Long Term Cell Immune Response to COVID-19 Vaccines Assessment Using a Delayed-Type Hypersensitivity (DTH) Cutaneous Test
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants, Vaccines, and Schedule
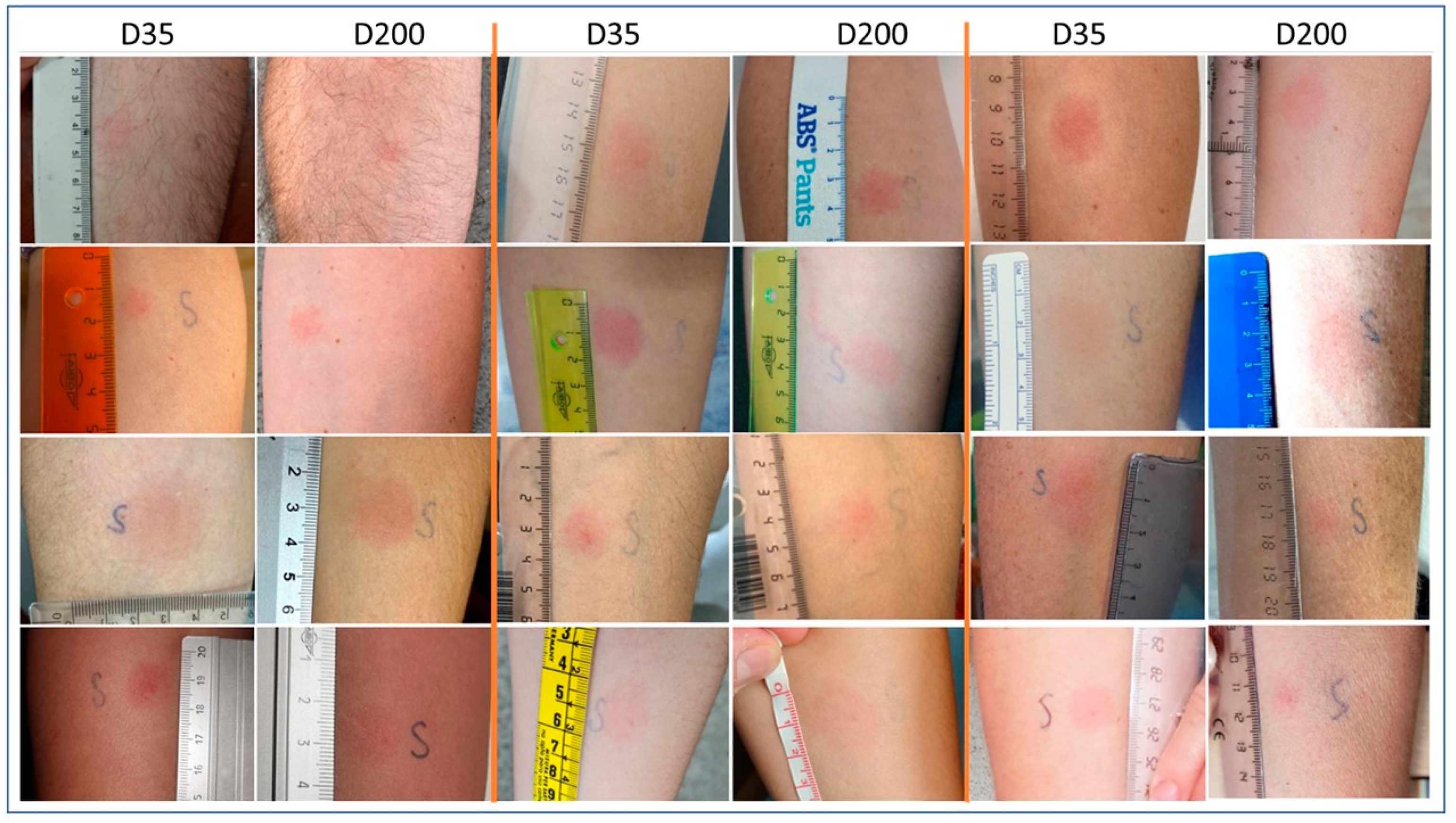
2.2. Delayed-Type Hypersensitivity (DTH) Skin Test
2.3. Serology
2.4. T-Cell Response by In Vitro Stimulation with SARS-CoV-2 Spike Protein
3. Results
3.1. Delayed-Type Hypersensitivity (DTH) Skin Test
3.2. Serology
3.3. IFN-Gamma Levels after Incubation of T-Cells with Spike RBD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Available online: www.who.int (accessed on 16 May 2022).
- Coronavirus: Commission Unveils EU Vaccines Strategy. Available online: https://ec.europa.eu/commission/presscorner/detail/en/ip_20_1103 (accessed on 16 May 2022).
- Esposito, M.; Salerno, M.; Scoto, E.; Di Nunno, N.; Sessa, F. The impact of the COVID-19 pandemic on the practice of forensic medicine: An overview. Healthcare 2022, 10, 319. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Available online: https://www.ema.europa.eu/en (accessed on 16 May 2022).
- Minidis, N.M.; Mesner, O.; Agan, B.K.; Okulicz, J.F. Delayed-type hypersensitivity (DTH) test anergy does not impact CD4 reconstitution or normalization of DTH responses during antiretroviral therapy. J. Int. AIDS Soc. 2014, 17, 18799. [Google Scholar] [CrossRef] [PubMed]
- Barrios, Y.; Franco, A.; Sánchez-Machín, I.; Poza-Guedes, P.; González-Pérez, R.; Matheu, V. A novel application of delayed-type hypersensitivity reaction to measure cellular immune response in SARS-CoV-2 exposed individuals. Clin. Immunol. 2021, 226, 108730. [Google Scholar] [CrossRef] [PubMed]
- Barrios, Y.; Franco, A.; Sánchez-Machín, I.; Poza-Guedes, P.; González-Pérez, R.; Matheu, V. The beauty of simplicity: Delayed-type hypersensitivity reaction to measure cellular Immune responses in RNA-SARS-CoV-2 vaccinated individuals. Vaccines 2021, 9, 575. [Google Scholar] [CrossRef] [PubMed]
- Meyer, B.; Torriani, G.; Yerly, S.; Mazza, L.; Calame, A.; Arm-Vernez, I.; Zimmer, G.; Agoritsas, T.; Stirnemann, J.; Spechbach, H.; et al. Validation of a commercially available SARS-CoV-2 serological immunoassay. Clin. Microbiol. Infect. Dis. 2020, 26, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- Hause, A.M.; Baggs, G.J.; Marquez, P.; Myers, T.R.; Shimabukuro, T.T.; Shay, D.K. Safety Monitoring of an Additional Dose of COVID-19 Vaccine—United States, 12 August–19 September 2021. Available online: https://www.cdc.gov/mmwr/index.html (accessed on 16 May 2022).
- Widge, A.T.; Rouphael, N.G.; Jackson, L.A.; Anderson, E.J.; Roberts, P.C.; Makhene, M.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; Pruijssers, A.J.; et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N. Engl. J. Med. 2021, 384, 80–82. [Google Scholar] [CrossRef] [PubMed]
- Lustig, Y.; Sapir, E.; Regev-Yochay, G.; Cohen, C.; Fluss, R.; Olmer, L.; Indenbaum, V.; Mandelboim, M.; Doolman, R.; Amit, S.; et al. BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: A prospective, single-centre, longitudinal cohort study in health-care workers. Lancet Respir. Med. 2021, 9, 999–1009. [Google Scholar] [CrossRef]
- Doria-Rose, N.; Suthar, M.S.; Makowski, M.; O’Connell, S.; McDermott, A.B.; Flach, B.; Ledgerwood, J.E.; Mascola, J.R.; Graham, B.S.; Lin, B.C.; et al. Antibody persistence through 6 months after the second dose of mRNA-1273 vaccine for COVID-19. N. Engl. J. Med. 2021, 384, 2259–2261. [Google Scholar] [CrossRef] [PubMed]
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Yu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021, 371, eabf4063. [Google Scholar] [CrossRef] [PubMed]
- Barrios, Y.; Rodriguez, A.; Franco, A.; Alava-Cruz, C.; Marrero-Miranda, D.; Perez-Tamajon, L.; Matheu, V. Optimizing a protocol to assess immune responses after SARS-CoV-2 vaccination in kidney-transplanted patients: In vivo DTH cutaneous test as the initial screening method. Vaccines 2021, 9, 1315. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barrios, Y.; Alava-Cruz, C.; Franco, A.; Matheu, V. Long Term Cell Immune Response to COVID-19 Vaccines Assessment Using a Delayed-Type Hypersensitivity (DTH) Cutaneous Test. Diagnostics 2022, 12, 1421. https://doi.org/10.3390/diagnostics12061421
Barrios Y, Alava-Cruz C, Franco A, Matheu V. Long Term Cell Immune Response to COVID-19 Vaccines Assessment Using a Delayed-Type Hypersensitivity (DTH) Cutaneous Test. Diagnostics. 2022; 12(6):1421. https://doi.org/10.3390/diagnostics12061421
Chicago/Turabian StyleBarrios, Yvelise, Cristina Alava-Cruz, Andres Franco, and Victor Matheu. 2022. "Long Term Cell Immune Response to COVID-19 Vaccines Assessment Using a Delayed-Type Hypersensitivity (DTH) Cutaneous Test" Diagnostics 12, no. 6: 1421. https://doi.org/10.3390/diagnostics12061421





