Abstract
Diagnosing and treating many infectious diseases depends on correctly identifying the causative pathogen. Characterization of pathogen-specific nucleic acid sequences by PCR is the most sensitive and specific method available for this purpose, although it is restricted to laboratories that have the necessary infrastructure and finance. Microscopy, rapid immunochromatographic tests for antigens, and immunoassays for detecting pathogen-specific antibodies are alternative and useful diagnostic methods with different advantages and disadvantages. Detection of ribosomal RNA molecules in the cytoplasm of bacterial and protozoan pathogens by fluorescence in-situ hybridization (FISH) using sequence-specific fluorescently labelled DNA probes, is cheaper than PCR and requires minimal equipment and infrastructure. A LED light source attached to most laboratory light microscopes can be used in place of a fluorescence microscope with a UV lamp for FISH. A FISH test hybridization can be completed in 30 min at 37 °C and the whole test in less than two hours. FISH tests can therefore be rapidly performed in both well-equipped and poorly-resourced laboratories. Highly sensitive and specific FISH tests for identifying many bacterial and protozoan pathogens that cause disease in humans, livestock and pets are reviewed, with particular reference to parasites causing malaria and babesiosis, and mycobacteria responsible for tuberculosis.
1. Background
The in-situ hybridization (ISH) technique for examining the formation and detection of RNA-DNA or DNA-DNA nucleotide complementary hybrids in cells utilizing radioactively labelled oligonucleotides as probes was first described in 1969 [1,2]. ISH permits the detection of nucleic acids in individual cells that contain specific nucleotide sequences among a heterogenous population of cells. It also allows the simultaneous determination of biochemical and morphological characteristics of the reactive cells. The hybridization of fluorescently labeled, chromosome-specific, composite DNA probe pools to cytological preparations, termed chromosome painting, has made major contributions to karyotyping and identifying chromosomal changes responsible for human pathology [3]. Fluorescence in situ hybridization (FISH) methods have since been developed to study chromosomal genomic changes at the kilobase level [4]. ISH was first utilized for bacteriology in 1983 with radioactively labeled DNA probes targeting ribosomal RNA (rRNA) [5]. Fluorescently labeled probes have subsequently replaced radioactive probes for FISH [6,7,8]. In 1989, DeLong and colleagues demonstrated that oligodeoxynucleotide probes, complementary to 16S rRNA, labelled with different fluorescent molecules used in FISH can detect single microbial cells and differentiate closely related organisms [9,10]. Shah et al. in 1990 established that FISH can detect and differentiate Pneumocystis carinii strains in sputum and tissue from patients [11]. FISH assays for detecting pathogens in clinical samples now use either peptide nucleic acid (PNA) probes (in which the sugar phosphate backbone is replaced with a more hydrolysis-resistant polyamide chain), locked nucleic acid (LNA) probes (where greater stability is achieved by a methylene bridge linking the 2′ oxygen to the 4′ carbon of the pentose) or, more commonly, DNA probes [12,13,14,15,16,17,18,19,20].
The application of FISH assays for detecting and identifying microbial pathogens has advanced considerably since the turn of the century [21]. FISH techniques have been applied to investigate the localization of viral nucleic acids within infected tissues and organs, e.g., for SARS-CoV-2 [22,23] and HIV [24], and rarely for identifying the infecting virus [25]. PCR tests, and the detection of viral antigens and specific antibodies to viral antigens, are more commonly and effectively used for diagnosing viral infections. In contrast, FISH tests have proved useful for identifying bacterial, fungal and protozoan disease-causing pathogens, particularly at the species level. Recent examples are listed in Table 1.

Table 1.
FISH Tests for Identifying Pathogens.
Ribosomal RNA molecules possess genus, species and strain-specific regions. Hence, 16S rRNA sequences have been used to establish phylogenetic relationships among bacteria [47]. The binding of rRNA-targeting probes can be visualized without nucleic acid-based amplification (NAA) of the target rRNA sequence because rRNA is present in each of the numerous ribosomes in the cytoplasm. Many FISH tests listed in Table 1 are based on DNA or PNA probes that hybridize to specific rRNA sequences in suitably permeabilized cells. Due to the complex three-dimensional structure of rRNA, not all nucleotide sequences within an rRNA molecule are equally accessible for hybridizing with FISH probes. Loop and hairpin formation as well as rRNA-protein interactions hinder hybridization and produce differential binding sensitivity with oligonucleotide probes [48,49]. It is therefore necessary to evaluate and optimize every newly designed probe with the respective reference organism and appropriate negative controls, before it is applied to test samples. Self-annealing and hairpin formation occurring within an oligonucleotide probe itself can lead to low signal intensities, and hence newly designed oligonucleotides also need to be checked for internal complementarity using appropriate software.
The World Health Organization estimated 241 million cases of malaria and 627,000 resulting deaths worldwide in 2020 [50]. Infection with Mycobacterium tuberculosis (MTB), which is primarily responsible for human tuberculosis, caused an estimated 1.4 million deaths worldwide in 2020 [51]. Malaria and tuberculosis can manifest as latent infections that rapidly become fulminant diseases with fatal consequences. Babesiosis is a potentially fatal, tick-borne, globally emerging human disease, that also afflicts livestock and domestic pets [52]. While the principle of FISH tests remains the same, their application to identify different pathogens can vary considerably. Recently developed FISH tests that can be easily used in resource-limited laboratories worldwide for identifying causative pathogens in malaria, tuberculosis and babesiosis are therefore selected for detailed consideration in this article. These FISH tests for malaria, tuberculosis and babesiosis have the following shared characteristics: (i) the assays are performed on thin smears on glass microscope slides, (ii) cells in the smear are rapidly permeabilized for hybridization which is then performed with fluorescently labelled DNA probes at 37 °C for 30 min, (iii) fluorescence can be viewed under LED illumination in common light microscopes as shown in Supplementary Figure S1, (iv) the test is completed in less than two hours, and (v) only living cells are labelled because rRNA is rapidly degraded in dying cells. Supplementary Figure S2 summarizes the published work–flow for tuberculosis FISH tests [28,29].
2. FISH Tests for Malaria
2.1. Background
Plasmodium falciparum is responsible for most of the annual 241 million global malaria infections, together with an estimated 4.5 million cases of Plasmodium vivax and fewer cases of Plasmodium malariae and Plasmodium ovale [50]. Plasmodium knowlesi, which normally infects macaque and leaf monkeys, causes a significant number of dead-end human infections in Southeast Asian countries [53]. Plasmodium knowlesi is difficult to differentiate from human malaria parasites in Giemsa-stained blood smears that are commonly used for diagnosing malaria in endemic areas [53]. Occasional zoonotic infections with Plasmodium cynomolgi and Plasmodium inui have also been reported in Southeast Asia [53]. Plasmodium brasilianum and Plasmodium simium infect platyrrhine monkeys in South and Central America, are genetically almost identical to the human malaria parasites P. malariae and P. vivax respectively, and likely to have been derived from the human parasites by anthroponosis [53]. P. simium and P. brasilianum can also infect humans by zoonosis but their differentiation from P. vivax and P. malariae respectively in stained blood smears is not possible [53].
There were 2171 US cases of malaria reported to the US Centers for Disease Control and Prevention (CDC) in 2017 according to the latest available CDC report [54]. Plasmodium falciparum accounted for 70.5%, P. vivax 10.0%, P. ovale 5.5%, and P. malariae 2.6% of the infections, all of which had been acquired outside the US [54]. Infections with two or more Plasmodium species were responsible for 1.0% of infections [54]. The identification of malaria parasites for diagnosis is therefore also needed in the US and other countries with no indigenous transmission of malaria.
Giemsa-stained thick and thin blood smear microscopy has been the most widely used technique globally for diagnosing malaria. It however requires time and an experienced microscopist for optimal sensitivity of detection and for identifying the infecting species of parasite. A sensitivity of >150 parasites per µL is typically achieved during routine microscopy [39,55]. Rapid diagnostic tests (RDTs), based on the immunochromatographic detection of antibodies to the histidine rich protein 2 of P. falciparum (PfHRP2) and pan Plasmodium-specific lactate dehydrogenase and aldolase, have more recently proved helpful in resource-limited locations [55]. However, selection for PfHRP2 gene deletions in P. falciparum in malaria-endemic areas of Africa has lately increased the false negativity rates for P. falciparum [55]. PCR-dependent NAA diagnostic tests have the best sensitivity and specificity and are able to identify Plasmodium at the species level, but the procedure is not suitable for resource-poor settings and field use [56]. The Loop-Mediated Isothermal Amplification (LAMP) technique has the desired sensitivity and specificity but is not widely utilized for routine malaria diagnosis and species identification [57]. Flow cytometric detection of malaria parasites in blood have also been recently described although details of its limit of detection and ability to identify different species remain to be established [58,59].
2.2. Genus-Specific FISH Test That Identifies All Common Human Malaria Parasites
Simple, rapid and specific FISH tests for malaria, that can be easily performed in resource-constrained diagnostic laboratories, have many advantages [39,42]. These FISH tests for malaria employ a similar protocol to FISH tests for tuberculosis shown in Figure S2. A standard laboratory microscope with a LED fluorescence unit attached to it can be used to read the processed smears on the slide (Figure S1). Results from a Plasmodium genus-specific FISH test utilizing a DNA probe hybridizing to 18S rRNA [39] are reproduced in Figure 1.
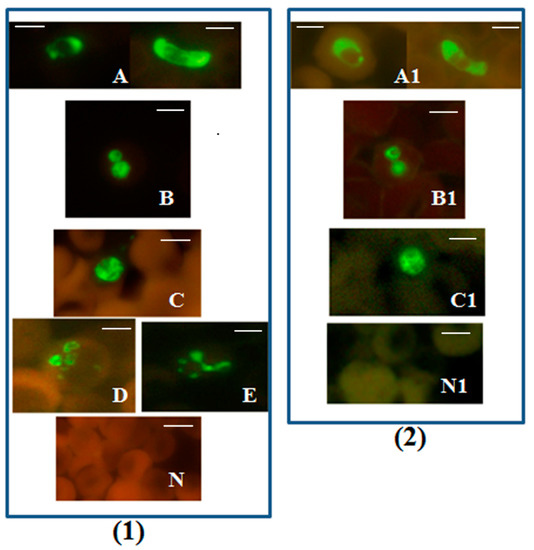
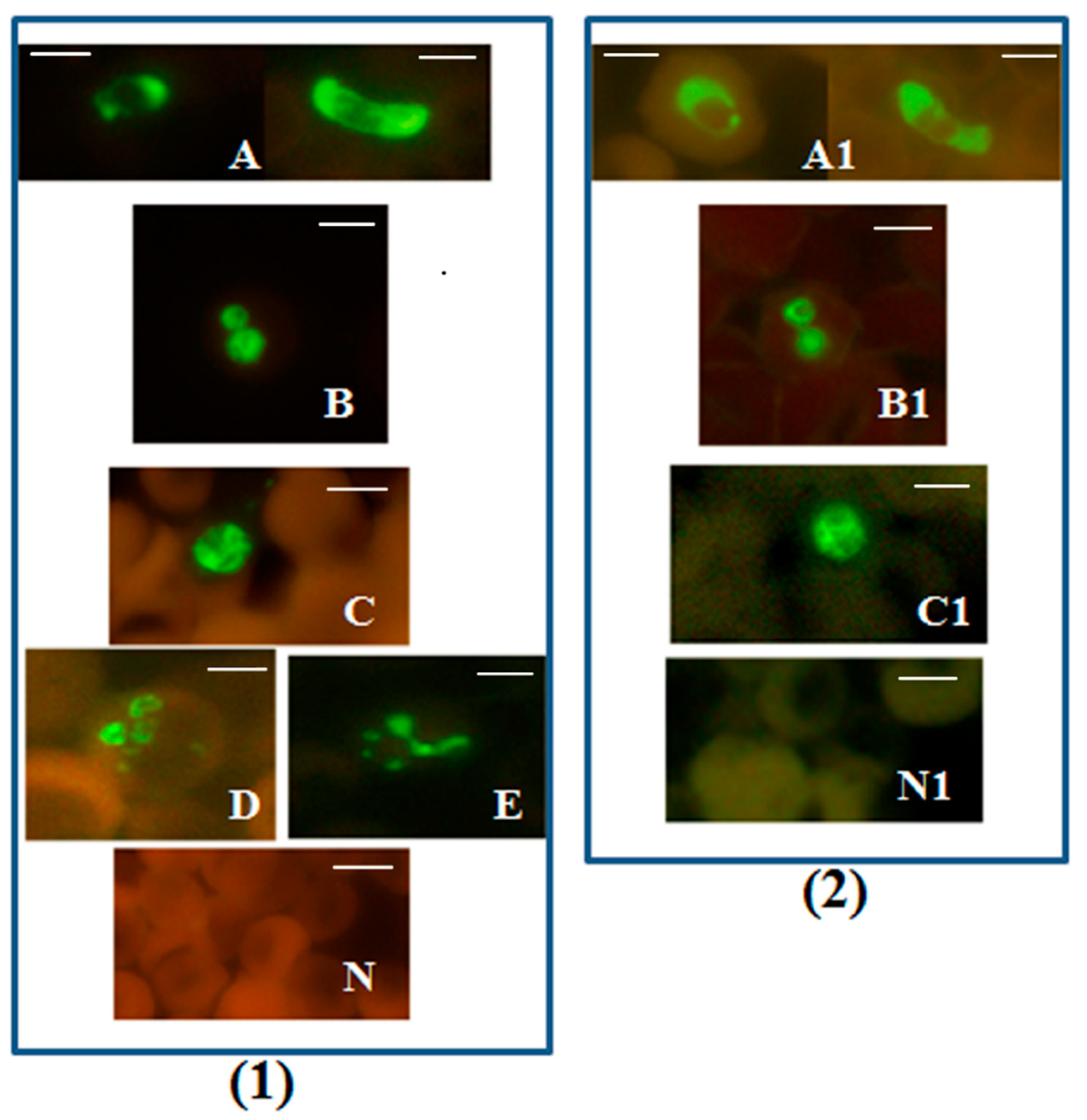
Figure 1.
Plasmodium genus-specific FISH test identifying all human malaria parasites. Photographs showing Plasmodium genus-specific FISH test results with blood smears from patients with confirmed P. falciparum, P. vivax, P. malariae, P. ovale and P. knowlesi infections. Fluorescence was viewed in (1)—fluorescence microscope with a UV light source, and (2)—microscope with LED light source illustrated in Figure S1. Green fluorescence demonstrated the presence of Plasmodium rRNA. (A,A1) P. falciparum including a crescent shaped gametocyte; (B,B1) P. vivax; (C,C1) P. knowlesi; (D) P. ovale; (E) P. malariae; and (N,N1) negative controls. Alexa 488 green was used to label the Plasmodium genus-specific probe in the FISH test. Only Plasmodium parasites fluoresce green in the assay. Scale bars represent approximately 5 µm. Figure reproduced with permission under the creative commons license from Reference [39].
The Plasmodium genus-specific FISH test identified all common species of human malaria parasites with 100% specificity when compared with several other common human blood-borne pathogens [39].
2.3. FISH Test for Specifically Identifying Plasmodium Falciparum
A PF-FISH test that complements the Plasmodium genus-specific FISH test, and designed to specifically identify P. falciparum, utilized a mixture of P. falciparum 18S rRNA-specific probes labeled with Alexa 488 green and the Plasmodium genus-specific probe labeled with a Texas Red in a multiplex format. Plasmodium falciparum fluoresced green (Figure 2), while all Plasmodium parasites, including P. falciparum, fluoresced red with appropriate light filters in the PF-FISH test [39].
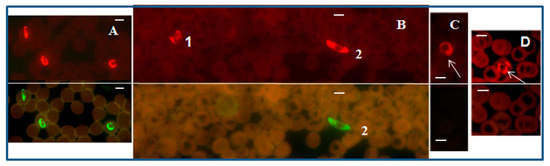
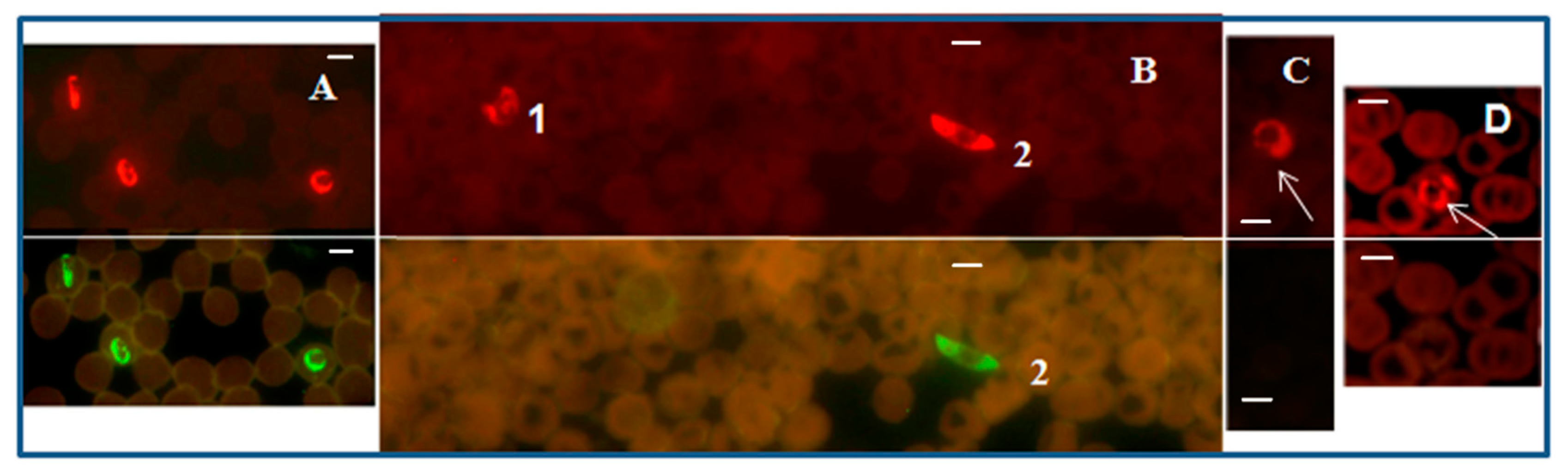
Figure 2.
P. falciparum-specific PF-FISH test. Photographs showing results from the PF-FISH test performed on thin blood smears in patients from Peru and Kenya infected with: (A) P. falciparum; (B) 1. P. malariae and 2. P. falciparum; (C) P. ovale; (D) P. vivax. The P. falciparum-specific probe and Plasmodium genus-specific probe fluoresce green and red, respectively, in the same field when viewed with appropriate light filters. The scale bars represent approximately 5 µm. Figure reproduced with permission under the creative commons license from Reference [39].
2.4. FISH Test for Specifically Identifying Plasmodium Vivax
In a second complementary FISH test, termed the PV-FISH test, a mixture of DNA probes that hybridize only to the 18S rRNA of P. vivax were labeled with the Alexa 488 green, and used in a multiplex format with the Plasmodium genus-specific probe labeled with Texas Red. Only P. vivax fluoresced green, and all Plasmodium species fluoresced red, with appropriate filters in the PV-FISH test [39] as shown in Figure 3.
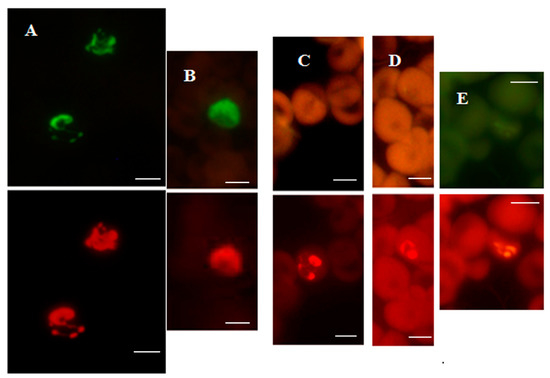
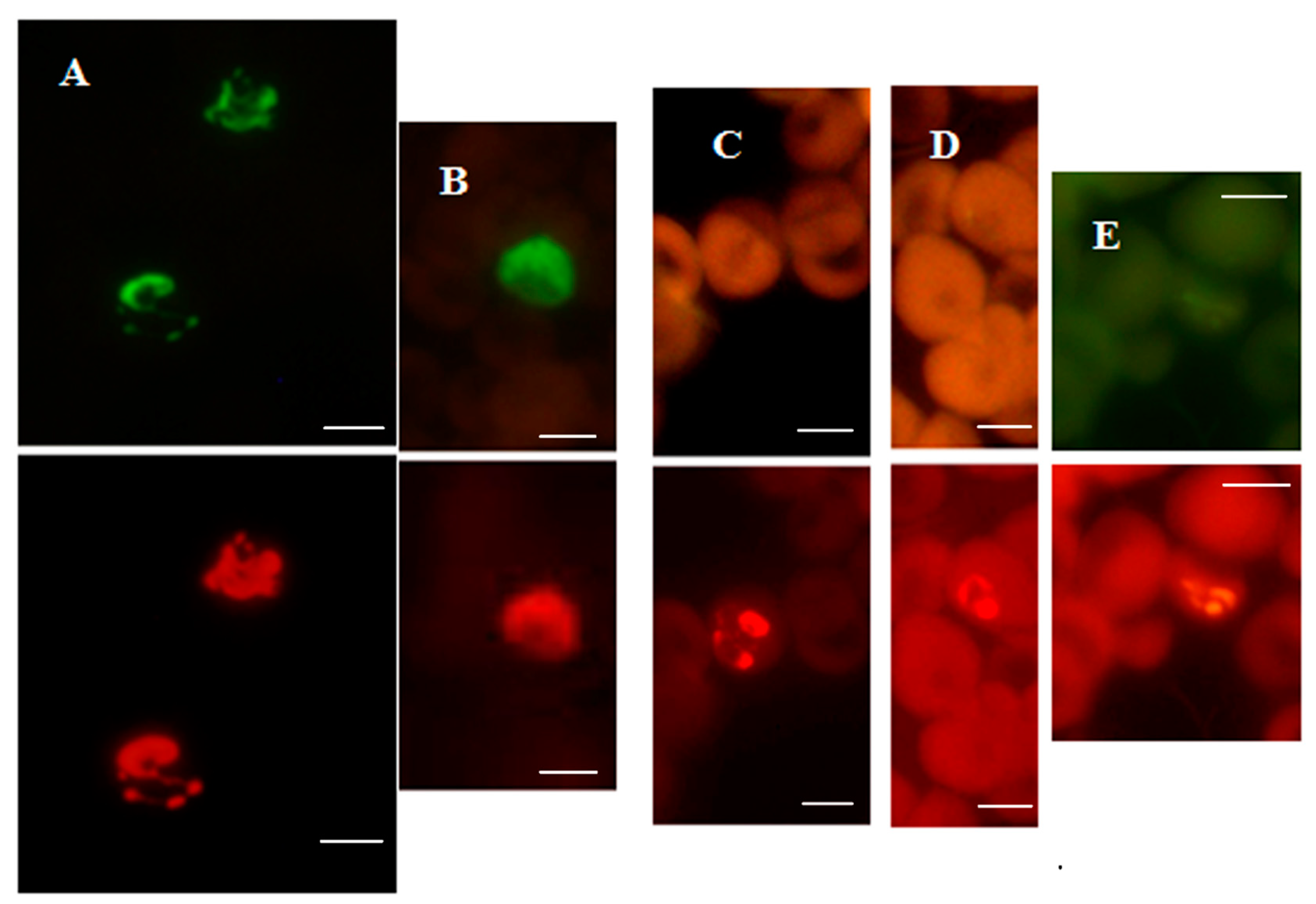
Figure 3.
P. vivax-specific FISH test.Photographs showing PV-FISH test results on patient blood samples with independently confirmed malaria infections from Peru (A), India (B) and Kenya (C–E). Patient blood positive for (A,B) P. vivax; (C) P. ovale; (D) P. malariae; (E) P. falciparum. Green and red fluorescence are due to hybridization with the P. vivax-specific probe and Plasmodium genus-specific probe, respectively, in the same field when viewed with appropriate light filters. The scale bars represent approximately 5 µm. Figure reproduced with permission under the creative commons license from Reference [39].
The two FISH tests for specifically identifying P. falciparum and P. vivax had greater analytical sensitivity, and also higher clinical sensitivity and specificity, compared to microscopic examination of Giemsa-stained blood smears [39].
2.5. FISH Test for Specifically Identifying Plasmodium Knowlesi
Zoonotic P. knowlesi infections in Southeast Asia are commonly misidentified as P. malariae or P. falciparum in Giemsa-stained human blood smears because of morphological similarities between the blood stages, so that PCR-based tests were needed for confirming P. knowlesi infections [60,61,62,63,64,65]. Correct diagnosis of P. knowlesi malaria is essential for two reasons: (i) understanding its epidemiology, and (ii) its pathogenicity and drug treatment options can differ from human malaria caused by P. falciparum, P. malariae, P. ovale and P. vivax that also occur in Southeast Asia. Reliable RDTs for specifically detecting P. knowlesi are not yet available [66]. However, a simple and specific FISH test using DNA probes targeting P. knowlesi 18S rRNA (termed the PK-FISH test) specifically identified P. knowlesi in blood smears [42], as shown in Figure 4.
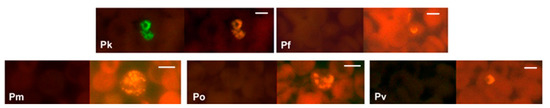
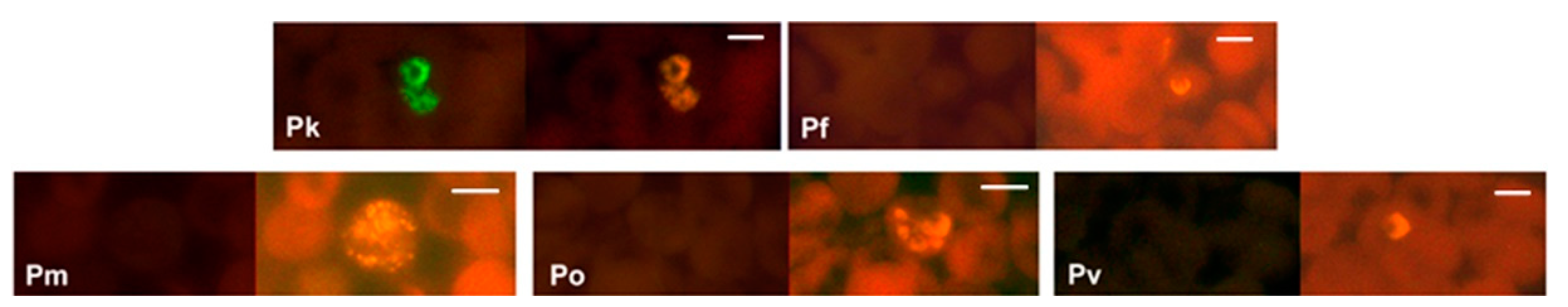
Figure 4.
Specificity of the PK-FISH test for P. knowlesi. Photographs showing PK-FISH test results with the P. knowlesi-specific probe (green fluorescence) and the Plasmodium genus-specific probe (orange fluorescence) in blood smears containing P. knowlesi from monkey blood (Pk), and from human blood with confirmed infections of P. falciparum (Pf), P. malariae (Pm), P. ovale (Po) and P. vivax (Pv). Each set of paired photographs shows fluorescence in the same field when viewed in a LED fluorescence microscope with appropriate light filters (Figure S1). The scale bars represent approximately 5 µm. Reproduced with permission under the creative commons license from Reference [42].
The PK-FISH test, like the analogous Plasmodium genus-specific test and the PF-FISH and PV-FISH tests [39], identified all asexual blood stages, i.e., rings, trophozoites and schizonts, as shown in Figure 5 for P. knowlesi [42]. The PK-FISH test also detected P. knowlesi at the low limit of 16 P. knowlesi parasites per µL, even in the concomitant presence of P. falciparum at approximately 500 parasites per µL [42], which is superior to that possible with routine microscopic examination of Giemsa-stained thin blood films [39,55]. This property is very useful in Southeast Asia where mixed infections of P. knowlesi and other human malaria parasite species are common [60,63]. The highly specific and sensitive PK-FISH therefore meets a widely—recognized diagnostic need of peripheral and district-level clinical laboratories in areas of Southeast Asia where P. knowlesi zoonosis is prevalent [53,60,61,62,63,64,65,66].
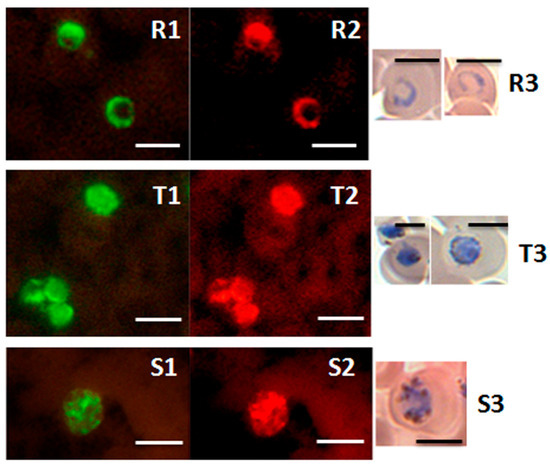
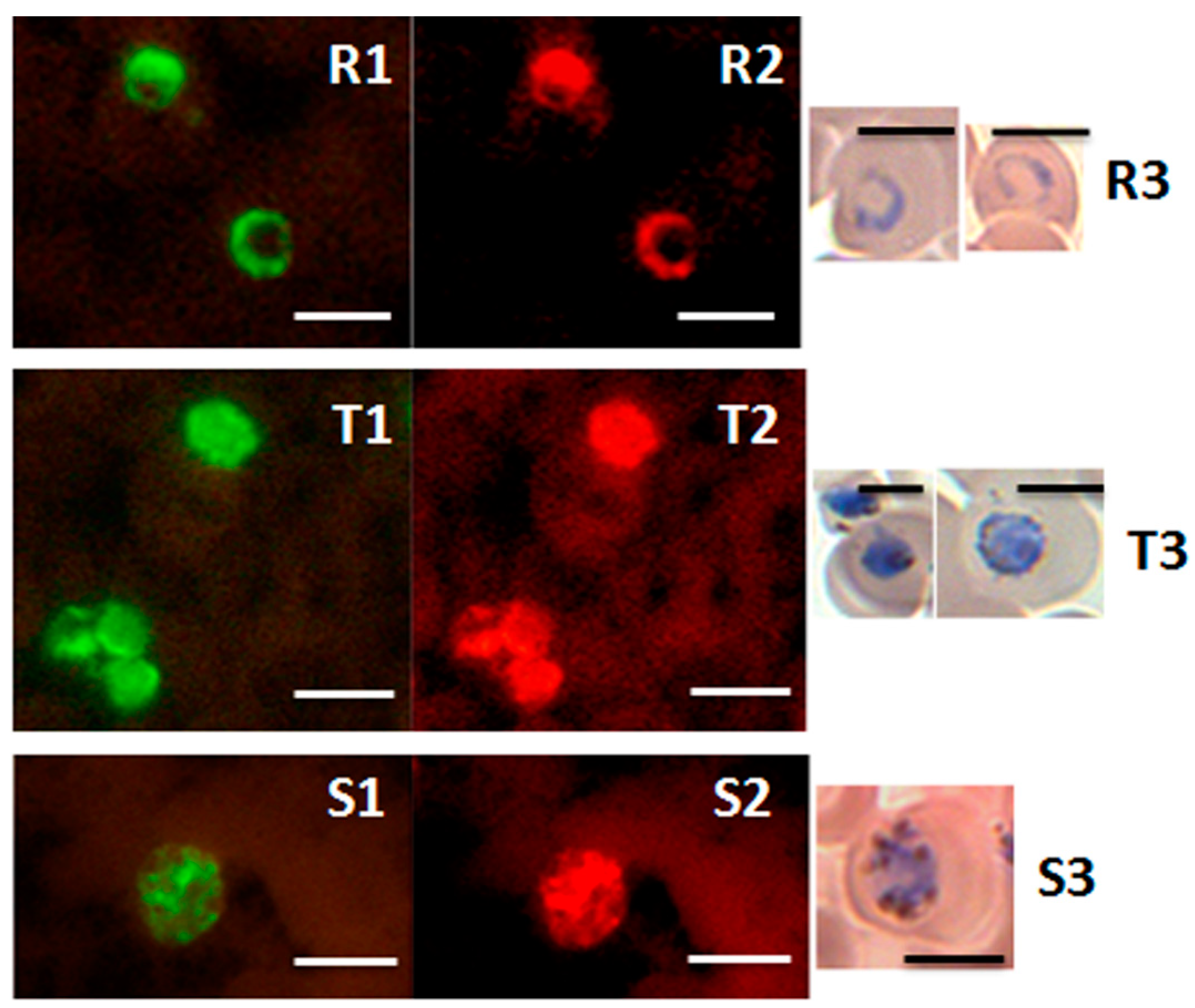
Figure 5.
Detection of ring, trophozoite and schizont stages of P. knowlesi in the PK-FISH test. Photographs showing results from the PK-FISH test with the P. knowlesi-specific probe (green fluorescence) and the Plasmodium genus-specific probe (orange fluorescence) on R—rings; T—trophozoites; S—schizonts. Dual colour fluorescence in the same field is shown in paired photographs R1 and R2, T1 and T2, and S1 and S2. Fluorescence was viewed in a LED fluorescence microscope with pertinent light filters (Figure S1). The ring, trophozoite and schizont-stage parasites were produced from synchronised in vitro cultures of P. knowlesi. Parasites stained with Giemsa from smears prepared in parallel to the corresponding smears used in the PK-FISH test are shown in R3, T3 and S3 respectively. The scale bars represent approximately 5 µm. Reproduced with permission under the creative commons license from Reference [42].
2.6. Conclusions
A large number of malaria tests are performed for diagnostic and screening purposes in malaria-endemic countries. Tests in malaria-free countries are utilized for (i) screening passengers arriving from malaria-endemic countries to prevent the reintroduction of malaria if mosquito vectors are present in the country of arrival, and (ii) confirming malaria in arriving travelers who have malaria-like symptoms. FISH tests are more costly and complex to perform than Giemsa-stained blood smear microscopy and RDTs, but significantly less so than NAA-dependent PCR and LAMP, for detecting Plasmodium infections (table in Section 5 below). FISH tests are particularly useful for identifying the species of infecting parasites, as illustrated here for P. falciparum, P. vivax and P. knowlesi. Therefore, the clinical diagnostic characteristics and simple methodology of the newly described FISH tests for malaria parasites, suggest that they can (i) usefully complement Giemsa-stained blood smear microscopy and RDTs for routine diagnosis and screening for malaria, and (ii) identify the species of infecting Plasmodium (including in mixed infections), in both endemic and non-endemic countries.
3. FISH Tests for Babesiosis
3.1. Background
Babesia are apicomplexan protozoan parasites, like Plasmodium, that infect and replicate within red blood cells to cause babesiosis in humans, livestock and pets [52,67,68,69]. Ticks acquiring Babesia from animal reservoirs function as vectors to transmit infections to humans [52,67,68,69]. Infections can also be transmitted congenitally and through blood transfusion [69,70]. The CDC recorded 2418 cases of babesiosis in 2019 in the 40 US states and the District of Columbia where babesiosis was a notifiable disease [70]. Babesia microti, B. duncani and B. divergens are largely responsible for human infections in the US [67,68,69,70]. Babesia microti, B. divergens, B. venatorum and B. crassa are responsible for human babesiosis in Eurasia [52,68,69,70]. Human babesiosis is also prevalent in Africa, South America and Australia [68,69]. Moreover, Babesia infect cattle (e.g., B. bovis, B. divergens, B. bigemina), horses (e.g., B. caballi), dogs (e.g., B. canis), cats (B. felis), deer (e.g., B. odocolei, B.venatorum), mice (e.g., B. microti, B. rodhani) and other animals [52,69]. The hard ticks, Ixodes scapularis, I. ricinus, I. persulcatus and Dermacentor albipictus are vectors that transmit Babesia to humans [69,70,71].
Laboratory tests commonly used for diagnosing babesiosis involve the detection of (i) parasites in stained blood smears by microscopy, (ii) serum antibodies to Babesia by immunoassays, and (iii) Babesia-specific nucleic acid sequences by PCR [69,70]. Babesiosis and borreliosis (a tick vector-borne disease caused by spirochete Borrelia bacteria), share many clinical manifestations, and occur as coinfections [72,73,74,75,76]. They have an overlapping geographical distribution [67,68,69,70,71,77,78], underscoring the importance of diagnostic laboratory tests for differentiating babesiosis and borreliosis. Early intra-erythrocytic stages of human—infecting Babesia species are not readily distinguished from the ring and trophozoite stages of P. falciparum by microscopy in areas where babesiosis and malaria are co-endemic [69]. Furthermore, the antibody assays for diagnosing human and veterinary babesiosis cannot easily differentiate between active and resolved Babesia infections [67,68,69,70]. PCR tests for babesiosis have high sensitivity [79,80,81] and are recommended for screening donor blood for babesiosis in the US [82]. However, cost and infrastructure requirements make PCR-based tests impractical for use in resource-limited laboratories and field settings.
3.2. Babesia Genus-Specific FISH Test
A FISH test that identifies all common species of Babesia parasites, with many advantages for use in resource-limited laboratories, has been developed [43,44]. Termed the Babesia genus FISH test, it is based on DNA probes that specifically hybridize to the multiple copies of Babesia 18S rRNA present in the parasite cytoplasm. Like other rRNA-directed FISH tests, the Babesia genus FISH test does not require NAA—a process that is sensitive to NAA inhibitors sometimes present in blood [83].
The Babesia genus-specific FISH test detects B. microti, B. duncani and B. divergens, as well as the two important parasites causing bovine babesiosis, B. bovis and B. bigemina [43], as illustrated in Figure 6.
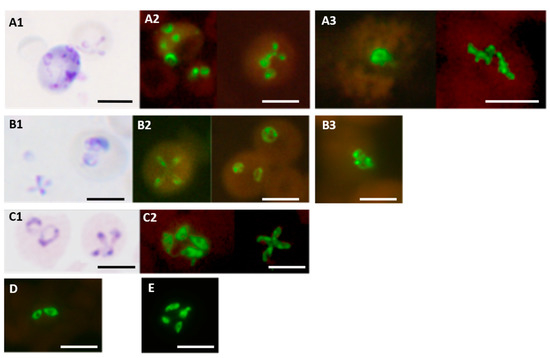
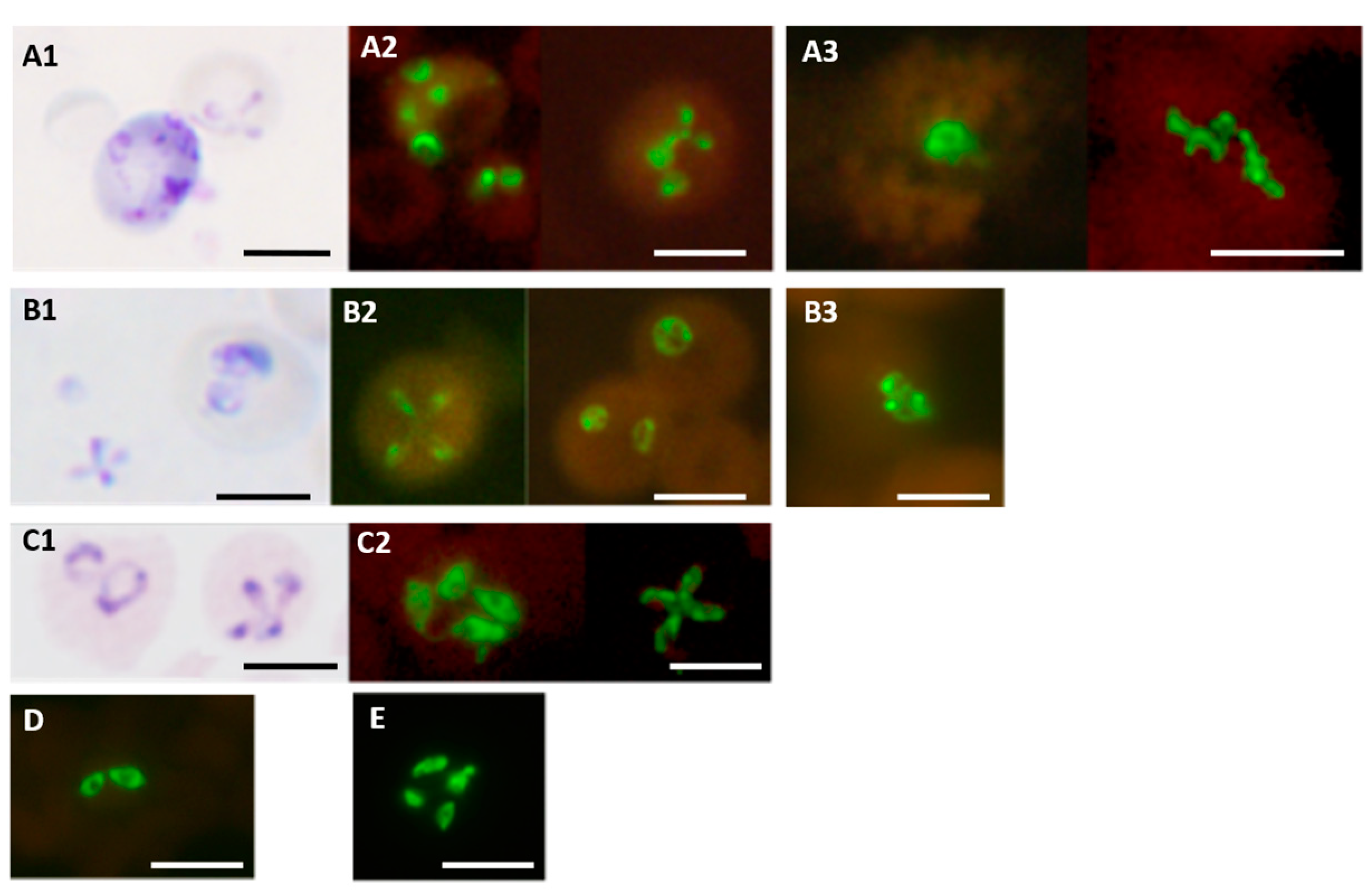
Figure 6.
Babesia genus-specific FISH test on different Babesia species. Parasites stained with Giemsa in smears used for FISH tests are shown in the case of (A1) B. microti; (B1) B. duncani; and (C1) B. divergens. Fluorescence observed in FISH tests on corresponding smears from the same preparations are shown in (A2) B. microti (from hamster blood); (B2) B. duncani (from hamster blood); (C2) B. divergens (from culture). Other FISH test results on smears of (D) B. bovis (from bovine blood); (E) B. bigemina (from bovine blood) are also shown. Fluorescence in FISH tests on blood smears from two patients with the infecting species confirmed by DNA sequencing are shown in (A3) for B. microti and (B3) for B. duncani. Scale bars represent approximately 5 μm. Reproduced with permission under the creative commons license from Reference [43].
The Babesia genus-specific FISH test, in conjunction with an IFA test for detecting serum antibodies to B. duncani and B. microti, on clinical samples originating from USA, Australia, Europe and elsewhere, showed that the global prevalence of B. duncani infections had hitherto been under-estimated [44]. Furthermore, the Babesia genus-specific FISH test was highly specific and did not detect other pertinent pathogens found in human blood [43], including different species of Borrelia and Plasmodium [43], as well as various species of Bartonella that infect humans and domestic pets [33,84].
3.3. Conclusions
The prevalence of human babesiosis has probably been underestimated throughout the world [44]. Babesiosis also afflicts livestock and pets [52]. The clinical diagnostic characteristics and simple methodology of the FISH tests show that they can complement existing diagnostic methods to meet an increasing need to specifically and easily identify Babesia infections in patients and animals. FISH tests can be used in mixed infections. FISH is also useful for histopathological investigations to identify Babesia parasites sequestered in tissues [45]. Species-specific Babesia FISH tests, that are presently being developed, can address more precise diagnostic requirements in babesiosis.
4. FISH Tests for Tuberculosis
4.1. Background
Pulmonary mycobacterial infections in humans are caused mostly by Mycobacterium tuberculosis (MTB) and the closely related species Mycobacterium bovis, both of which belong to the Mycobacterium tuberculosis complex (MTBC) [51]. Infections with non-tuberculous mycobacteria (NTM), including the Mycobacterium avium complex (MAC), M. kansasii, M. fortuitum, M. xenopi, M. abscessus, and M. simiae also occur worldwide, making their differential diagnosis important for clinical purposes [51,85,86,87]. Infections with MAC are common in late-stage human immunodeficiency virus infections, where the mycobacteria are often restricted to lymphoid tissue. FISH provides a sensitive and specific method for detecting MAC by in biopsied tissues, which is important because the management and treatment of patients with MTBC and NTM infections are different [30]. Norcardiosis, caused by related Norcadia species widely distributed in the environment, also needs to be differentiated from MTB during lung infections [88].
Microscopic examination for acid-fast staining (AFS) bacilli, e.g., with the Ziehl-Neelsen stain, in sputum or tissue plays an important role in the diagnosis of tuberculosis [89]. AFS does not differentiate between mycobacterial species. It also lacks sufficient sensitivity with sputum smears and tissue samples. Sensitivity is increased in sputum smears and biopsied tissue by staining with auramine and detecting fluorescence in a LED fluorescence microscope [90], similar to that used for FISH tests (Figure S1). The Xpert® MTB/RIF system or Xpert (Cepheid, Sunnyvale, CA, USA), is a PCR-based nucleic acid amplification (NAA) technique that detects specific DNA sequences of MTB [91,92,93]. Xpert is recommended by the WHO for identifying MTB and rifampicin resistance in the sputum of adults and children presumed to have tuberculosis [91,92,93]. It is approximately 100 times more sensitive for detecting MTB than conventional AFS, but Xpert identifies only MTB, and its use in many resource-constrained endemic countries is limited by cost. Culturing clinical specimens continues to have an important role in identifying infecting mycobacteria in tuberculosis-like disease, especially in smear negative, pediatric or extra pulmonary infections and resource-limited laboratories. Culture techniques that significantly reduce culture times for identifying mycobacteria are becoming available to facilitate diagnosis [94]. Immunochromatographic tests to detect specific proteins produced by MTBC are more cost-effective than PCR tests, but, as yet, do not have the desired specificity and sensitivity [92,93].
4.2. FISH Tests for Identifying the Genus Mycobacterium as Well as the Mycobacterium Tuberculosis and Mycobacterium Avium Complexes in Culture
A simple and rapid test for directly identifying MTB and NTM in sputum and tissues that can be used by resource-limited laboratories in endemic countries is therefore expected to greatly aid tuberculosis control worldwide [92,93]. Two dual color FISH tests, with simple protocols (Figure S2), and requiring only a LED fluorescence microscope (Figure S1), meet this need [28,29,30]. The MN Genus-MTBC FISH test used an orange fluorescent DNA probe that specifically hybridizes to the 23S rRNA of the Mycobacterium tuberculosis complex (MTBC) and a green fluorescent probe specific for the Mycobacterium and Nocardia genera (MN Genus) 16S rRNA to detect and distinguish MTBC from other mycobacteria and Nocardia species. A complementary MTBC-MAC FISH test used green and orange fluorescent probes for 23S rRNA that respectively differentiate MTBC and MAC [28,29,30].
All Mycobacterium species from reference cultures, except M. wolinskyi, reacted positively with the MN Genus-specific probe and only the M. tuberculosis complex species reacted positively with the MTBC- specific probe in the MN Genus—MTBC FISH test. Only the M. tuberculosis complex species reacted positively with the MTBC-specific probe and only the M. avium complex species reacted positively with the MAC–specific probe in the MTBC-MAC FISH test [28]. Nocardia reacted positively with the MN Genus probe but not with the MTBC- and MAC-specific probes in the MN Genus-MTBC and the MTBC-MAC tests [28]. The estimated specificity of the two FISH tests for MTBC and MAC in reference cultures was 100%, with a limit of detection of 1.5–5.1 × 104 bacteria per ml [28]. Results from the two FISH tests with reference strain cultures of M. tuberculosis, M. avium and M. kansasii [28] are reproduced in Figure 7.
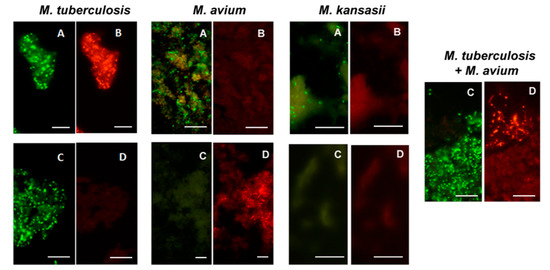
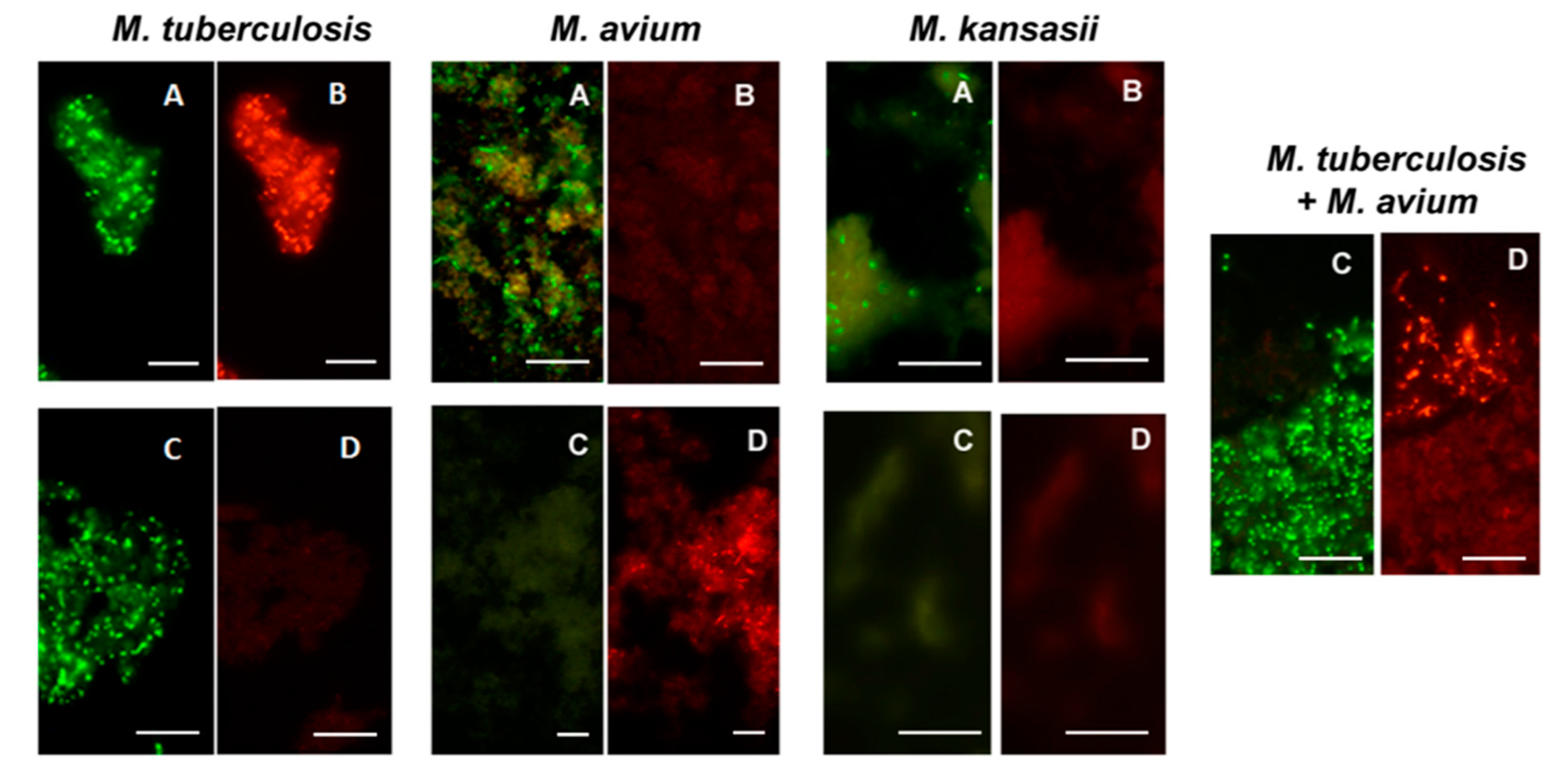
Figure 7.
Dual color fluorescence reactivity of Mycobacterium tuberculosis, Mycobacterium avium and Mycobacterium kansasii in the MN Genus-MTBC FISH and MTBC-MAC FISH tests. Paired photographs showing dual colour fluorescence in the same microscopic field with A-MN Genus- specific probe (green fluorescence) and B-MTBC-specific probe (orange fluorescence) in the MN Genus-MTBC FISH test; and C-MTBC- specific probe (green fluorescence) and D-MAC-specific probe (orange fluorescence) in the MTBC-MAC FISH test. Mycobacteria used in the FISH tests were reference cultures of M. tuberculosis, M. avium, and M. kansasii, as well as an artificially mixed culture of M. tuberculosis and M. avium. Scale bars represent approximately 50 µm. Reproduced with permission under the creative commons license from Reference [28].
4.3. FISH Tests for Identifying MTBC and MAC in Sputum
The FISH tests used for culture identification can also be used for directly detecting mycobacteria in sputum [29]. Figure 8 reproduces results obtained with the MN Genus-MTBC FISH test performed directly on a sputum smear containing MTBC which reacted with the MN Genus- and MTBC-specific probes and a different smear containing M. abscessus, an NTM, that reacted only with the MN Genus-specific probe.
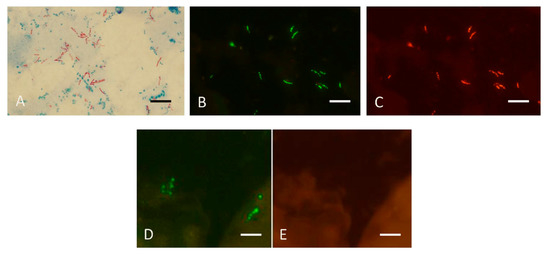
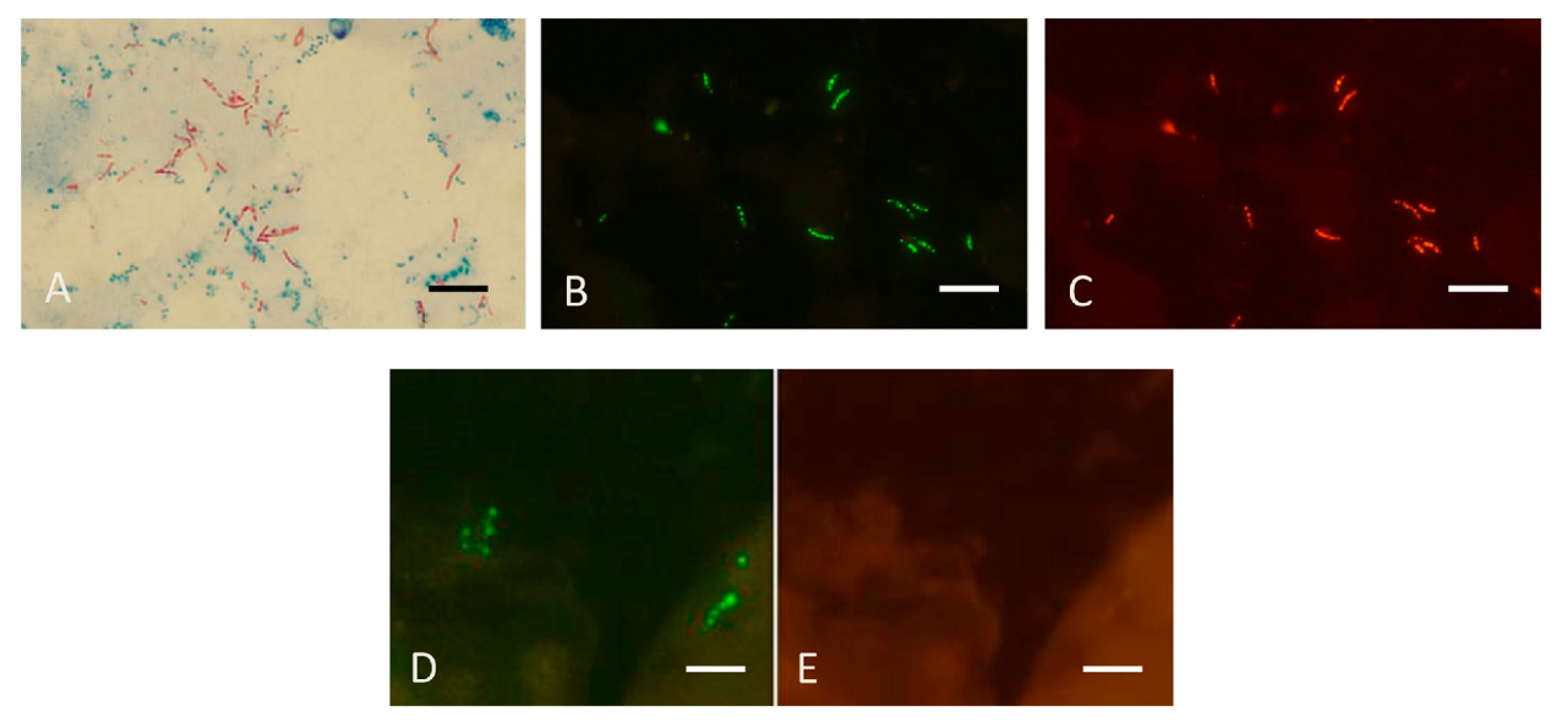
Figure 8.
Sputum smears in the MN Genus-MTBC FISH test. Photographs showing dual colour fluorescence reactivity of sputum smears from patients with Mycobacterium tuberculosis and Mycobacterium abscessus infections in the MN Genus-MTBC FISH test. A shows Ziehl–Neelsen staining for acid-fast bacilli in sputum from a patient with Mycobacterium tuberculosis infection. MN Genus-MTBC FISH test results with the MN Genus- and MTBC-specific probes on the sputum of the same patient are shown in B (green fluorescence) and C (orange fluorescence), respectively. Reactions with the MN Genus- and MTBC-specific probes in the same field in a sputum smear from another patient with Mycobacterium abscessus infection are shown in D (green fluorescence) and E (orange fluorescence), respectively. Scale bars represent approximately 5 µm. Reproduced with permission under the creative commons license from Reference [29].
4.4. Other FISH Tests for Tuberculosis
Another FISH test specific for MTBC in sputum targeting the rpoB gene coding for the β subunit of RNA polymerase has been described [95]. The rpoB FISH test however required enzyme digestion of sputum, concentration of mycobacteria by centrifugation, hybridization overnight and a UV fluorescence microscope for visualizing results [95]. Other PNA or DNA probe-based FISH tests described for MTBC and MAC [12,13,14,15,16] also require long and more stringent hybridization procedures, and a UV fluorescence microscope for viewing test results. They have not been used yet for routine diagnosis in endemic countries. The MN Genus-MTBC and MTBC-MAC FISH tests on the other hand, cost < US$5 per test, provide results in <2 h after sputum collection, do not require enzyme treatment and centrifugation, can use LED fluorescence microscopy (Figure S1), and utilize reagents that are stable at ambient temperature [29]. They are used in India [30]. All FISH assays have the advantage that they are unaffected by inhibitors in respiratory samples which reduce sensitivity and require elaborate controls for NAA tests [96,97].
4.5. Conclusions
FISH tests for tuberculosis meet internationally-expressed needs for diagnosing tuberculosis in respiratory samples [92,93]. They can complement AFS microscopy and NAA methods to detect and differentiate MTBC from MAC and other NTM in sputum and cultures. FISH is also useful in detecting MTB and MAC in biopsied tissues [30]. Table in Section 5 below compares NAA and FISH tests for tuberculosis.
5. Comparison of NAA and FISH Tests for Diagnosing Malaria and Tuberculosis
Tests that depend on amplifying specific nucleic acid sequences of pathogens and the subsequent detection and/or sequencing of the amplified nucleic acids are widely regarded as the gold standard for diagnostic tests because of their high sensitivity and specificity. Two NAA techniques that can be used for RNA and DNA, are based on PCR [98] and LAMP [99]. Diagnostic test needs vary considerably for different pathogens and the diseases caused by them. Malaria and tuberculosis are parasitic and bacterial diseases respectively of great global clinical concern [50,51]. The use of PCR and LAMP tests for identifying pathogens causing the two diseases are therefore compared with FISH tests in Table 2.

Table 2.
Comparison of two NAA and FISH tests for malaria and tuberculosis.
6. Overall Conclusions and Future Prospects
The simplicity, cost, modest infrastructure/equipment/reagent requirements, reagent stability, good diagnostic parameters, and the ability to identify pathogens at the species level, suggest that FISH tests can be used in advanced as well as resource-constrained diagnostic laboratories throughout the world. FISH tests, therefore, can complement existing diagnostic tests in both disease-endemic and non-endemic countries. FISH tests are particularly advantageous for identifying pathogens at the species level. Flow cytometry combined with FISH has been shown able to rapidly identify potentially pathogenic bacteria present in food, water, air and biofilms formed on various abiotic surfaces [110]. The use of the Flow-FISH methodology [24,110] for identifying causative pathogens in infectious diseases therefore merits further investigation. FISH may also be usefully interfaced with advanced optical and microscopic techniques [22,23,25,111,112] to further expand its scope for identifying infecting pathogens for research and diagnostic purposes.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/diagnostics12051286/s1, Figure S1: Microscope with LED & filter attachment; Figure S2: Procedure for TB FISH.
Author Contributions
J.S.S. and R.R. wrote and approved the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
JSS is stockholder and RR is science advisor at IGeneX Inc., and IDFISH Inc.
Abbreviations
AFS–acid-fast staining; FISH—fluorescence in situ hybridization; ISH—in situ hybridization; LAMP—loop-mediated isothermal amplification; LED—light emitting diode; LNA—locked nuclei acid; MAC—Mycobacterium avium complex; MDR—multi drug resistant; MN—Mycobacterium and Nocardia; MTB—Mycobacterium tuberculosis; MTBC—Mycobacterium tuberculosis complex; NAA—nuclei acid amplification; NTM—non-tuberculous mycobacteria; PCR—polymerase chain reaction; rRNA—ribosomal RNA; SARS-CoV-2—severe acute respiratory syndrome coronavirus 2.
References
- Gall, J.G.; Pardue, M.L. Formation and detection of RNA-DNA hybrid molecules in cytological preparations. Proc. Natl. Acad. Sci. USA 1969, 63, 378–383. [Google Scholar] [CrossRef] [PubMed]
- John, H.; Birnstiel, M.; Jones, K. RNA: DNA hybrids at the cytological level. Nature 1969, 223, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Ried, T.; Schröck, E.; Ning, Y.; Wienberg, J. Chromosome painting: A useful art. Hum. Mol. Genet. 1998, 7, 1619–1626. [Google Scholar] [CrossRef]
- Hu, Q.; Maurais, E.G.; Ly, P. Cellular and genomic approaches for exploring structural chromosomal rearrangements. Chromosome Res. 2020, 28, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, S.J.; DeLong, E.F.; Olsen, G.J.; Pace, N.R. Phylogenetic group-specific oligodeoxynucleotide probes for identification of single microbial cells. J. Bacteriol. 1988, 170, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Landegent, J.E.; Jansen in de Wal, N.; Baan, R.A.; Hoeijmakers, J.H.J.; van der Ploeg, M. 2-Acetylaminofluorene-modified probes for the indirect hybridocytochemical detection of specific nucleic acid sequences. Exp. Cell Res. 1984, 153, 61–72. [Google Scholar] [CrossRef]
- Pinkel, D.; Straume, T.; Gray, J.W. Cytogenetic analysis using quantitative, high-density fluorescence hybridization. Proc. Natl. Acad. Sci. USA 1986, 83, 2934–2938. [Google Scholar] [CrossRef]
- Pinkel, D.; Ladegent, J.; Collins, C.; Fuscoe, J.; Segraves, R.; Lucas, J.; Gray, J. Fluorescence in situ hybridization with human chromosome-specific libraries: Detection of trisomy 21 and translocations of chromosome 4. Proc. Natl. Acad. Sci. USA 1988, 85, 9138–9142. [Google Scholar] [CrossRef]
- DeLong, E.F.; Wickham, G.S.; Pace, N.R. Phylogenetic stains: Ribosomal RNA-based probes for the identification of single cells. Science 1989, 243, 1360–1363. [Google Scholar] [CrossRef]
- DeLong, E.F.; Shah, J. Fluorescent, ribosomal RNA probes for clinical application: A research review. Diagn. Clin. Test. 1990, 28, 41–44. [Google Scholar]
- Shah, J.S.; Pieciak, W.; Liu, J.; Buharin, A.; Lane, D.J. Diversity of host species and strains of Pneumocystis carinii is based on rRNA sequences. Clin. Diagn. Lab. Immunol. 1996, 3, 119–127. [Google Scholar] [CrossRef]
- Stender, H.; Lund, K.; Petersen, K.H.; Rasmussen, O.F.; Hongmanee, P.; Miorner, H.; Godtfredsen, S.E. Fluorescence in situ hybridization assay using peptide nucleic acid probes for differentiation between tuberculosis and non-tuberculosis Mycobacterium species in smears of Mycobacterium cultures. J. Clin. Microbiol. 1999, 37, 2760–2765. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Ke, Z.; Ma, J.; Liu, L.; Li, Y. The fluorescence in situ hybridization for diagnosis of Mycobacterium tuberculosis complex in sputum samples. Ann. Clin. Lab. Sci. 2015, 45, 631–638. [Google Scholar]
- St. Amand, A.L.; Frank, D.N.; De Groote, M.A.; Basaraba, R.J.; Orme, I.M.; Pace, N.R. Use of specific rRNA oligonucleotide probes for microscopic detection of Mycobacterium tuberculosis in culture and tissue specimens. J. Clin. Microbiol. 2005, 43, 5369–5371. [Google Scholar] [CrossRef] [PubMed]
- St. Amand, A.L.; Frank, D.N.; De Groote, M.A.; Pace, N.R. Use of specific rRNA oligonucleotide probes for microscopic detection of Mycobacterium avium complex organisms in tissue. J. Clin. Microbiol. 2005, 43, 1505–1514. [Google Scholar] [CrossRef]
- Lefmann, M.; Schweickert, B.; Buchholz, P.; Göbel, U.B.; Ulrichs, T.; Seiler, P.; Theegarten, D.; Moter, A. Evaluation of peptide nucleic acid-fluorescence in situ hybridization for identification of clinically relevant mycobacteria in clinical specimens and tissue sections. J. Clin. Microbiol. 2006, 44, 3760–3767. [Google Scholar] [CrossRef] [PubMed]
- Rigby, S.; Procop, G.W.; Haase, G.; Wilson, D.; Hall, G.; Kurtzman, C.; Oliveira, K.; Von Oy, S.; Hyldig-Nielsen, J.J.; Coull, J.; et al. Fluorescence in situ hybridization with peptide nucleic acid probes for rapid identification of Candida albicans directly from blood culture bottles. J. Clin. Microbiol. 2000, 40, 2182–2186. [Google Scholar] [CrossRef]
- Machado, A.; Almeida, C.; Salgueiro, D.; Henriques, A.; Vaneechoutte, M.; Haesebrouck, F.; Vieira, M.J.; Rodrigues, L.; Azevedo, N.F.; Cerca, N. Fluorescence in situ hybridization method using peptide nucleic acid probes for rapid detection of Lactobacillus and Gardnerella spp. BMC Microbiol. 2013, 13, 82. [Google Scholar] [CrossRef]
- Shah, J.S.; Harris, N.S. In Situ- Hybridization for Detecting Target Nucleic Acid. US Patent No. 6,165,723, 2000. [Google Scholar]
- Silahtaroglu, A.; Pfundheller, H.; Koshkin, A.; Tommerup, N.; Kauppinen, S. LNA-modified oligonucleotides are highly efficient as FISH probes. Cytogenet. Genome Res. 2004, 107, 2–37. [Google Scholar] [CrossRef]
- Moter, A.; Göbel, U.B. Fluorescence in situ hybridization (FISH) for direct visualization of microorganisms. J. Microbiol. Methods 2000, 41, 85–112. [Google Scholar] [CrossRef]
- Khan, M.; Yoo, S.J.; Clijsters, M.; Backaert, W.; Vanstapel, A.; Speleman, K.; Lietaer, C.; Choi, S.; Hether, T.D.; Marcelis, L.; et al. Visualizing in deceased COVID-19 patients how SARS-CoV-2 attacks the respiratory and olfactory mucosae but spares the olfactory bulb. Cell 2021, 184, 5932–5949.e15. [Google Scholar] [CrossRef] [PubMed]
- Rensen, E.; Pietropaoli, S.; Mueller, F.; Weber, C.; Souquere, S.; Sommer, S.; Isnard, P.; Rabant, M.; Gibier, J.B.; Terzi, F.; et al. Sensitive visualization of SARS-CoV-2 RNA with CoronaFISH. Life Sci. Alliance 2022, 5, e202101124. [Google Scholar] [CrossRef]
- Baxter, A.E.; Niessl, J.; Morou, A.; Kaufmann, D.E. RNA flow cytometric FISH for investigations into HIV immunology, vaccination and cure strategies. AIDS Res. Ther. 2017, 14, 40. [Google Scholar] [CrossRef]
- Annunziato, P.; Lungu, O.; Gershon, A.; Silvers, D.N.; LaRussa, P.; Silverstein, S.J. In situ hybridization detection of varicella zoster virus in paraffin-embedded skin biopsy samples. Clin. Diagn. Virol. 1996, 7, 69–76. [Google Scholar] [CrossRef]
- Kim, N.; Lee, S.H.; Yi, J.; Chang, C.L. Evaluation of dual-color fluorescence in situ hybridization with peptide nucleic acid probes for the detection of Mycobacterium tuberculosis and non-tuberculous mycobacteria in clinical specimens. Ann. Lab. Med. 2015, 35, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Nuñez, J.; Avelar, F.J.; Marquez, F.; Rivas-Santiago, B.; Quiñones, C.; Guerrero-Barrera, A.L. Mycobacterium tuberculosis complex detected by modified fluorescent in situ hybridization in lymph nodes of clinical samples. J. Infect. Dev. Ctries. 2012, 6, 58–66. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shah, J.; Weltman, H.; Narciso, P.; Murphy, C.; Poruri, A.; Baliga, S.; Sharon, L.; York, M.; Cunningham, G.; Miller, S.; et al. Dual color fluorescence in situ hybridization (FISH) assays for detecting Mycobacterium tuberculosis and Mycobacterium avium complexes and related pathogens in cultures. PLoS ONE 2017, 12, e0174989. [Google Scholar] [CrossRef]
- Baliga, S.; Murphy, C.; Sharon, L.; Shenoy, S.; Biranthabail, D.; Weltman, H.; Miller, S.; Ramasamy, R.; Shah, J. Rapid method for detecting and differentiating Mycobacterium tuberculosis complex and non-tuberculous mycobacteria in sputum by fluorescence in situ hybridization with DNA probes. Int. J. Infect. Dis. 2018, 75, 1–7. [Google Scholar] [CrossRef]
- Prabhu, V.; Coelho, S.; Achappa, B.; Baliga, S.; Sharon, L.; Shah, J. Role of fluorescence in situ hybridization in detecting Mycobacterium avium complex presenting as fever in treatment failure HIV. J. Clin. Tuberc. Other Mycobact. Dis. 2020, 21, 100188. [Google Scholar] [CrossRef]
- Millogo, A.; Loukil, A.; Fellag, M.; Diallo, B.; Ouedraogo, A.S.; Godreuil, S.; Drancourt, M. Fluorescent Hybridization of Mycobacterium leprae in skin samples collected in Burkina Faso. J. Clin. Microbiol. 2020, 58, e02130-19. [Google Scholar] [CrossRef]
- Swidsinski, A.; Mendling, W.; Loening-Baucke, V.; Swidsinski, S.; Dörffel, Y.; Scholze, J.; Lochs, H.; Verstraelen, H. An adherent Gardnerella vaginalis biofilm persists on the vaginal epithelium after standard therapy with oral metronidazole. Am. J. Obstet. Gynecol 2008, 198, 97.e1–97.e6. [Google Scholar] [CrossRef]
- IGeneX. Diagnostic Tests for Bartonella. Available online: https://igenex.com/disease/bartonella/ (accessed on 4 April 2022).
- Graczyk, T.K.; Grimes, B.H.; Knight, R.; Da Silva, A.J.; Pieniazek, N.J.; Veal, D.A. Detection of Cryptosporidium parvum and Giardia lamblia carried by synanthropic flies by combined fluorescent in situ hybridization and a monoclonal antibody. Am. J. Trop. Med. Hyg. 2003, 68, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Radwanska, M.; Magez, S.; Perry-O’Keefe, H.; Stender, H.; Coull, J.; Sternberg, J.M.; Büscher, P.; Hyldig-Nielsen, J.J. Direct detection and identification of African trypanosomes by fluorescence in situ hybridization with peptide nucleic acid probes. J. Clin. Microbiol. 2002, 40, 4295–4297. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Frickmann, H.; Alnamar, Y.; Essig, A.; Clos, J.; Racz, P.; Barth, T.F.; Hagen, R.M.; Fischer, M.; Poppert, S. Rapid identification of Leishmania spp. in formalin-fixed, paraffin-embedded tissue samples by fluorescence in situ hybridization. Trop. Med. Int. Health 2012, 17, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Jayasena Kaluarachchi, T.; Wickremasinghe, R.; Weerasekera, M.; Yasawardene, S.; McBain, A.J.; Yapa, B.; De Silva, H.; Menike, C.; Jayathilake, S.; Munasinghe, A.; et al. Diagnosing human cutaneous leishmaniasis using fluorescence in situ hybridization. Pathog. Glob. Health 2021, 115, 307–314. [Google Scholar] [CrossRef]
- Muresu, R.; Rubino, S.; Rizzu, P.; Baldini, A.; Colombo, M.; Cappuccinelli, P. A new method for identification of Trichomonas vaginalis by fluorescent DNA in situ hybridization. J. Clin. Microbiol. 1994, 32, 1018–1022. [Google Scholar] [CrossRef]
- Shah, J.; Mark, O.; Weltman, H.; Barcelo, N.; Lo, W.; Wronska, D.; Kakkilaya, S.; Rao, A.; Bhat, S.T.; Sinha, R.; et al. Fluorescence in situ hybridization (FISH) assays for diagnosing malaria in endemic areas. PLoS ONE 2015, 10, e0136726. [Google Scholar] [CrossRef]
- Osoga, J.; Waitumbi, J.; Guyah, B.; Sande, J.; Arima, C.; Ayaya, M.; Moseti, C.; Morang’a, C.; Wahome, M.; Achilla, R.; et al. Comparative evaluation of fluorescent in situ hybridization and Giemsa microscopy with quantitative real-time PCR technique in detecting malaria parasites in a holoendemic region of Kenya. Malar. J. 2017, 16, 297. [Google Scholar] [CrossRef]
- Kandie, R.; Ochola, R.; Njaanake, K. Evaluation of fluorescent in-situ hybridization technique for diagnosis of malaria in Ahero Sub-County hospital, Kenya. BMC Infect. Dis. 2018, 18, 22. [Google Scholar] [CrossRef]
- Shah, J.; Poruri, A.; Mark, O.; Khadilka, U.; Mohring, F.; Moon, R.W.; Ramasamy, R. A dual colour fluorescence in situ hybridization (FISH) assay for identifying the zoonotic malaria parasite Plasmodium knowlesi with a potential application for the specific diagnosis of knowlesi malaria in peripheral-level laboratories of Southeast Asia. Parasit. Vectors 2017, 10, 342. [Google Scholar] [CrossRef]
- Shah, J.S.; Mark, O.; Caoili, E.; Poruri, A.; Horowitz, R.I.; Ashbaugh, A.D.; Ramasamy, R. A fluorescence in situ hybridization (FISH) test for diagnosing babesiosis. Diagnostics 2020, 10, 377. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.S.; Caoili, E.; Patton, M.F.; Tamhankar, S.; Myint, M.M.; Poruri, A.; Mark, O.; Horowitz, R.I.; Ashbaugh, A.D.; Ramasamy, R. Combined immunofluorescence (IFA) and fluorescence in situ hybridization (FISH) assays for diagnosing babesiosis in patients from the USA, Europe and Australia. Diagnostics 2020, 10, 761. [Google Scholar] [CrossRef] [PubMed]
- Albertyńska, M.; Okła, H.; Jasik, K.; Urbańska-Jasik, D.; Pol, P. Interactions between Babesia microti merozoites and rat kidney cells in a short-term in vitro culture and animal model. Sci. Rep. 2021, 11, 23663. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.F.; Zheng, Y.C.; Jiang, R.R.; Li, H.; Huo, Q.B.; Jiang, B.G.; Sun, Y.; Jia, N.; Wang, Y.W.; Ma, L.; et al. Epidemiological, clinical, and laboratory characteristics of 48 cases of “Babesia venatorum” infection in China: A descriptive study. Lancet Infect. Dis. 2015, 15, 196–203. [Google Scholar] [CrossRef]
- Woese, C.R. Bacterial evolution. Microbiol. Rev. 1987, 51, 221–271. [Google Scholar] [CrossRef]
- Frischer, M.E.; Floriani, P.J.; Nierzwicki-Bauer, S.A. Differential sensitivity of 16S rRNA targeted oligonucleotide probes used for fluorescence in situ hybridization is a result of ribosomal higher order structure. Can. J. Microbiol. 1996, 42, 1061–1071. [Google Scholar] [CrossRef]
- Fuchs, B.M.; Wallner, G.; Beisker, W.; Schwippl, I.; Ludwig, W.; Amann, R. Flow cytometric analysis of the in situ accessibility of Escherichia coli 16S rRNA for fluorescently labeled oligonucleotide probes. Appl. Environ. Microbiol. 1998, 64, 4973–4982. [Google Scholar] [CrossRef]
- World Malaria Report 2021; World Health Organization: Geneve, Switzerland; Available online: www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2021 (accessed on 2 April 2022).
- Global Tuberculosis Report 2021; World Health Organization: Geneve, Switzerland; Available online: https://www.who.int/publications/digital/global-tuberculosis-report-2021 (accessed on 4 April 2022).
- Bajer, A.; Beck, A.; Beck, R.; Behnke, J.M.; Dwużnik-Szarek, D.; Eichenberger, R.M.; Farkas, R.; Fuehrer, H.-P.; Heddergott, M.; Jokelainen, P.; et al. Babesiosis in Southeastern, Central and Northeastern Europe: An emerging and re-emerging tick-borne disease of humans and animals. Microorganisms 2022, 10, 945. [Google Scholar] [CrossRef]
- Ramasamy, R. Zoonotic malaria–Global overview and research and policy needs. Front. Public Health 2014, 2, 123. [Google Scholar] [CrossRef]
- Mace, K.E.; Lucchi, N.W.; Tan, K.R. Malaria Surveillance—United States, 2017. MMWR Surveill. Summ. 2021, 70, 1–35. [Google Scholar] [CrossRef]
- Gimenez, A.M.; Marques, R.F.; Regiart, M.; Bargieri, D.Y. Diagnostic methods for non-falciparum malaria. Front. Cell Infect. Microbiol. 2021, 11, 681063. [Google Scholar] [CrossRef] [PubMed]
- Snounou, G.; Viriyakosol, S.; Zhu, X.P.; Jarra, W.; Pinheiro, L.; do Rosario, V.E.; Thaithong, S.; Brown, K.N. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol. Biochem. Parasitol. 1993, 61, 315–320. [Google Scholar] [CrossRef]
- Polley, S.D.; González, I.J.; Mohamed, D.; Daly, R.; Bowers, K.; Watson, J.; Mewse, E.; Armstrong, M.; Gray, C.; Perkins, M.D.; et al. Clinical evaluation of a loop-mediated amplification kit for diagnosis of imported malaria. J. Infect. Dis. 2013, 208, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Eshel, Y.; Houri-Yafin, A.; Benkuzari, H.; Lezmy, N.; Soni, M.; Charles, M.; Swaminathan, J.; Solomon, H.; Sampathkumar, P.; Premji, Z.; et al. Evaluation of the Parasight platform for malaria diagnosis. J. Clin. Microbiol. 2017, 55, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Pillay, E.; Khodaiji, S.; Bezuidenhout, B.C.; Litshie, M.; Coetzer, T.L. Evaluation of automated malaria diagnosis using the Sysmex XN-30 analyser in a clinical setting. Malar. J. 2019, 18, 15. [Google Scholar] [CrossRef]
- Singh, B.; Kim Sung, L.; Matusop, A.; Radhakrishnan, A.; Shamsul, S.S.; Cox-Singh, J.; Thomas, A.; Conway, D. A large focus of naturally acquired Plasmodium knowlesi infections in human beings. Lancet 2004, 363, 1017–1024. [Google Scholar] [CrossRef]
- Vythilingam, I.; Noorazian, Y.M.; Huat, T.C.; Jiram, A.I.; Yusri, Y.M.; Azahari, A.H.; Norparina, I.; Noorrain, A.; Lokmanhakim, S. Plasmodium knowlesi in humans, macaques and mosquitoes in peninsular Malaysia. Parasit. Vectors. 2008, 1, 26. [Google Scholar] [CrossRef]
- Cox-Singh, J.; Davis, T.M.; Lee, K.S.; Shamsul, S.S.; Matusop, A.; Ratnam, S.; Rahman, H.A.; Conway, D.J.; Singh, B. Plasmodium knowlesi malaria in humans is widely distributed and potentially life threatening. Clin. Infect. Dis. 2008, 46, 165–171. [Google Scholar] [CrossRef]
- Yusof, R.; Lau, Y.L.; Mahmud, R.; Fong, M.Y.; Jelip, J.; Ngian, H.U.; Mustakim, S.; Hussin, H.M.; Marzuki, N.; Mohd Ali, M. High proportion of knowlesi malaria in recent malaria cases in Malaysia. Malar. J. 2014, 13, 168. [Google Scholar] [CrossRef]
- Moyes, C.L.; Henry, A.J.; Golding, N.; Huang, Z.; Singh, B.; Baird, J.K.; Newton, P.N.; Huffman, M.; Duda, K.A.; Drakeley, C.J.; et al. Defining the geographical range of the Plasmodium knowlesi reservoir. PLoS Neg. Trop. Dis. 2014, 8, e2780. [Google Scholar] [CrossRef]
- Komaki-Yasuda, K.; Vincent, J.P.; Nakatsu, M.; Kato, Y.; Ohmagari, N.; Kano, S. A novel PCR-based system for the detection of four species of human malaria parasites and Plasmodium knowlesi. PLoS ONE 2018, 13, e0191886. [Google Scholar] [CrossRef] [PubMed]
- Foster, D.; Cox-Singh, J.; Mohamad, D.S.A.; Krishna, S.; Chin, P.P.; Singh, B. Evaluation of three rapid diagnostic tests for the detection of human infections with Plasmodium knowlesi. Malar. J. 2014, 13, 60. [Google Scholar] [CrossRef] [PubMed]
- Vannier, E.; Gewurz, B.E.; Krause, P.J. Human babesiosis. Infect. Dis. Clin. N. Am. 2008, 22, 469–488. [Google Scholar] [CrossRef]
- Ord, R.L.; Lobo, C.A. Human babesiosis: Pathogens, prevalence, diagnosis and treatment. Curr. Clin. Microbiol. Rep. 2015, 2, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Krause, P.J. Human babesiosis. Int. J. Parasitol. 2019, 49, 165–174. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Surveillance for Babesiosis—United States, 2019 Annual Summary. Available online: https://www.cdc.gov/parasites/babesiosis/resources/Surveillance_Babesiosis_US_2019.pdf (accessed on 3 April 2022).
- Swei, A.; O’Connor, K.E.; Couper, L.I.; Thekkiniath, J.; Conrad, P.A.; Padgett, K.A.; Burns, J.; Yoshimizu, M.H.; Gonzales, B.; Munk, B.; et al. Evidence for transmission of the zoonotic apicomplexan parasite Babesia duncani by the tick Dermacentor albipictus. Int. J. Parasitol. 2019, 49, 95–103. [Google Scholar] [CrossRef]
- Dunn, J.M.; Krause, P.J.; Davis, S.; Vannier, E.G.; Fitzpatrick, M.C.; Rollend, L.; Belperron, A.A.; States, S.L.; Stacey, A.; Bockenstedt, L.K.; et al. Borrelia burgdorferi promotes the establishment of Babesia microti in the northeastern United States. PLoS ONE 2014, 9, e115494. [Google Scholar] [CrossRef]
- Hersh, M.H.; Ostfeld, R.S.; McHenry, D.J.; Tibbetts, M.; Brunner, J.L.; Killilea, M.E.; LoGiudice, K.; Schmidt, K.A.; Keesing, F. Co-Infection of blacklegged ticks with Babesia microti and Borrelia burgdorferi is higher than expected and acquired from small mammal hosts. PLoS ONE 2014, 9, e99348. [Google Scholar] [CrossRef]
- Diuk-Wasser, M.A.; Vannier, E.; Krause, P.J. Coinfection by Ixodes tick-borne pathogens: Ecological, epidemiological, and clinical consequences. Trends Parasitol. 2016, 32, 30–42. [Google Scholar] [CrossRef]
- Djokic, V.; Primus, S.; Akoolo, L.; Chakraborti, M.; Parveen, N. Age-related differential stimulation of immune response by Babesia microti and Borrelia burgdorferi during acute phase of infection affects disease severity. Front. Immunol. 2018, 9, 2891. [Google Scholar] [CrossRef]
- Primus, S.; Akoolo, L.; Schlachter, S.; Gedroic, K.; Rojtman, A.D.; Parveen, N. Efficient detection of symptomatic and asymptomatic patient samples for Babesia microti and Borrelia burgdorferi infection by multiplex qPCR. PLoS ONE 2018, 13, e0196748. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Tick-Borne Relapsing Fever (TBRF). Available online: https://www.cdc.gov/relapsing-fever/index.html (accessed on 4 April 2022).
- Centers for Disease Control and Prevention. Lyme Disease. Available online: https://www.cdc.gov/lyme/index.html (accessed on 4 April 2022).
- Wang, G.; Villafuerte, P.; Zhuge, J.; Visintainer, P.; Wormser, G.P. Comparison of a quantitative PCR assay with peripheral blood smear examination for detection and quantitation of Babesia microti infection in humans. Diagn. Microbiol. Infect. Dis. 2015, 82, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Akoolo, L.; Schlachter, S.; Khan, R.; Alter, L.; Rojtman, A.D.; Gedroic, K.; Bhanot, P.; Parveen, N. A novel quantitative PCR detects Babesia infection in patients not identified by currently available non-nucleic acid amplification tests. BMC Microbiol. 2017, 17, 16. [Google Scholar] [CrossRef] [PubMed]
- Souza, S.S.; Bishop, H.S.; Sprinkle, P.; Qvarnstrom, Y. Comparison of Babesia microti real-time polymerase chain reaction assays for confirmatory diagnosis of babesiosis. Am. J. Trop. Med. Hyg. 2016, 95, 1413–1416. [Google Scholar] [CrossRef]
- Ward, S.J.; Stramer, S.L.; Szczepiorkowski, Z.M. Assessing the risk of Babesia to the United States blood supply using a risk-based decision-making approach: Report of AABB’s ad hoc Babesia policy working group. Transfusion 2018, 58, 1916–1923. [Google Scholar] [CrossRef]
- Shah, J.S.; D’Cruz, I.; Ward, S.; Harris, N.S.; Ramasamy, R. Development of a sensitive PCR-dot blot assay to supplement serological tests for diagnosing Lyme disease. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 701–709. [Google Scholar] [CrossRef]
- Mada, P.K.; Zulfiqar, H.; Joel Chandranesan, A.S. Bartonellosis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK430874/ (accessed on 6 May 2022).
- Winthrop, K.L.; McNelley, E.; Kendall, B.; Marshall-Olson, A.; Morris, C.; Cassidy, M.; Saulson, A.; Hedberg, K. Pulmonary nontuberculous mycobacterial disease prevalence and clinical features: An emerging public health disease. Am. J. Respir. Crit. Care Med. 2010, 182, 977–982. [Google Scholar] [CrossRef]
- O’Brien, R.J.; Geiter, L.J.; Snider, D.E. The epidemiology of nontuberculous mycobacterial diseases in the United States. Results from a national survey. Am. Rev. Respir. Dis. 1987, 135, 1007–1014. [Google Scholar]
- Prince, D.S.; Peterson, D.D.; Steiner, R.M.; Gottlieb, J.E.; Scott, R.; Israel, H.L.; Figueroa, W.G.; Fish, J.E. Infection with Mycobacterium avium complex in patients without predisposing conditions. N. Engl. J. Med. 1989, 321, 863–868. [Google Scholar] [CrossRef]
- Nocardiosis; Centre for Disease Control: Atlanta, GA, USA, 2017; Available online: https://www.cdc.gov/nocardiosis/index.html (accessed on 4 April 2022).
- Koch, M.L.; Cote, R.A. Comparison of fluorescence microscopy with Ziehl-Neelsen stain for demonstration of acid-fast bacilli in smear preparations and tissue sections. Am. Rev. Resp Dis. 1965, 91, 283–284. [Google Scholar]
- Assefa, G.; Desta, K.; Araya, S.; Girma, S.; Mihret, A.; Hailu, T.; Atnafu, A.; Endalafer, N.; Abera, A.; Bekele, S.; et al. Diagnostic efficacy of light-emitting diode (LED) fluorescence based microscope for the diagnosis of tuberculous lymphadenitis. PLoS ONE 2021, 16, e0255146. [Google Scholar] [CrossRef] [PubMed]
- Xpert MTB/RIF Implementation Manual; World Health Organization: Geneve, Switzerland, 2014; Available online: https://apps.who.int/iris/bitstream/handle/10665/112469/9789241506700_eng.pdf (accessed on 5 May 2022).
- Pai, M.; Schito, M. Tuberculosis diagnosis 2015: Landscape, priorities, needs and prospects. J. Infect. Dis. 2015, 211, S21–S28. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. UNITAID–Tuberculosis Diagnostics Technology and Market Landscape, 4th ed.; WHO: Geneva, Switzerland, 2015; pp. 1–88.
- Asmar, S.; Drancourt, M. Rapid culture-based diagnosis of pulmonary tuberculosis in developed and developing countries. Front. Microbiol. 2015, 6, 1184. [Google Scholar] [CrossRef]
- Loukil, A.; Kirtania, P.; Bedotto, M.; Drancourt, M. FISHing Mycobacterium tuberculosis complex using a rpoB DNA probe bait. J. Clin. Microbiol. 2018, 56, e00568-18. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Updated guidelines for the use of nucleic acid amplification tests in the diagnosis of tuberculosis. Morb. Mortal. Wkly. Rep. 2009, 58, 7–10. [Google Scholar]
- Centers for Disease Control and Prevention USA. Report of an Expert Consultation on the Uses of Nucleic Acid Amplification Tests for the Diagnosis of Tuberculosis. 2012. Available online: https://www.cdc.gov/tb/publications/guidelines/amplification_tests/amplificationtests.pdf (accessed on 4 April 2022).
- Mullis, K.B.; Faloona, F.A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987, 155, 335–350. [Google Scholar] [CrossRef] [PubMed]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, E63. [Google Scholar] [CrossRef]
- Pooran, A.; Theron, G.; Zijenah, L.; Chanda, D.; Clowes, P.; Mwenge, L.; Mutenherwa, F.; Lecesse, P.; Metcalfe, J.; Sohn, H.; et al. Point of care Xpert MTB/RIF versus smear microscopy for tuberculosis diagnosis in southern African primary care clinics: A multicentre economic evaluation. Lancet Glob. Health 2019, 7, e798–e807. [Google Scholar] [CrossRef]
- Chitpim, N.; Jittikoon, J.; Udomsinprasert, W.; Mahasirimongkol, S.; Chaikledkaew, U. Cost-utility analysis of molecular testing for tuberculosis diagnosis in suspected pulmonary tuberculosis in Thailand. Clinicoecon. Outcomes Res. 2022, 14, 61–73. [Google Scholar] [CrossRef]
- Vassall, A.; Siapka, M.; Foster, N.; Cunnama, L.; Ramma, L.; Fielding, K.; McCarthy, K.; Churchyard, G.; Grant, A.; Sinanovic, E. Cost-effectiveness of Xpert MTB/RIF for tuberculosis diagnosis in South Africa: A real-world cost analysis and economic evaluation. Lancet Glob. Health 2017, 5, e710–e719. [Google Scholar] [CrossRef]
- Zwerling, A.A.; Sahu, M.; Ngwira, L.G.; Khundi, M.; Harawa, T.; Corbett, E.L.; Chaisson, R.E.; Dowdy, D.W. Screening for tuberculosis among adults newly diagnosed with hiv in sub-saharan Africa: A cost-effectiveness analysis. J. Acquir. Immun. Defic. Syndr. 2015, 70, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Chakravorty, S.; Simmons, A.M.; Rowneki, M.; Parmar, H.; Cao, Y.; Ryan, J.; Banada, P.P.; Deshpande, S.; Shenai, S.; Gall, A.; et al. The new Xpert MTB/RIF Ultra: Improving detection of Mycobacterium tuberculosis and resistance to rifampin in an assay suitable for point-of-care testing. mBio 2017, 8, e00812–e00817. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Miyake, H.; Kim, H.S.; Tanabe, M.; Arai, M.; Kawai, S.; Yamane, A.; Wataya, Y. Species-specific PCR detection of malaria parasites by microtiter plate hybridization: Clinical study with malaria patients. J. Clin. Microbiol. 1995, 33, 2342–2346. [Google Scholar] [CrossRef] [PubMed]
- Selvarajah, D.; Naing, C.; Htet, N.H.; Mak, J.W. Loop-mediated isothermal amplification (LAMP) test for diagnosis of uncomplicated malaria in endemic areas: A meta-analysis of diagnostic test accuracy. Malar. J. 2020, 19, 211. [Google Scholar] [CrossRef] [PubMed]
- Horne, D.J.; Kohli, M.; Zifodya, J.S.; Schiller, I.; Dendukuri, N.; Tollefson, D.; Schumacher, S.G.; Ochodo, E.A.; Pai, M.; Steingart, K.R. Xpert MTB/RIF and Xpert MTB/RIF Ultra for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst. Rev. 2019, 6, CD009593. [Google Scholar] [CrossRef] [PubMed]
- Shete, P.B.; Farr, K.; Strnad, L.; Gray, C.M.; Cattamanchi, A. Diagnostic accuracy of TB-LAMP for pulmonary tuberculosis: A systematic review and meta-analysis. BMC Infect. Dis. 2019, 19, 268. [Google Scholar] [CrossRef]
- Miotto, P.; Bigoni, S.; Migliori, G.B.; Matteelli, A.; Cirillo, D.M. Early tuberculosis treatment monitoring by Xpert(R) MTB/RIF. Eur. Respir. J. 2012, 39, 1269–1271. [Google Scholar] [CrossRef]
- Zand, E.; Froehling, A.; Schoenher, C.; Zunabovic-Pichler, M.; Schlueter, O.; Jaeger, H. Potential of flow cytometric approaches for rapid microbial detection and characterization in the food industry-a review. Foods 2021, 10, 3112. [Google Scholar] [CrossRef]
- Lukic, D.; Karygianni, L.; Flury, M.; Attin, T.; Thurnheer, T. Endodontic-like oral biofilms as models for multispecies interactions in endodontic diseases. Microorganisms 2020, 8, 674. [Google Scholar] [CrossRef]
- Meleppat, R.K.; Shearwood, C.; Seah, L.K.; Matham, M.V. Quantitative optical coherence microscopy for the in situ investigation of the biofilm. J. Biomed. Opt. 2016, 21, 127002. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).