Role of LI-RADS Indeterminate Observations in the Risk of Hepatocellular Carcinoma after HCV Eradication with Direct-Acting Antivirals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Clinical Data
2.3. CT/MRI Technique
2.4. Imaging Analysis
2.5. Statistical Analysis
3. Results
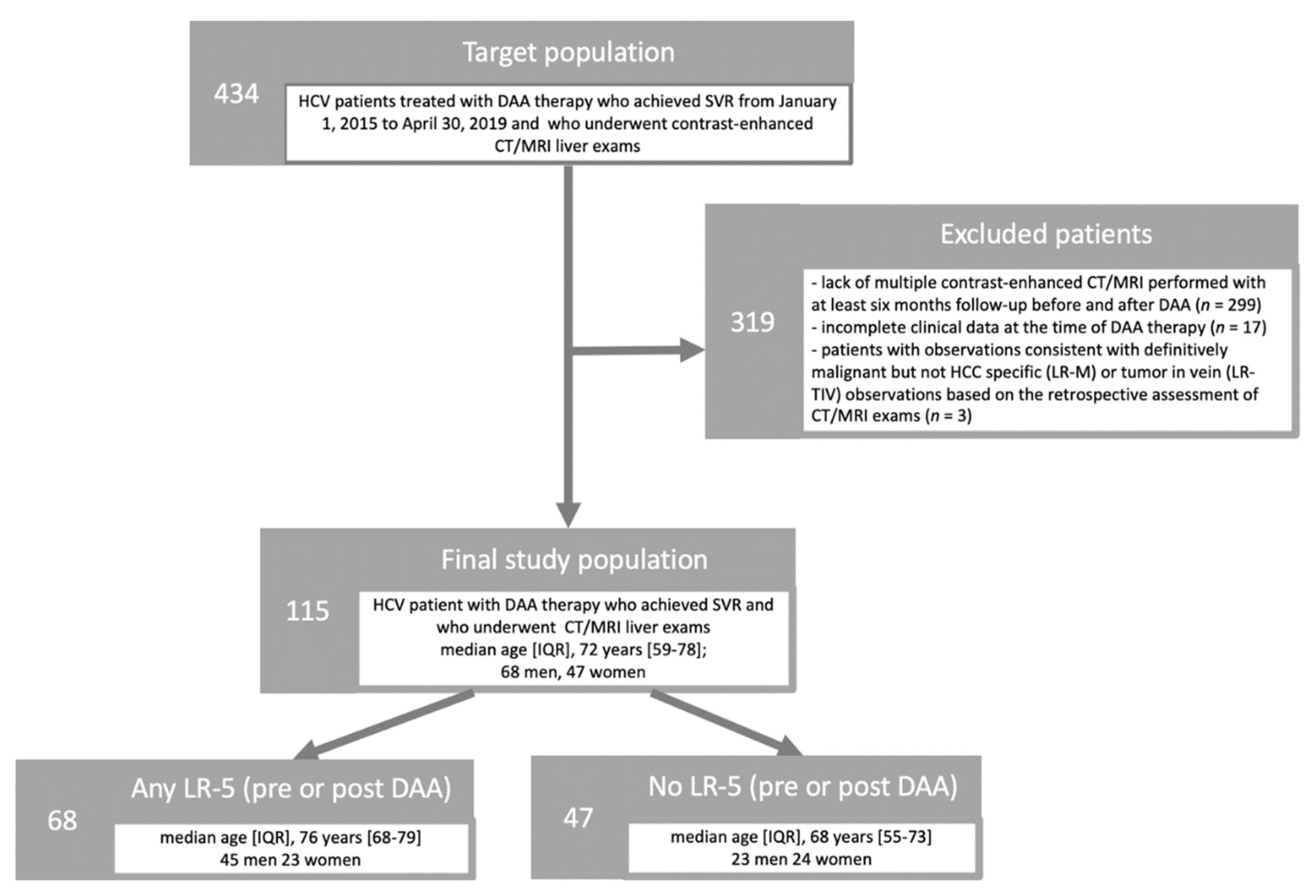
3.1. Study Cohort
3.2. Time to LR-5 Occurrence after DAAs
3.3. Risk Factors for LR-5 after DAAs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Tzartzeva, K.; Obi, J.; Rich, N.E.; Parikh, N.D.; Marrero, J.A.; Yopp, A.; Waljee, A.K.; Singal, A.G. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients with Cirrhosis: A Meta-analysis. Gastroenterology 2018, 154, 1706–1718.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afdhal, N.; Zeuzem, S.; Kwo, P.; Chojkier, M.; Gitlin, N.; Puoti, M.; Romero-Gomez, M.; Zarski, J.-P.; Agarwal, K.; Buggisch, P.; et al. Ledipasvir and Sofosbuvir for Untreated HCV Genotype 1 Infection. N. Engl. J. Med. 2014, 370, 1889–1898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowdley, K.V.; Gordon, S.C.; Reddy, K.R.; Rossaro, L.; Bernstein, D.E.; Lawitz, E.; Shiffman, M.L.; Schiff, E.; Ghalib, R.; Ryan, M.; et al. Ledipasvir and Sofosbuvir for 8 or 12 Weeks for Chronic HCV without Cirrhosis. N. Engl. J. Med. 2014, 370, 1879–1888. [Google Scholar] [CrossRef] [Green Version]
- Conti, F.; Buonfiglioli, F.; Scuteri, A.; Crespi, C.; Bolondi, L.; Caraceni, P.; Foschi, F.G.; Lenzi, M.; Mazzella, G.; Verucchi, G.; et al. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J. Hepatol. 2016, 65, 727–733. [Google Scholar] [CrossRef]
- Calvaruso, V.; Cabibbo, G.; Cacciola, I.; Petta, S.; Madonia, S.; Bellia, A.; Tinè, F.; Distefano, M.; Licata, A.; Giannitrapani, L.; et al. Incidence of Hepatocellular Carcinoma in Patients with HCV-Associated Cirrhosis Treated with Direct-Acting Antiviral Agents. Gastroenterology 2018, 155, 411–421.e4. [Google Scholar] [CrossRef] [Green Version]
- Cabibbo, G.; Celsa, C.; Calvaruso, V.; Petta, S.; Cacciola, I.; Cannavò, M.R.; Madonia, S.; Rossi, M.; Magro, B.; Rini, F.; et al. Direct-acting antivirals after successful treatment of early hepatocellular carcinoma improve survival in HCV-cirrhotic patients. J. Hepatol. 2019, 71, 265–273. [Google Scholar] [CrossRef]
- Persico, M.; Aglitti, A.; Aghemo, A.; Rendina, M.; Lleo, A.; Ciancio, A.; Di Marco, V.; Lampertico, P.; Brunetto, M.R.; Zuin, M.; et al. High efficacy of direct-acting anti-viral agents in hepatitis C virus-infected cirrhotic patients with successfully treated hepatocellular carcinoma. Aliment. Pharmacol. Ther. 2018, 47, 1705–1712. [Google Scholar] [CrossRef] [Green Version]
- Galati, G.; Muley, M.; Viganò, M.; Iavarone, M.; Vitale, A.; Dell’Unto, C.; Lai, Q.; Cabibbo, G.; Sacco, R.; Villa, E.; et al. Occurrence of hepatocellular carcinoma after direct-acting antiviral therapy for hepatitis C virus infection: Literature review and risk analysis. Expert Opin. Drug Saf. 2019, 18, 603–610. [Google Scholar] [CrossRef]
- Ogawa, E.; Nomura, H.; Nakamuta, M.; Furusyo, N.; Kajiwara, E.; Dohmen, K.; Kawano, A.; Ooho, A.; Azuma, K.; Takahashi, K.; et al. Incidence of Hepatocellular Carcinoma after Treatment with Sofosbuvir-Based or Sofosbuvir-Free Regimens in Patients with Chronic Hepatitis C. Cancers 2020, 12, 2602. [Google Scholar] [CrossRef]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reig, M.; Mariño, Z.; Perelló, C.; Iñarrairaegui, M.; Ribeiro, A.; Lens, S.; Díaz, A.; Vilana, R.; Darnell, A.; Varela, M.; et al. Unexpected early tumor recurrence in patients with hepatitis C virus-related hepatocellular carcinoma undergoing interferon-free therapy: A note of caution. J. Hepatol. 2016, 65, 719–726. [Google Scholar] [CrossRef] [Green Version]
- Kozbial, K.; Moser, S.; Schwarzer, R.; Laferl, H.; Al-Zoairy, R.; Stauber, R.; Stättermayer, A.F.; Beinhardt, S.; Graziadei, I.; Freissmuth, C.; et al. Unexpected high incidence of hepatocellular carcinoma in cirrhotic patients with sustained virologic response following interferon-free direct-acting antiviral treatment. J. Hepatol. 2016, 65, 856–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sapena, V.; Enea, M.; Torres, F.; Celsa, C.; Rios, J.; Rizzo, G.E.M.; Nahon, P.; Mariño, Z.; Tateishi, R.; Minami, T.; et al. Hepatocellular carcinoma recurrence after direct-acting antiviral therapy: An individual patient data meta-analysis. Gut 2021, 71, 593–604. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [Green Version]
- Korean Liver Cancer Association-National Cancer Center (KLCANCC). 2018 KLCA-NCC Korea Practice Guideline for the Management of Hepatocellular Carcinoma. Korean J. Radiol. 2019, 20, 1042–1113. [Google Scholar] [CrossRef] [Green Version]
- American College of Radiology (ACR). Liver Imaging Reporting and Data System Version 2018. Available online: https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/LI-RADS (accessed on 7 November 2021).
- Organ Procurement and Transplantation Network (OPTN) Policies. Available online: https://optn.transplant.hrsa.gov/media/1200/optn_policies.pdf (accessed on 21 February 2021).
- Lee, S.; Kim, M.-J.; Kim, S.-S.; Shin, H.; Kim, D.Y.; Choi, J.-Y.; Park, M.-S.; Mitchell, D.G. Retrospective comparison of EASL 2018 and LI-RADS 2018 for the noninvasive diagnosis of hepatocellular carcinoma using magnetic resonance imaging. Hepatol. Int. 2019, 14, 70–79. [Google Scholar] [CrossRef]
- van der Pol, C.; Lim, C.S.; Sirlin, C.B.; McGrath, T.A.; Salameh, J.-P.; Bashir, M.R.; Tang, A.; Singal, A.G.; Costa, A.F.; Fowler, K.; et al. Accuracy of the Liver Imaging Reporting and Data System in Computed Tomography and Magnetic Resonance Image Analysis of Hepatocellular Carcinoma or Overall Malignancy—A Systematic Review. Gastroenterology 2019, 156, 976–986. [Google Scholar] [CrossRef] [Green Version]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE)statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, E.M.; Sulkowski, M.S.; Gane, E.J.; Herring, R.W., Jr.; Ratziu, V.; Ding, X.; Wang, J.; Chuang, S.M.; Ma, J.; McNally, J.; et al. Concordance of sustained virological response 4, 12, and 24 weeks post-treatment with sofosbuvir-containing regimens for hepatitis C virus. Hepatology 2015, 61, 41–45. [Google Scholar] [CrossRef]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; Sulkowski, M.S.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L.; et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Child, C.G.; Turcotte, J.G. Surgery and portal hypertension. In The Liver and Portal Hypertension; Child, C.G., Ed.; Saunders: Philadelphia, PA, USA, 1964; pp. 50–64. [Google Scholar]
- Pugh, R.N.H.; Murray-Lyon, I.M.; Dawson, J.L.; Pietroni, M.C.; Williams, R. Transection of the esophagus in bleeding oesophageal varices. Br. J. Surg. 1973, 60, 648–652. [Google Scholar]
- Kamath, P.S.; Wiesner, R.H.; Malinchoc, M.; Kremers, W.K.; Therneau, T.M.; Kosberg, C.L.; D’Amico, G.; Dickson, E.R.; Kim, W.R. A model to predict survival in patients with end-stage liver disease. Hepatology 2001, 33, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Kambadakone, A.R.; Fung, A.; Gupta, R.T.; Hope, T.A.; Fowler, K.; Lyshchik, A.; Ganesan, K.; Yaghmai, V.; Guimaraes, A.R.; Sahani, D.V.; et al. LI-RADS technical requirements for CT, MRI, and contrast-enhanced ultrasound. Abdom. Radiol. 2017, 43, 56–74. [Google Scholar] [CrossRef]
- Waziry, R.; Hajarizadeh, B.; Grebely, J.; Amin, J.; Law, M.; Danta, M.; George, J.; Dore, G.J. Hepatocellular carcinoma risk following direct-acting antiviral HCV therapy: A systematic review, meta-analyses, and meta-regression. J. Hepatol. 2017, 67, 1204–1212. [Google Scholar] [CrossRef]
- Kanwal, F.; Kramer, J.; Asch, S.M.; Chayanupatkul, M.; Cao, Y.; El-Serag, H.B. Risk of Hepatocellular Cancer in HCV Patients Treated with Direct-Acting Antiviral Agents. Gastroenterology 2017, 153, 996–1005.e1. [Google Scholar] [CrossRef] [Green Version]
- Singal, A.G.; Lim, J.K.; Kanwal, F. AGA Clinical Practice Update on Interaction Between Oral Direct-Acting Antivirals for Chronic Hepatitis C Infection and Hepatocellular Carcinoma: Expert Review. Gastroenterology 2019, 156, 2149–2157. [Google Scholar] [CrossRef] [Green Version]
- Erkan, B.; Meier, J.; Clark, T.J.; Kaplan, J.; Lambert, J.R.; Chang, S. Non-invasive diagnostic criteria of hepatocellular carcinoma: Comparison of diagnostic accuracy of updated LI-RADS with clinical practice guidelines of OPTN-UNOS, AASLD, NCCN, EASL-EORTC, and KLSCG-NCC. PLoS ONE 2019, 14, e0226291. [Google Scholar] [CrossRef]
- Cannella, R.; Vernuccio, F.; Sagreiya, H.; Choudhury, K.R.; Iranpour, N.; Marin, D.; Furlan, A. Liver Imaging Reporting and Data System (LI-RADS) v2018: Diagnostic value of ancillary features favoring malignancy in hypervascular observations ≥ 10 mm at intermediate (LR-3) and high probability (LR-4) for hepatocellular carcinoma. Eur. Radiol. 2020, 30, 3770–3781. [Google Scholar] [CrossRef]
- Vernuccio, F.; Cannella, R.; Meyer, M.; Choudhoury, K.R.; Gonzáles, F.; Schwartz, F.R.; Gupta, R.T.; Bashir, M.R.; Furlan, A.; Marin, D. LI-RADS: Diagnostic Performance of Hepatobiliary Phase Hypointensity and Major Imaging Features of LR-3 and LR-4 Lesions Measuring 10-19 mm With Arterial Phase Hyperenhancement. Am. J. Roentgenol. 2019, 213, W57–W65. [Google Scholar] [CrossRef]
- Mariño, Z.; Darnell, A.; Lens, S.; Sapena, V.; Díaz, A.; Belmonte, E.; Perelló, C.; Calleja, J.L.; Varela, M.; Rodriguez, M.; et al. Time association between hepatitis C therapy and hepatocellular carcinoma emergence in cirrhosis: Relevance of non-characterized nodules. J. Hepatol. 2019, 70, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Angeli, P.; Piovesan, S.; Noventa, F.; Anastassopoulos, G.; Chemello, L.; Cavalletto, L.; Gambato, M.; Russo, F.P.; Burra, P.; et al. Newly diagnosed hepatocellular carcinoma in patients with advanced hepatitis C treated with DAAs: A prospective population study. J. Hepatol. 2018, 69, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Ooka, Y.; Miho, K.; Shuntaro, O.; Nakamura, M.; Ogasawara, S.; Suzuki, E.; Yasui, S.; Chiba, T.; Arai, M.; Kanda, T.; et al. Prediction of the very early occurrence of HCC right after DAA therapy for HCV infection. Hepatol. Int. 2018, 12, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, N.; Miura, K.; Watanabe, S.; Tsukui, M.; Takaoka, Y.; Nomoto, H.; Murayama, K.; Hirosawa, T.; Goka, R.; Kunitomo, N.; et al. Usefulness of Gd-EOB-DTPA-enhanced MRI for evaluating the potential for early development of hepatocellular carcinoma after HCV eradication by direct-acting antiviral treatment. J. Rural Med. 2019, 14, 78–86. [Google Scholar] [CrossRef] [Green Version]
- Sangiovanni, A.; Alimenti, E.; Gattai, R.; Filomia, R.; Parente, E.; Valenti, L.; Marzi, L.; Pellegatta, G.; Borgia, G.; Gambato, M.; et al. Undefined/non-malignant hepatic nodules are associated with early occurrence of HCC in DAA-treated patients with HCV-related cirrhosis. J. Hepatol. 2020, 73, 593–602. [Google Scholar] [CrossRef]
- Corwin, M.T.; Lee, A.Y.; Fananapazir, G.; Loehfelm, T.W.; Sarkar, S.; Sirlin, C.B. Nonstandardized Terminology to Describe Focal Liver Lesions in Patients at Risk for Hepatocellular Carcinoma: Implications Regarding Clinical Communication. Am. J. Roentgenol. 2018, 210, 85–90. [Google Scholar] [CrossRef]
- Cannella, R.; Vernuccio, F.; Celsa, C.; Cabibbo, G.; Calvaruso, V.; Greco, S.; Battaglia, S.; Choudhury, K.R.; Tang, A.; Midiri, M.; et al. Long-term evolution of LI-RADS observations in HCV-related cirrhosis treated with direct-acting antivirals. Liver Int. 2021, 41, 2179–2188. [Google Scholar] [CrossRef]
- Kudo, M. Immune Checkpoint Inhibition in Hepatocellular Carcinoma: Basics and Ongoing Clinical Trials. Oncology 2017, 92 (Suppl. 1), 50–62. [Google Scholar] [CrossRef]
- Huppert, L.A.; Gordan, J.D.; Kelley, R.K. Checkpoint Inhibitors for the Treatment of Advanced Hepatocellular Carcinoma. Clin. Liver Dis. 2020, 15, 53–58. [Google Scholar] [CrossRef]
- Kamath, A.; Roudenko, A.; Hecht, E.; Sirlin, C.; Chernyak, V.; Fowler, K.; Mitchell, D.G. CT/MR LI-RADS 2018: Clinical implications and management recommendations. Abdom. Radiol. 2019, 44, 1306–1322. [Google Scholar] [CrossRef]




| Characteristics | Total (n = 115) | Any LR-5 (n = 68) | Lack of LR-5 (n = 47) | p Value |
|---|---|---|---|---|
| Age (years) | 72 (59.0, 78.0) | 76 (68.2, 78.7) | 68.0 (55.0, 73.0) | <0.001 |
| Sex | ||||
| Men | 68 (59.1) | 45 (66.2) | 23 (48.9) | 0.064 |
| Women | 47 (40.9) | 23 (33.8) | 24 (51.1) | |
| HCV genotype | ||||
| 1a | 4 (3.5) | 1 (1.5) | 3 (6.4) | 0.586 |
| 1b | 86 (74.8) | 51 (75.0) | 35 (74.5) | |
| 2 | 9 (7.8) | 6 (8.8) | 3 (6.4) | |
| 3 | 15 (13.0) | 9 (13.2) | 6 (12.8) | |
| 4 | 1 (0.9) | 1 (1.5) | 0 (0) | |
| BMI (kg/m2) | 24.2 (22.0, 27.6) | 25.9 (24.2, 27.6) | 25.3 (23.8, 27.3) | 0.391 |
| AST (IU/L) | 64.0 (44.5, 94.7) | 69.0 (50.0, 90.0) | 59.0 (40.0, 107.0) | 0.896 |
| ALT (IU/L) | 64.5 (41.5, 86.0) | 65.0 (49.0, 84.0) | 64.0 (37.0, 97.0) | 0.871 |
| Hemoglobin (g/dL) | 13.3 (11.9, 14.6) | 13.1 (11.9, 14.7) | 13.4 (11.6, 14.5) | 0.852 |
| WBC (×103/mL) | 4900 (3885, 6055) | 4810 (4000, 6313) | 5050 (3607, 5997) | 0.675 |
| INR | 1.0 (1.0, 1.2) | 1.0 (1.0, 1.2) | 1.0 (1.0, 1.2) | 0.120 |
| Albumin (g/dL) | 3.6 (3.3, 4.0) | 3.6 (3.1, 4.0) | 3.7 (3.5, 4.0) | 0.204 |
| Creatinine (mg/dL) | 0.8 (0.7, 1.0) | 0.8 (0.7, 1.0) | 0.8 (0.6, 0.9) | 0.604 |
| Bilirubin (mg/dL) | 1.0 (0.7, 1.3) | 1.0 (0.6, 1.3) | 1.0 (0.7, 1.8) | 0.352 |
| Platelet count (×103/μL) | 92.0 (73.0, 138.0) | 89.0 (70.0, 128.0) | 103.0 (73.0, 145.5) | 0.133 |
| Diabetes, n (%) | 27 (23.5) | 17 (25.0) | 10 (21.3) | 0.643 |
| Hypertension, n (%) | 55 (47.8) | 34 (61.8) | 21 (44.7) | 0.575 |
| TE (kPa) | 13.2 (17.2, 29.6) | 20.0 (15.0, 33.8) | 16.0 (12.7, 29.0) | 0.108 |
| Child-Pugh Score | 6.0 (5.0, 6.0) | 5.0 (5.0, 6.0) | 6.0 (5.0, 6.0) | 0.979 |
| Child-Pugh Class | ||||
| A | 90 (78.3) | 53 (77.9) | 37 (78.7) | 0.920 |
| B | 25 (21.7) | 15 (22.1) | 10 (21.3) | |
| FIB-4 score | 6.45 (3.86, 9.37) | 7.60 (4.34, 10.28) | 5.46 (2.86, 8.60) | 0.034 |
| MELD score | 8.0 (6.0, 10.0) | 8.0 (6.0, 10.0) | 7.0 (6.0, 10.0) | 0.431 |
| LR-3/LR-4 | 45 (39.1) | 34 (50.0) | 11 (23.4) | 0.004 |
| Characteristics | LR-5 Post-DAA (n = 29) | Lack of LR-5 Post-DAA (n = 86) | p Value |
|---|---|---|---|
| Age (years) | 77.0 (63.0, 78.0) | 71.5 (58.0, 72.7) | 0.057 |
| Sex | |||
| Men | 21 (72.4) | 47 (57.7) | 0.092 |
| Women | 8 (27.6) | 39 (45.3) | |
| HCV genotype | |||
| 1a | 0 (0) | 4 (4.7) | 0.303 |
| 1b | 22 (75.9) | 64 (74.4) | |
| 2 | 3 (10.3) | 6 (7.0) | |
| 3 | 3 (10.3) | 12 (14.0) | |
| 4 | 1 (1.4) | 0 (0) | |
| BMI (kg/m2) | 25.0 (24.2, 27.6) | 25.7 (24.2, 27.6) | 0.841 |
| AST (IU/L) | 73.0 (57.5, 99.5) | 70.0 (46.0, 106.0) | 0.521 |
| ALT (IU/L) | 68.0 (49.0, 85.5) | 68.0 (50.0, 104.5) | 0.747 |
| Hemoglobin (g/dL) | 13.1 (12.0, 14.4) | 13.4 (11.9, 14.5) | 0.695 |
| WBC (×103/mL) | 4130 (3715, 5850) | 4950 (3847, 6130) | 0.658 |
| INR | 1.0 (1.0, 1.2) | 1.0 (1.0, 1.3) | 0.105 |
| Albumin (g/dL) | 3.5 (3.0, 3.9) | 3.7 (3.4, 4.0) | 0.002 |
| Creatinine (mg/dL) | 0.8 (0.7, 1.0) | 0.8 (0.7, 0.9) | 0.448 |
| Bilirubin (mg/dL) | 1.0 (0.5, 1.6) | 1.0 (0.7, 1.3) | 0.377 |
| Platelet count (×103/μL) | 80.0 (62.0, 114.0) | 98.0 (70.7, 156.0) | 0.063 |
| Diabetes, n (%) | 10 (34.4) | 17 (19.8) | 0.106 |
| Hypertension, n (%) | 13 (48.8) | 42 (48.8) | 0.709 |
| TE (kPa) | 26.0 (16.9, 35.1) | 17.0 (13.0, 29.0) | 0.015 |
| Child-Pugh Score | 6.0 (5.0, 7.0) | 5.0 (5.0, 6.0) | 0.010 |
| Child-Pugh Class | |||
| A | 19 (65.5) | 71 (82.6) | 0.054 |
| B | 10 (34.5) | 15 (17.4) | |
| FIB-4 score | 8.84 (5.46, 11.65) | 5.59 (3.25, 8.80) | 0.009 |
| MELD score | 8.0 (7.0, 11.0) | 8.0 (6.0, 9.0) | 0.031 |
| LR-5 pre-DAA | 13 (44.8) | 39 (45.3) | 0.961 |
| LR-3/LR-4 | 21 (72.4) | 33 (38.4) | 0.001 |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Characteristics | Hazard Ratio | 95% CI | p Value | Hazard Ratio | 95% CI | p Value |
| Age | 1.03 | 0.99, 1.07 | 0.093 | 1.03 | 0.99, 1.08 | 0.093 |
| Male sex | 1.52 | 0.67, 3.46 | 0.314 | |||
| Transient elastography | 1.01 | 0.98, 1.51 | 0.215 | |||
| Child-Pugh Class B | 2.13 | 0.98, 4.62 | 0.055 | 2.62 | 1.13, 6.02 | 0.023 |
| FIB-4 score | 1.11 | 1.03, 1.20 | 0.005 | 1.07 | 0.98, 1.16 | 0.103 |
| MELD score | 1.09 | 0.98, 1.26 | 0.100 | |||
| LR-5 pre-DAA | 0.787 | 0.37, 1.64 | 0.522 | |||
| LR-3 or LR-4 observations | 2.47 | 1.09, 5.61 | 0.030 | 2.40 | 1.03, 5.74 | 0.048 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vernuccio, F.; Cannella, R.; Cabibbo, G.; Greco, S.; Celsa, C.; Matteini, F.; Giuffrida, P.; Midiri, M.; Di Marco, V.; Cammà, C.; et al. Role of LI-RADS Indeterminate Observations in the Risk of Hepatocellular Carcinoma after HCV Eradication with Direct-Acting Antivirals. Diagnostics 2022, 12, 1187. https://doi.org/10.3390/diagnostics12051187
Vernuccio F, Cannella R, Cabibbo G, Greco S, Celsa C, Matteini F, Giuffrida P, Midiri M, Di Marco V, Cammà C, et al. Role of LI-RADS Indeterminate Observations in the Risk of Hepatocellular Carcinoma after HCV Eradication with Direct-Acting Antivirals. Diagnostics. 2022; 12(5):1187. https://doi.org/10.3390/diagnostics12051187
Chicago/Turabian StyleVernuccio, Federica, Roberto Cannella, Giuseppe Cabibbo, Silvia Greco, Ciro Celsa, Francesco Matteini, Paolo Giuffrida, Massimo Midiri, Vito Di Marco, Calogero Cammà, and et al. 2022. "Role of LI-RADS Indeterminate Observations in the Risk of Hepatocellular Carcinoma after HCV Eradication with Direct-Acting Antivirals" Diagnostics 12, no. 5: 1187. https://doi.org/10.3390/diagnostics12051187






