 818–829. [Google Scholar] [CrossRef]
- Timmermans, A.; Opmeer, B.; Veersema, S.; Mol, B. Patients’ preferences in the evaluation of postmenopausal bleeding. BJOG: Int. J. Obstet. Gynaecol. 2007, 114, 1146–1149. [Google Scholar] [CrossRef]
- Vitale, S.G.; Caruso, S.; Carugno, J.; Ciebiera, M.; Barra, F.; Ferrero, S.; Cianci, A. Quality of life and sexuality of postmenopausal women with intrauterine pathologies: A recommended three-step multidisciplinary approach focusing on the role of hysteroscopy. Minim. Invasive Ther. Allied Technol. 2021, 30, 317–325. [Google Scholar] [CrossRef]
- Sima, R.-M.; Pleş, L.; Socea, B.; Sklavounos, P.; Negoi, I.; Stănescu, A.-D.; Iordache, I.-I.; Hamoud, B.H.; Radosa, M.P.; Juhasz-Boess, I.; et al. Evaluation of the SF-36 questionnaire for assessment of the quality of life of endometriosis patients undergoing treatment: A systematic review and meta-analysis. Exp. Ther. Med. 2021, 22, 1283. [Google Scholar] [CrossRef]
- Watrowski, R.; Rohde, A. Psychological well-being of gynecologic and obstetric patients: A validation of the 12-item Well-Being Questionnaire (W-BQ12). Wien. Klin. Wochenschr. 2014, 126, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, F.; Petito, A.; Angioni, S.; D’Antonio, F.; Severo, M.; Solazzo, M.C.; Tinelli, R.; Nappi, L. Impact of anxiety levels on the perception of pain in patients undergoing office hysteroscopy. Arch. Gynecol. Obstet. 2021, 303, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Kokanali, M.K.; Cavkaytar, S.; Guzel, A.I.; Topçu, H.O.; Eroğlu, E.; Aksakal, O.; Doğanay, M. Impact of preprocedural anxiety levels on pain perception in patients undergoing office hysteroscopy. J. Chin. Med. Assoc. 2014, 77, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Carta, G.; Palermo, P.; Marinangeli, F.; Piroli, A.; Necozione, S.; De Lellis, V.; Patacchiola, F. Waiting Time and Pain During Office Hysteroscopy. J. Minim. Invasive Gynecol. 2012, 19, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Vitale, S.G.; Caruso, S.; Ciebiera, M.; Török, P.; Tesarik, J.; Vilos, G.A.; Cholkeri-Singh, A.; Gulino, F.A.; Kamath, M.S.; Cianci, A. Management of anxiety and pain perception in women undergoing office hysteroscopy: A systematic review. Arch. Gynecol. Obstet. 2020, 301, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Akca, A.; Yilmaz, G.; Esmer, A.; Yuksel, S.; Koroglu, N.; Cetin, B.A. Use of video-based multimedia information to reduce anxiety before office hysteroscopy. Videosurgery Other Miniinvasive Tech. 2020, 15, 329–336. [Google Scholar] [CrossRef]
- El-Nashar, S.A.; Hopkins, M.R.; Barnes, S.A.; Pruthi, R.K.; Gebhart, J.B.; Cliby, W.A.; Famuyide, A.O. Health-related quality of life and patient satisfaction after global endometrial ablation for menorrhagia in women with bleeding disorders: A follow-up survey and systematic review. Am. J. Obstet. Gynecol. 2010, 202, 348.e1–348.e7. [Google Scholar] [CrossRef]
- Nathani, F.; Clark, T.J. Uterine polypectomy in the management of abnormal uterine bleeding: A systematic review. J. Minim. Invasive Gynecol. 2006, 13, 260–268. [Google Scholar] [CrossRef]
- Rubino, R.J.; Lukes, A.S. Twelve-Month Outcomes for Patients Undergoing Hysteroscopic Morcellation of Uterine Polyps and Myomas in an Office or Ambulatory Surgical Center. J. Minim. Invasive Gynecol. 2015, 22, 285–290. [Google Scholar] [CrossRef]
- Laughlin-Tommaso, S.K.; Lu, D.; Thomas, L.; Diamond, M.P.; Wallace, K.; Wegienka, G.; Vines, A.I.; Anchan, R.M.; Wang, T.; Maxwell, G.L.; et al. Short-term quality of life after myomectomy for uterine fibroids from the COMPARE-UF Fibroid Registry. Am. J. Obstet. Gynecol. 2020, 222, 345.e1–345.e22. [Google Scholar] [CrossRef]
- Trento, S.R.S.S.; Madeiro, A.; Rufino, A.C. Sexual Function and Associated Factors in Postmenopausal Women. Rev. Bras. Ginecol. Obstet. 2021, 43, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Berra, M.; De Musso, F.; Matteucci, C.; Martelli, V.; Perrone, A.M.; Pelusi, C.; Pelusi, G.; Meriggiola, M.C. The Impairment of Sexual Function Is Less Distressing for Menopausal than for Premenopausal Women. J. Sex. Med. 2010, 7, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Kuppermann, M.; Summitt, R.L.; Varner, R.E.; McNeeley, S.G.; Goodman-Gruen, D.; Learman, L.A.; Ireland, C.C.; Vittinghoff, E.; Lin, F.; Richter, H.E.; et al. Sexual Functioning After Total Compared with Supracervical Hysterectomy: A Randomized Trial. Obstet. Gynecol. 2005, 105, 1309–1318. [Google Scholar] [CrossRef]
- Marnach, M.L.; Long, M.E.; McGree, M.E.; Weaver, A.L.; Casey, P.M. Female Sexual Function Improves After Endometrial Ablation. J. Women’s Health 2016, 25, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gu, X.; Meng, Y.; Guo, H.; Du, J.; Xing, W. Analysis of the effect of laparoscopy and hysteroscopy on ovarian function, immune function and quality of sexual life of patients with hysteromyoma at different ages. Oncol. Lett. 2018, 15, 2929–2934. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.A.; Naji, A.A.; Pinion, S.B.; Mollison, J.; Kitchener, H.C.; E Parkin, D.; Abramovich, D.R.; Russell, I.T. Randomised trial comparing hysterectomy with endometrial ablation for dysfunctional uterine bleeding: Psychiatric and psychosocial aspects. BMJ 1996, 312, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Crosignani, P.G.; Vercellini, P.; Apolone, G.; De Giorgi, O.; Cortesi, I.; Meschia, M. Endometrial resection versus vaginal hysterectomy for menorrhagia: Long-term clinical and quality-of-life outcomes. Am. J. Obstet. Gynecol. 1997, 177, 95–101. [Google Scholar] [CrossRef]
- Aberdeen Endometrial Ablation Trials Group A randomised trial of endometrial ablation versus hysterectomy for the treatment of dysfunctional uterine bleeding: Outcome at four years. BJOG: Int. J. Obstet. Gynaecol. 1999, 106, 360–366. [CrossRef]
- O’Connor, H.; Broadbent, J.A.M.; Magos, A.L.; McPherson, K. Medical Research Council randomised trial of endometrial resection versus hysterectomy in management of menorrhagia. Lancet 1997, 349, 897–901. [Google Scholar] [CrossRef]
- Rahkola-Soisalo, P.; Brummer, T.; Jalkanen, J.; Sjöberg, J.; Sintonen, H.; Roine, R.P.; Härkki, P. Hysterectomy Provides Benefit in Health-Related Quality of Life: A 10-Year Follow-up Study. J. Minim. Invasive Gynecol. 2020, 27, 868–874. [Google Scholar] [CrossRef]
- Lonnée-Hoffmann, R.; Pinas, I. Effects of Hysterectomy on Sexual Function. Curr. Sex. Health Rep. 2014, 6, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Radosa, J.C.; Meyberg-Solomayer, G.; Kastl, C.; Radosa, C.G.; Mavrova, R.; Gräber, S.; Baum, S.; Radosa, M.P. Influences of Different Hysterectomy Techniques on Patients’ Postoperative Sexual Function and Quality of Life. J. Sex. Med. 2014, 11, 2342–2350. [Google Scholar] [CrossRef] [PubMed]
- Berlit, S.; Tuschy, B.; Wuhrer, A.; Jürgens, S.; Buchweitz, O.; Kircher, A.-T.; Sütterlin, M.; Lis, S.; Hornemann, A. Sexual functioning after total versus subtotal laparoscopic hysterectomy. Arch. Gynecol. Obstet. 2018, 298, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Janda, M.; Armfield, N.R.; Kerr, G.; Kurz, S.; Jackson, G.; Currie, J.; Page, K.; Weaver, E.; Yazdani, A.; Obermair, A. Patient-Reported Experiences After Hysterectomy: A Cross-Sectional Study of the Views of Over 2300 Women. J. Patient Exp. 2020, 7, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Edris, F.; Vilos, G.A.; Al-Mubarak, A.; Ettler, H.C.; Hollett-Caines, J.; Abu-Rafea, B. Resectoscopic surgery may be an alternative to hysterectomy in high-risk women with atypical endometrial hyperplasia. J. Minim. Invasive Gynecol. 2007, 14, 68–73. [Google Scholar] [CrossRef]
- Gallo, A.; Catena, U.; Saccone, G.; Sardo, A.D.S. Conservative Surgery in Endometrial Cancer. J. Clin. Med. 2021, 11, 183. [Google Scholar] [CrossRef]
- Watrowski, R.; Jäger, C.; Möckel, J.; Kurz, P.; Schmidt, D.; Freudenberg, N. Hysteroscopic treatment of uterine tumor resembling ovarian sex cord-like tumor (UTROSCT). Gynecol. Endocrinol. 2015, 31, 856–859. [Google Scholar] [CrossRef]
- Giampaolino, P.; Sardo, A.D.S.; Mollo, A.; Raffone, A.; Travaglino, A.; Boccellino, A.; Zizolfi, B.; Insabato, L.; Zullo, F.; De Placido, G.; et al. Hysteroscopic Endometrial Focal Resection followed by Levonorgestrel Intrauterine Device Insertion as a Fertility-Sparing Treatment of Atypical Endometrial Hyperplasia and Early Endometrial Cancer: A Retrospective Study. J. Minim. Invasive Gynecol. 2019, 26, 648–656. [Google Scholar] [CrossRef]
- Casadio, P.; La Rosa, M.; Alletto, A.; Magnarelli, G.; Arena, A.; Fontana, E.; Fabbri, M.; Giovannico, K.; Virgilio, A.; Raimondo, D.; et al. Fertility Sparing Treatment of Endometrial Cancer with and without Initial Infiltration of Myometrium: A Single Center Experience. Cancers 2020, 12, 3571. [Google Scholar] [CrossRef]
- Cavaliere, A.F.; Perelli, F.; Zaami, S.; D’Indinosante, M.; Turrini, I.; Giusti, M.; Gullo, G.; Vizzielli, G.; Mattei, A.; Scambia, G.; et al. Fertility Sparing Treatments in Endometrial Cancer Patients: The Potential Role of the New Molecular Classification. Int. J. Mol. Sci. 2021, 22, 12248. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).