Deep Learning Paradigm for Cardiovascular Disease/Stroke Risk Stratification in Parkinson’s Disease Affected by COVID-19: A Narrative Review
Abstract
:1. Introduction
2. Search Strategy
3. Pathophysiology of Lung and Parkinson’s Disease during COVID-19
3.1. Acute Respiratory Distress Syndrome, Imaging, and Lung Lesions during COVID-19

3.2. Vascular Damage Due to COVID-19
3.3. Dopamine in Parkinson’s Disease with or without COVID-19
4. The Relationship between Parkinson’s Disease, Heart, Brain, and COVID-19
4.1. The Relationship between Parkinson’s Disease and CVD
4.2. The Relationship between Parkinson’s Disease and Stroke without COVID-19
4.3. The Relationship between Parkinson’s Disease and COVID-19
| SN | Citations | PS | ME | Relation * | Outcome | TRE |
|---|---|---|---|---|---|---|
| 1 | Li et al. [112] (2018) | 63 | LBBM | Stroke and CAD in PD | When it comes to reducing the risk for heart disease, exercise may be useful in some cases. It has been discovered that having high amounts of blood cholesterol, smoking cigarettes, and having a high BMI are all connected with the development of PD. | NR |
| 2 | Studer et al. [133] (2017) | 73 | LBBM | Heart-rate variability and skin resonance in PD | Both SSR and HRV tests are effective in detecting ANS failure in PD patients, not only in the later stages but also in the early stages. Patients with PD may benefit from utilizing these tests to rule out autonomic dysfunction. | NR |
| 3 | Liu et al. [134] (2014) | 32 | Self-reporting | Stroke in PD | Since cerebrovascular and neurodegenerative diseases coexist, cerebral infarction is linked to PD. However, even though levodopa raises homocysteine levels, it is the most effective and required symptomatic treatment for many PD patients. | NR |
| 4 | Becker et al. [20] (2009) | NR | LBBM | Risk of stroke in PD | Homocysteine levels that are too high in people who have PD may make them more likely to have a stroke. There has been a link between high levels of homocysteine and a higher likelihood of stroke and heart disease. Vascular disease and dementia, as well as a rise in homocysteine levels in the blood after taking levodopa, are some of the side effects. | NR |
| 5 | Levine et al. [105] (2009) | NR | LBBM | Traumatic brain injury in PD | Patients with neurological problems can benefit from exercise training by feeling less physically and mentally worn out all the time. People with PD who engage in cardiovascular activity report less fatigue as a result of their efforts. | NR |
| 6 | Rickards [135] (2005) | NR | NR | Stroke in PD | Patients with chronic neurological illnesses are more likely than the general population to experience debilitating depressive symptoms. It is unclear what causes them, but they may be multifactorial in some cases. | NR |
| 7 | Mastaglia et al. [136] (2002) | 100 | Self-reporting | Prevalence of stroke in PD | Findings were not directly compared with those of prior investigations of stroke-related mortality and morbidity in the PD group following postmortem examination. | NR |
4.4. Effect of Comorbidities on Parkinson’s Disease
| SN | Author | Year | Demographics | Age | Sex | Type | Data Size | Non-PD | PD | PD w/s COVID | PD Years | Gold Standard |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Antonini et al. [56] (2020) | 2020 | European | 68 | MF | PD with COVID | 10 | 0 | 10 | 10 | 20 | PD + COVID-19 + Respiratory dysfunctions |
| 2 | Baschi et al. [7] (2020) | 2020 | European | 60 | MF | PD with COVID | 34 | 0 | 34 | 34 | 6 | PD + COVID-19 + Pneumonia |
| 3 | Brown et al. [163] (2020) | 2020 | European | 70 | MF | PD with COVID | 102 | 40 | 62 | 51 | 4 | PD + COVID-19 + Respiratory dysfunctions |
| 4 | Cella et al. [2] (2020) | 2020 | European | 65 | MF | PD with COVID | 141 | 0 | 12 | 12 | 4 | PD + COVID-19 + Respiratory dysfunctions |
| 5 | Starmbi et al. [129] (2021) | 2021 | European | 65 | MF | PD with COVID | 105 | 0 | 32 | 32 | 4 | PD + COVID-19 + Pneumonia |
| 6 | Helmich et al. [6] (2020) | 2020 | European | NR | NR | PD with Coved | NR | NR | NR | NR | NR | PD + COVID-19 + Respiratory dysfunctions |
| 7 | Khoshnood et al. [5] (2021) | 2021 | European | NR | NR | PD with COVID | NR | NR | NR | NR | NR | PD + COVID-19 + Pneumonia |
| 8 | Lau et al. [16] (2021) | 2021 | European | NR | NR | PD with COVID | NR | NR | NR | NR | 12 | PD + COVID-19 + Respiratory dysfunctions |
| 9 | Sulzer et al. [4] (2021) | 2021 | NR | NR | NR | PD with COVID | NR | NR | NR | NR | NR | PD + COVID-19 + Respiratory dysfunctions |
| 10 | Tsivgoulis et al. [131] (2021) | 2021 | NR | NR | NR | PD with COVID | NR | NR | NR | NR | 6 | PD + COVID-19 + Pneumonia |
| 11 | Sorbera et al. [130] (2021) | 2021 | European | 65 | MF | PD with COVID | 18 | 5 | 13 | 9 | 3 | PD + COVID-19 + Pneumonia |
4.5. The Relationship between Combined Parkinson’s Disease and COVID-19 on CVD/Stroke
5. Deep Learning for CVD/Stroke Risk Assessment in PD Patients with COVID-19
5.1. Deep Learning for COVID-19 Lesion Segmentation and Its Quantification in CT
5.2. Deep Learning for CVD/Stroke Risk Assessment for Joint PD and COVID-19 Patients
| Attributes (Left to Right) | C1 | C2 | C3 | C4 | C5 | C6 | C7 | C8 | C9 |
|---|---|---|---|---|---|---|---|---|---|
| Citations | IP | AI | CLS | ACC | SEN | SPEC | AUC | MCC | F1 |
| Hoq et al. [229] (2021) | Voice | HDL | SVM | 94.0 | NR | NR | NR | 0.71 | 0.91 |
| Kamble et al. [230] (2021) | HW | ML | SVM | 96.0 | NR | NR | 0.87 | NR | 0.8 |
| Alzubaidi et al. [231] (2021) | Tremor | HDL | DT | 87.9 | NR | NR | NR | 89.34 | 1.17 |
| Khedr et al. [232] (2021) | Voice | ML | SVM | 95.8 | 90.24 | 92.3 | NR | 92.03 | 96 |
| Mei et al. [53] (2021) | Voice | ML | KNN | 83.07 | NR | NR | 0.91 | NR | NR |
| Singamaneni et al. [1] (2021) | Voice | ML | SVM | 94.86 | NR | NR | NR | NR | NR |
| Jayachandran et al. [233] (2020) | Voice | ML | NB | 78.34 | NR | NR | NR | NR | NR |
| Anitha et al. [234] (2020) | Voice | ML | SVM | 90.21 | 1.8 | 4.39 | 2.49 | NR | 1.17 |
| Maitín et al. [235] (2020) | EEG | ML | LR | 62.99 | 0.9067 | 0.981 | NR | NR | NR |
| Poorjam et al. [236] (2019) | Voice | HDL | SVM | 96.00 | NR | NR | NR | NR | NR |
| Aseer et al. [237] (2019) | HW | SDL | SVM | 98.28 | NR | NR | NR | NR | NR |
| Naghsh et al. [35] (2019) | EEG | SDL | DT | 97.38 | NR | NR | NR | NR | NR |
| Wang et al. [234] (2017) | BM | HDL | KNN | 96.12 | NR | NR | NR | NR | NR |
5.3. Deep Learning LSTM Architecture
5.4. The Comparative Analysis of AI Systems with a Different Set of Input Covariates
- (i)
- AI systems that use office-based biomarkers as input covariate
- (ii)
- AI systems that use laboratory-based biomarkers as input covariate
- (iii)
- AI systems that use carotid ultrasound image phenotype as a covariate
- (iv)
- AI systems that use Parkinson’s disease symptoms as input covariate

- (v)
- AI systems that use COVID-19 as input covariate
| SN | Citations | Year | Input Covariates | GT | PS | AI | FE | CLS | ACC % | AUC | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OBBM | LBBM | CUSIP | MedUSE | PD | COV | ||||||||||
| 1 | Yan et. al. [268] | 2019 | ✓ | ✓ | ✕ | ✓ | ✕ | ✕ | CVD | NA | NA | NA | NA | NA | NA |
| 2 | Park et al. [249] | 2017 | ✓ | ✓ | ✕ | ✕ | ✓ | ✕ | Stroke | 18 | ML | RF | SVM | 88.00 | NR |
| 3 | Suri et al. [248] | 2022 | ✓ | ✓ | ✓ | ✕ | ✓ | ✕ | CVD/stroke | NR | ML | NR | NR | NR | NR |
| 4 | Zimmerman et al. [252] | 2020 | ✓ | ✓ | ✕ | ✕ | ✕ | ✓ | CVD | 32 | DL | LDA | CNN | 87.23 | NR |
| 5 | Aljameel et al. [269] | 2021 | ✓ | ✓ | ✕ | ✕ | ✕ | ✓ | CVD/stroke | 287 | ML | KNN | SVM | 95.00 | 0/99 |
| 6 | Suri et al. [54] | 2020 | ✓ | ✓ | ✓ | ✕ | ✕ | ✓ | CVD/stroke | NR | ML/DL | NR | NR | NR | NR |
| 7 | Handy et al. [253] | 2021 | ✓ | ✓ | ✓ | ✕ | ✕ | ✓ | CVD/stroke | NR | ML/DL | LSTM | SVM | 84.00 | NR |
| 8 | Unnikrishnan et al. [245] | 2016 | ✓ | ✓ | ✕ | ✕ | ✕ | ✕ | CVD | 3654 | ML | LR | SVM | 83.00 | NR |
| 9 | Mouridsen et al. [270] | 2020 | ✓ | ✓ | ✕ | ✕ | ✕ | ✕ | Stroke, MRI | 16 | DL | NR | KNN | 74.00 | 0.74 |
| 10 | Bergamaschi et al. [254] | 2021 | ✓ | ✓ | ✕ | ✕ | ✕ | ✕ | CVD | 237 | NA | NA | NA | NA | NA |
| 11 | Reva et al. [244] | 2021 | ✓ | ✓ | ✕ | ✕ | ✕ | ✕ | Stroke, CT | 200 | ML | NB | DT, RF, SVM | 85.32 | NR |
| 12 | Kakadiaris et al. [243] | 2022 | ✓ | ✓ | ✕ | ✕ | ✕ | ✕ | CVD | 6459 | ML | DT, RF | SVM | 86.00 | 0.92 |
| 13 | Proposed study | 2022 | ✓ | ✓ | ✓ | ✕ | ✓ | ✓ | CVD/stroke | NA | NA | NA | NA | NA | NA |
5.5. Implementation and Maintenance of AI-Based CVD Risk Stratification System
- (i)
- Implementation of Training System
- (ii)
- Implementation of Prediction System
- (iii)
- Performance
- (iv)
- Maintenance
5.6. Distribution Strategies of the Potential Benefits of the ML/AI Model
6. Critical Discussion
6.1. Benchmarking
6.2. Bias in Deep Learning Systems
6.3. The Economic Aspect of AI-Based Diagnosis
6.4. Strengths, Weakness, and Extensions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SN | Abb | Definition | SN | Abb | Definition |
| 1 | A1c | Glycated hemoglobin | 34 | VCAM | Vascular cell adhesion molecule |
| 2 | ANS | Autonomic nervous system | 35 | LBBM | Laboratory-based biomarker |
| 3 | ANN | Artificial neural network | 36 | LDL | Low-density lipoprotein |
| 4 | ACE2 | Angiotensin converting enzyme 2 | 37 | LSTM | Long short-term memory |
| 5 | AUC | Area under the curve | 38 | MedUSE | Medication use |
| 6 | AI | Artificial intelligence | 39 | ML | Machine learning |
| 7 | ARDS | Acute respiratory distress syndrome | 40 | MRI | Magnetic resonance imaging |
| 8 | BMI | Body mass index | 41 | NPV | Negative predictive value |
| 9 | CAD | Coronary artery disease | 42 | NB | Naive byes |
| 10 | CAS | Coronary artery syndrome | 43 | nOH | Neurogenic orthostatic hypotension |
| 11 | CCA | circumflex coronary artery | 44 | Non-ML | Non-machine learning |
| 12 | CPD | Chorionic pulmonary disease | 45 | NN | Neural networks |
| 13 | COPD | Chronic obstructive pulmonary disease | 46 | NR | Not reported |
| 14 | CKD | Chronic kidney disease | 47 | NO | Nitric oxide |
| 15 | CT | Computed tomography | 48 | OBBM | Office-based biomarker |
| 16 | CUSIP | Carotid ultrasound image phenotype | 49 | OH | Orthostatic hypotension |
| 17 | CV | Cross-validation | 50 | PD | Parkinson’s disease |
| 18 | CVD | Cardiovascular disease | 51 | PE | Performance evaluation matrices |
| 19 | CNN | Convolution neural network | 52 | PPV | Positive predictive value |
| 20 | DA | Endogenous dopamine | 53 | PCA | Principal component analysis |
| 21 | DL | Deep learning | 54 | RA | Rheumatoid arthritis |
| 22 | DM | Diabetes mellitus | 55 | RF | Random forest |
| 23 | DBP | Diastolic blood pressure | 56 | RoB | Risk of bias |
| 24 | DT | Decision tree | 57 | RoS | Reactive oxygen species |
| 25 | EMG | Electromyography | 58 | ROC | Receiver operating characteristics |
| 26 | EPB | Increased blood pressure | 59 | RNN | Recurrent neural network |
| 27 | FoG | Freezing of gait | 60 | SCORE | Systematic coronary risk evaluation |
| 28 | GGO | Ground-glass opacities | 61 | SBP | Spontaneous bacterial peritonitis |
| 29 | GT | Ground truth | 62 | RNA | Ribonucleic acid |
| 30 | HTN | Hypertension | 63 | SMOTE | Synthetic minority over-sampling technique |
| 31 | HDL | Hybrid deep learning | 64 | SVM | Support vector machine |
| 32 | ICU | Intensive care unit | 65 | TMD | Temporomandibular disorder |
| 33 | ICAM | Intercellular adhesion molecule | 66 | TMJ | Temporomandibular joint |
| 67 | US | Ultrasound |
Appendix A
Appendix A.1. Deep Convolutional Neural Network Architecture
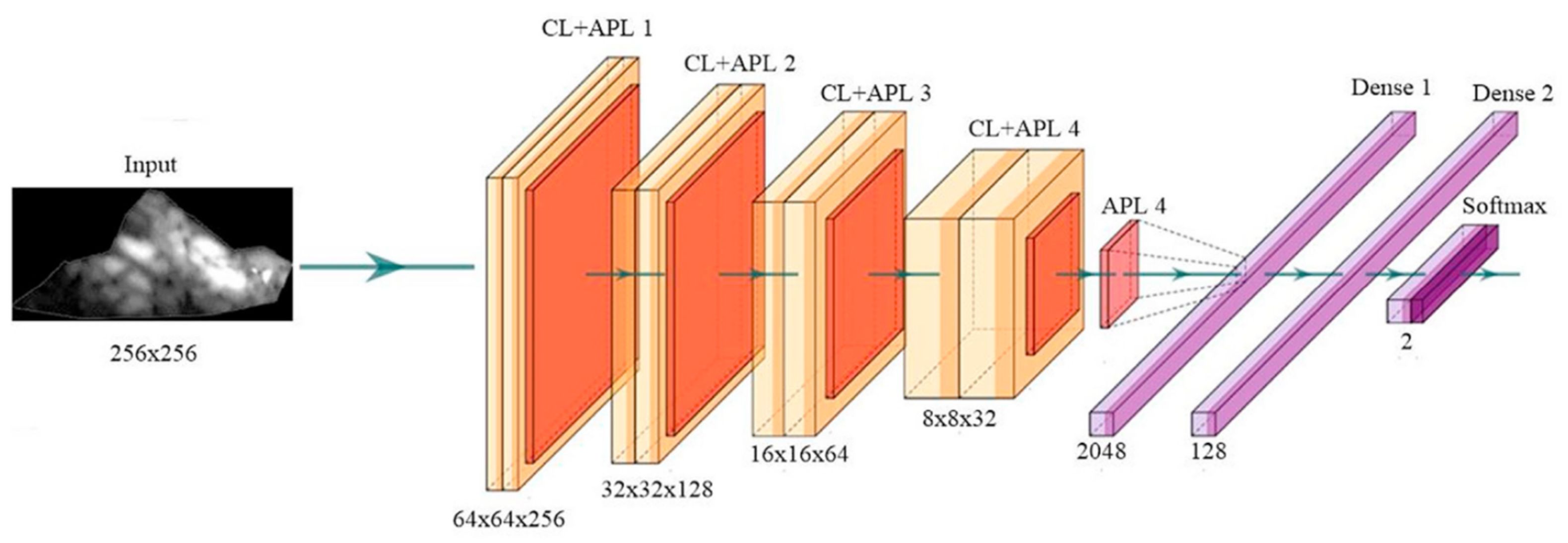
Appendix A.2. DenseNet Architecture

Appendix A.3. Inception V3 Architecture

Appendix A.4. Xception Net Architecture

Appendix A.5. Resnet50 Architecture

Appendix A.6. MobileNet Architecture

Appendix A.7. AlexNet Architecture

Appendix A.8. Suri Net Architecture

References
- Bhat, S.; Acharya, U.R.; Hagiwara, Y.; Dadmehr, N.; Adeli, H. Parkinson’s disease: Cause factors, measurable indicators, and early diagnosis. Comput. Biol. Med. 2018, 102, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Cilia, R.; Bonvegna, S.; Straccia, G.; Andreasi, N.G.; Elia, A.E.; Romito, L.M.; Devigili, G.; Cereda, E.; Eleopra, R. Effects of COVID-19 on Parkinson’s disease clinical features: A community-based case-control study. J. Mov. Disord. 2020, 35, 1287–1292. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, A.; Li, Y.; Hayashi, H.; Kondo, N. Dementia risks identified by vocal features via telephone conversations: A novel machine learning prediction model. PLoS ONE 2021, 16, e0253988. [Google Scholar] [CrossRef] [PubMed]
- Sulzer, D.; Antonini, A.; Leta, V.; Nordvig, A.; Smeyne, R.J.; Goldman, J.E.; Al-Dalahmah, O.; Zecca, L.; Sette, A.; Bubacco, L. COVID-19 and possible links with Parkinson’s disease and parkinsonism: From bench to bedside. npj Parkinson’s Dis. 2020, 6, 18. [Google Scholar] [CrossRef]
- Khoshnood, R.J.; Zali, A.; Tafreshinejad, A.; Ghajarzadeh, M.; Ebrahimi, N.; Safari, S.; Mirmosayyeb, O. Parkinson’s disease and COVID-19: A systematic review and meta-analysis. Neurol. Sci. 2021, 43, 775–783. [Google Scholar] [CrossRef]
- Helmich, R.C.; Bloem, B.R. The impact of the COVID-19 pandemic on Parkinson’s disease: Hidden sorrows and emerging opportunities. J. Parkinson’s Dis. 2020, 10, 351. [Google Scholar] [CrossRef] [Green Version]
- Baschi, R.; Luca, A.; Nicoletti, A.; Caccamo, M.; Cicero, C.E.; D’Agate, C.; di Giorgi, L.; la Bianca, G.; Castro, T.L.; Zappia, M. Changes in motor, cognitive, and behavioral symptoms in Parkinson’s disease and mild cognitive impairment during the COVID-19 lockdown. J. Front. Psychiatry 2020, 11, 590134. [Google Scholar] [CrossRef]
- Maniruzzaman, M.; Kumar, N.; Abedin, M.M.; Islam, M.S.; Suri, H.S.; El-Baz, A.S.; Suri, J.S. Comparative approaches for classification of diabetes mellitus data: Machine learning paradigm. Comput. Methods Programs Biomed. 2017, 152, 23–34. [Google Scholar] [CrossRef]
- Schrag, A.; Jahanshahi, M.; Quinn, N.P. What contributes to depression in Parkinson’s disease? Psychol. Med. 2001, 31, 65–73. [Google Scholar] [CrossRef] [Green Version]
- Reijnders, J.S.; Ehrt, U.; Weber, W.E.; Aarsland, D.; Leentjens, A.F. A systematic review of prevalence studies of depression in Parkinson’s disease. Mov. Disord. 2008, 23, 183–189. [Google Scholar] [CrossRef] [Green Version]
- McKeith, I.G.; Burn, D. Spectrum of Parkinson’s disease, Parkinson’s dementia, and Lewy body dementia. Neurol. Clin. 2000, 18, 865–883. [Google Scholar] [CrossRef]
- Noe, E.; Marder, K.; Bell, K.L.; Jacobs, D.M.; Manly, J.J.; Stern, Y. Comparison of dementia with Lewy bodies to Alzheimer’s disease and Parkinson’s disease with dementia. Mov. Disord. 2004, 19, 60–67. [Google Scholar] [CrossRef]
- Minagi, S.; Matsunaga, T.; Shibata, T.; Sato, T.S. An appliance for management of TMJ pain as a complication of Parkinson’s disease. CRANIO® 1998, 16, 57–59. [Google Scholar] [CrossRef]
- Van Camp, G.; Flamez, A.; Cosyns, B.; Weytjens, C.; Muyldermans, L.; van Zandijcke, M.; de Sutter, J.; Santens, P.; Decoodt, P.; Moerman, C. Treatment of Parkinson’s disease with pergolide and relation to restrictive valvular heart disease. Lancet 2004, 363, 1179–1183. [Google Scholar] [CrossRef]
- Devos, D.; Kroumova, M.; Bordet, R.; Vodougnon, H.; Guieu, J.; Libersa, C.; Destee, A. Heart rate variability and Parkinson’s disease severity. J. Neural Transm. 2003, 110, 997–1011. [Google Scholar]
- Lau, Y.H.; Lau, K.M.; Ibrahim, N.M. Management of Parkinson’s Disease in the COVID-19 Pandemic and Future Perspectives in the Era of Vaccination. J. Mov. Disord. 2021, 14, 177. [Google Scholar] [CrossRef]
- Porcu, M.; Sanfilippo, R.; Montisci, R.; Balestrieri, A.; Suri, J.S.; Wintermark, M.; Saba, L. White-matter hyperintensities in patients with carotid artery stenosis: An exploratory connectometry study. Neuroradiol. J. 2020, 33, 486–493. [Google Scholar] [CrossRef]
- Elbaz, A.; Bower, J.H.; Peterson, B.J.; Maraganore, D.M.; McDonnell, S.K.; Ahlskog, J.E.; Schaid, D.J.; Rocca, W.A. Survival study of Parkinson disease in Olmsted county, Minnesota. Arch. Neurol. 2003, 60, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Pohar, S.L.; Jones, C.A. The burden of Parkinson disease (PD) and concomitant comorbidities. Arch. Gerontol. Geriatr. 2009, 49, 317–321. [Google Scholar] [CrossRef]
- Becker, C.; Jick, S.S.; Meier, C.R. Risk of stroke in patients with idiopathic Parkinson disease. Parkinsonism Relat. Disord. 2010, 16, 31–35. [Google Scholar] [CrossRef]
- Driver, J.; Kurth, T.; Buring, J.; Gaziano, J.; Logroscino, G. Parkinson disease and risk of mortality: A prospective comorbidity-matched cohort study. Neurology 2008, 70, 1423–1430. [Google Scholar] [CrossRef]
- Van, W.B.; Vanholder, R.; Verbeke, F.; Lameire, N. Is peritoneal dialysis associated with increased cardiovascular morbidity and mortality? Perit. Dial. Int. 2006, 26, 429–434. [Google Scholar]
- Nam, G.E.; Kim, S.M.; Han, K.; Kim, N.H.; Chung, H.S.; Kim, J.W.; Han, B.; Cho, S.J.; Yu, J.H.; Park, Y.G. Metabolic syndrome and risk of Parkinson disease: A nationwide cohort study. PLoS Med. 2018, 15, e1002640. [Google Scholar] [CrossRef] [Green Version]
- Qiu, C.; Hu, G.; Kivipelto, M.; Laatikainen, T.; Antikainen, R.; Fratiglioni, L.; Jousilahti, P.; Tuomilehto, J. Association of blood pressure and hypertension with the risk of Parkinson disease: The National FINRISK Study. Hypertension 2011, 57, 1094–1100. [Google Scholar] [CrossRef] [Green Version]
- Bots, M.L.; Grobbee, D.E.; Hofman, A.; Witteman, J.C. Common carotid intima-media thickness and risk of acute myocardial infarction: The role of lumen diameter. Stroke 2005, 36, 762–767. [Google Scholar] [CrossRef] [Green Version]
- Aleyasin, H.; Rousseaux, M.W.; Phillips, M.; Kim, R.H.; Bland, R.J.; Callaghan, S.; Slack, R.S.; During, M.J.; Mak, T.W.; Park, D.S. The Parkinson’s disease gene DJ-1 is also a key regulator of stroke-induced damage. Proc. Natl. Acad. Sci. USA 2007, 104, 18748–18753. [Google Scholar] [CrossRef] [Green Version]
- Kurl, S.; Laukkanen, J.A.; Rauramaa, R.; Lakka, T.A.; Sivenius, J.; Salonen, J.T. Cardiorespiratory fitness and the risk for stroke in men. Arch. Intern. Med. 2003, 163, 1682–1688. [Google Scholar] [CrossRef] [Green Version]
- Park, J.-H.; Kim, D.-H.; Park, Y.-G.; Kwon, D.-Y.; Choi, M.; Jung, J.-H.; Han, K. Association of Parkinson disease with risk of cardiovascular disease and all-cause mortality: A nationwide, population-based cohort study. Circulation 2020, 141, 1205–1207. [Google Scholar] [CrossRef]
- Tsivgoulis, G.; Palaiodimou, L.; Zand, R.; Lioutas, V.A.; Krogias, C.; Katsanos, A.H.; Shoamanesh, A.; Sharma, V.K.; Shahjouei, S.; Baracchini, C. COVID-19 and cerebrovascular diseases: A comprehensive overview. Ther. Adv. Neurol. 2020, 13, 1756286420978004. [Google Scholar] [CrossRef]
- El-Baz, A.S.; Acharya, R.; Mirmehdi, M.; Suri, J.S. Multi Modality State-of-the-Art Medical Image Segmentation and Registration Methodologies: Volume 1; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Suri, J.S.; Rangayyan, R.M. Breast Imaging, Mammography, and Computer-Aided Diagnosis of Breast Cancer; SPIE: Bellingham, WA, USA, 2006. [Google Scholar]
- Saba, L.; Biswas, M.; Kuppili, V.; Godia, E.C.; Suri, H.S.; Edla, D.R.; Omerzu, T.; Laird, J.R.; Khanna, N.N.; Mavrogeni, S. The present and future of deep learning in radiology. Eur. J. Radiol. 2019, 114, 14–24. [Google Scholar] [CrossRef]
- Kuppili, V.; Biswas, M.; Sreekumar, A.; Suri, H.S.; Saba, L.; Edla, D.R.; Marinhoe, R.T.; Sanches, J.M.; Suri, J.S. Extreme learning machine framework for risk stratification of fatty liver disease using ultrasound tissue characterization. J. Med. Syst. 2017, 41, 152. [Google Scholar] [CrossRef] [PubMed]
- Tandel, G.S.; Balestrieri, A.; Jujaray, T.; Khanna, N.N.; Saba, L.; Suri, J.S. Multiclass magnetic resonance imaging brain tumor classification using artificial intelligence paradigm. Comput. Biol. Med. 2020, 122, 103804. [Google Scholar] [CrossRef] [PubMed]
- Acharya, R.; Ng, Y.E.; Suri, J.S. Image Modeling of the Human Eye; Artech House: New York, NY, USA, 2008. [Google Scholar]
- Viswanathan, V.; Puvvula, A.; Jamthikar, A.D.; Saba, L.; Johri, A.M.; Kotsis, V.; Khanna, N.N.; Dhanjil, S.K.; Majhail, M.; Misra, D.P. Bidirectional link between diabetes mellitus and coronavirus disease 2019 leading to cardiovascular disease: A narrative review. World J. Diabetes 2021, 12, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Biswas, M.; Kuppili, V.; Saba, L.; Edla, D.R.; Suri, H.S.; Sharma, A.; Cuadrado-Godia, E.; Laird, J.R.; Nicolaides, A.; Suri, J.S. Deep learning fully convolution network for lumen characterization in diabetic patients using carotid ultrasound: A tool for stroke risk. Med. Biol. Eng. Comput. 2018, 57, 543–564. [Google Scholar] [CrossRef]
- Jamthikar, A.D.; Gupta, D.; Mantella, L.E.; Saba, L.; Laird, J.R.; Johri, A.M.; Suri, J.S. Multiclass machine learning vs. conventional calculators for stroke/CVD risk assessment using carotid plaque predictors with coronary angiography scores as gold standard: A 500 participants study. Int. J. Cardiovasc. Imaging 2020, 37, 1171–1187. [Google Scholar] [CrossRef]
- Acharya, U.R.; Sree, S.V.; Krishnan, M.M.R.; Molinari, F.; Garberoglio, R.; Suri, J.S. Non-invasive automated 3D thyroid lesion classification in ultrasound: A class of ThyroScan™ systems. Ultrasonics 2012, 52, 508–520. [Google Scholar] [CrossRef]
- Acharya, U.R.; Sree, S.V.; Krishnan, M.M.R.; Molinari, F.; ZieleŸnik, W.; Bardales, R.H.; Witkowska, A.; Suri, J.S. Computer-Aided Diagnostic System for Detection of Hashimoto Thyroiditis on Ultrasound Images From a Polish Population. J. Ultrasound Med. 2014, 33, 245–253. [Google Scholar] [CrossRef]
- Liu, K.; Suri, J.S. Automatic Vessel Indentification for Angiographic Screening. U.S. Patent 6,845,260, 18 January 2005. [Google Scholar]
- Acharya, U.R.; Sree, S.V.; Saba, L.; Molinari, F.; Guerriero, S.; Suri, J.S. Ovarian Tumor Characterization and Classification Using Ultrasound—A New Online Paradigm. J. Digit. Imaging 2012, 26, 544–553. [Google Scholar] [CrossRef] [Green Version]
- Wolfram, J.; Suri, K.; Huang, Y.; Molinaro, R.; Borsoi, C.; Scott, B.; Boom, K.; Paolino, D.; Fresta, M.; Wang, J.; et al. Evaluation of anticancer activity of celastrol liposomes in prostate cancer cells. J. Microencapsul. 2014, 31, 501–507. [Google Scholar] [CrossRef] [Green Version]
- Acharya, U.R.; Faust, O.; Sree, S.V.; Molinari, F.; Saba, L.; Nicolaides, A.; Suri, J.S. An accurate and generalized approach to plaque characterization in 346 carotid ultrasound scans. IEEE Trans. Instrum. Meas. 2011, 61, 1045–1053. [Google Scholar] [CrossRef]
- Suri, J.S.; Agarwal, S.; Carriero, A.; Paschè, A.; Danna, P.S.C.; Columbu, M.; Saba, L.; Viskovic, K.; Mehmedović, A.; Agarwal, S.; et al. COVLIAS 1.0 vs. MedSeg: Artificial Intelligence-Based Comparative Study for Automated COVID-19 Computed Tomography Lung Segmentation in Italian and Croatian Cohorts. Diagnostics 2021, 11, 2367. [Google Scholar] [CrossRef]
- Battineni, G.; Chintalapudi, N.; Amenta, F.; Traini, E. A Comprehensive Machine-Learning Model Applied to Magnetic Resonance Imaging (MRI) to Predict Alzheimer’s Disease (AD) in Older Subjects. J. Clin. Med. 2020, 9, 2146. [Google Scholar] [CrossRef]
- Saba, L.; Suri, J.S. Multi-Detector CT Imaging: Abdomen, Pelvis, and CAD Applications; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Sanches, J.M.; Laine, A.F.; Suri, J.S. Ultrasound Imaging; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Agarwal, M.; Saba, L.; Gupta, S.K.; Carriero, A.; Falaschi, Z.; Paschè, A.; Danna, P.; El-Baz, A.; Naidu, S.; Suri, J.S. A Novel Block Imaging Technique Using Nine Artificial Intelligence Models for COVID-19 Disease Classification, Characterization and Severity Measurement in Lung Computed Tomography Scans on an Italian Cohort. J. Med. Syst. 2021, 45, 28. [Google Scholar] [CrossRef]
- Suri, J.S.; Agarwal, S.; Gupta, S.K.; Puvvula, A.; Viskovic, K.; Suri, N.; Alizad, A.; El-Baz, A.; Saba, L.; Fatemi, M.; et al. Systematic Review of Artificial Intelligence in Acute Respiratory Distress Syndrome for COVID-19 Lung Patients: A Biomedical Imaging Perspective. IEEE J. Biomed. Health Inform. 2021, 25, 4128–4139. [Google Scholar] [CrossRef]
- Senturk, Z.K. Early diagnosis of Parkinson’s disease using machine learning algorithms. Med. Hypotheses 2020, 138, 109603. [Google Scholar] [CrossRef]
- Janghel, R.R.; Shukla, A.; Rathore, C.P.; Verma, K.; Rathore, S. A comparison of soft computing models for Parkinson’s disease diagnosis using voice and gait features. Netw. Model. Anal. Health Inform. Bioinform. 2017, 6, 14. [Google Scholar] [CrossRef]
- Mei, J.; Desrosiers, C.; Frasnelli, J. Machine learning for the diagnosis of parkinson’s disease: A review of literature. Front. Aging Neurosci. 2021, 13, 184. [Google Scholar] [CrossRef]
- Suri, J.S.; Puvvula, A.; Biswas, M.; Majhail, M.; Saba, L.; Faa, G.; Singh, I.M.; Oberleitner, R.; Turk, M.; Chadha, P.S.; et al. COVID-19 pathways for brain and heart injury in comorbidity patients: A role of medical imaging and artificial intelligence-based COVID severity classification: A review. Comput. Biol. Med. 2020, 124, 103960. [Google Scholar] [CrossRef]
- Saba, L.; Gerosa, C.; Wintermark, M.; Hedin, U.; Fanni, D.; Suri, J.S.; Balestrieri, A.; Faa, G. Can COVID19 trigger the plaque vulnerability—A Kounis syndrome warning for asymptomatic subjects. Cardiovasc. Diagn. Ther. 2020, 10, 1352. [Google Scholar] [CrossRef]
- Antonini, A.; Leta, V.; Teo, J.; Chaudhuri, K.R. Outcome of Parkinson’s disease patients affected by COVID-19. Mov. Disord. 2020. [Google Scholar] [CrossRef]
- Antonelli, A.; Bennardo, F.; Brancaccio, Y.; Barone, S.; Femiano, F.; Nucci, L.; Minervini, G.; Fortunato, L.; Attanasio, F.; Giudice, A. Can Bone Compaction Improve Primary Implant Stability? An In Vitro Comparative Study with Osseodensification Technique. Appl. Sci. 2020, 10, 8623. [Google Scholar] [CrossRef]
- Ciceri, F.; Castagna, A.; Rovere-Querini, P.; De Cobelli, F.; Ruggeri, A.; Galli, L.; Conte, C.; De Lorenzo, R.; Poli, A.; Ambrosio, A.; et al. Early predictors of clinical outcomes of COVID-19 outbreak in Milan, Italy. Clin. Immunol. 2020, 217, 108509. [Google Scholar] [CrossRef] [PubMed]
- Derespina, K.; Kaushik, S.; Plichta, A.; Conway, E.E.; Bercow, A.; Choi, J.; Eisenberg, R.; Gillen, J.; Sen, A.I.; Hennigan, C.M.; et al. Clinical Manifestations and Outcomes of Critically Ill Children and Adolescents with Coronavirus Disease 2019 in New York City. J. Pediatr. 2020, 226, 55–63.e2. [Google Scholar] [CrossRef] [PubMed]
- Meerson, F.Z.; Kagan, V.E.; Kozlov, Y.P.; Belkina, L.M.; Arkhipenko, Y.V. The role of lipid peroxidation in pathogenesis of ischemic damage and the antioxidant protection of the heart. Basic Res. Cardiol. 1982, 77, 465–485. [Google Scholar] [CrossRef]
- Jazieh, A.-R.; Alenazi, T.H.; Alhejazi, A.; Al Safi, F.; Al Olayan, A. Outcome of Oncology Patients Infected with Coronavirus. JCO Glob. Oncol. 2020, 6, 471–475. [Google Scholar] [CrossRef]
- Pathak, Y.; Shukla, P.; Tiwari, A.; Stalin, S.; Singh, S. Deep Transfer Learning Based Classification Model for COVID-19 Disease. IRBM 2020, 43, 87–92. [Google Scholar] [CrossRef]
- Salehi, S.; Abedi, A.; Balakrishnan, S.; Gholamrezanezhad, A. Coronavirus Disease 2019 (COVID-19): A Systematic Review of Imaging Findings in 919 Patients. Am. J. Roentgenol. 2020, 215, 87–93. [Google Scholar] [CrossRef]
- Cozzi, D.; Cavigli, E.; Moroni, C.; Smorchkova, O.; Zantonelli, G.; Pradella, S.; Miele, V. Ground-glass opacity (GGO): A review of the differential diagnosis in the era of COVID-19. Jpn. J. Radiol. 2021, 39, 721–732. [Google Scholar] [CrossRef]
- Xie, X.; Zhong, Z.; Zhao, W.; Zheng, C.; Wang, F.; Liu, J. Chest CT for typical 2019-nCoV pneumonia: Relationship to negative RT-PCR testing. Radiology 2020, 200343. [Google Scholar] [CrossRef] [Green Version]
- Gozes, O.; Frid-Adar, M.; Greenspan, H.; Browning, P.D.; Zhang, H.; Ji, W.; Bernheim, A.; Siegel, E. Rapid ai development cycle for the coronavirus (COVID-19) pandemic: Initial results for automated detection & patient monitoring using deep learning ct image analysis. arXiv 2020, arXiv:2003.05037. [Google Scholar]
- Aslan, M.F.; Unlersen, M.F.; Sabanci, K.; Durdu, A. CNN-based transfer learning–BiLSTM network: A novel approach for COVID-19 infection detection. Appl. Soft Comput. 2020, 98, 106912. [Google Scholar] [CrossRef]
- Wu, Y.-H.; Gao, S.-H.; Mei, J.; Xu, J.; Fan, D.-P.; Zhang, R.-G.; Cheng, M.-M. JCS: An Explainable COVID-19 Diagnosis System by Joint Classification and Segmentation. IEEE Trans. Image Process. 2021, 30, 3113–3126. [Google Scholar] [CrossRef] [PubMed]
- Munjral, S.; Ahluwalia, P.; Jamthikar, A.D.; Puvvula, A.; Saba, L.; Faa, G.; Singh, I.M.; Chadha, P.S.; Turk, M.; Johri, A.M.; et al. Nutrition, atherosclerosis, arterial imaging, cardiovascular risk stratification, and manifestations in COVID-19 framework: A narrative review. Front. Biosci. 2021, 26, 1312. [Google Scholar] [CrossRef]
- Hakim, A.M.; Ng, J.B.; Turek, M. Heart disease as a risk factor for dementia. Clin. Epidemiol. 2013, 5, 135–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amarenco, P.; Cohen, A.; Tzourio, C.; Bertrand, B.; Hommel, M.; Besson, G.; Chauvel, C.; Touboul, P.-J.; Bousser, M.-G. Atherosclerotic disease of the aortic arch and the risk of ischemic stroke. N. Engl. J. Med. 1994, 331, 1474–1479. [Google Scholar] [CrossRef]
- Ikeda, N.; Gupta, A.; Dey, N.; Bose, S.; Shafique, S.; Arak, T.; Godia, E.C.; Saba, L.; Laird, J.R.; Nicolaides, A. Improved correlation between carotid and coronary atherosclerosis SYNTAX score using automated ultrasound carotid bulb plaque IMT measurement. Ultrasound Med. Biol. 2015, 41, 1247–1262. [Google Scholar] [CrossRef]
- Peterson, E.; Lo, K.B.; DeJoy, R.; Salacup, G.; Pelayo, J.; Bhargav, R.; Gul, F.; Albano, J.; Azmaiparashvili, Z.; Amanullah, A. The relationship between coronary artery disease and clinical outcomes in COVID-19: A single-center retrospective analysis. Coron. Artery Dis. 2020, 32, 367–371. [Google Scholar] [CrossRef]
- Szarpak, L.; Mierzejewska, M.; Jurek, J.; Kochanowska, A.; Gasecka, A.; Truszewski, Z.; Pruc, M.; Blek, N.; Rafique, Z.; Filipiak, K. Effect of Coronary Artery Disease on COVID-19—Prognosis and Risk Assessment: A Systematic Review and Meta-Analysis. Biology 2022, 11, 221. [Google Scholar] [CrossRef]
- Saba, L.; Sanfilippo, R.; Sannia, S.; Anzidei, M.; Montisci, R.; Mallarini, G.; Suri, J.S. Association Between Carotid Artery Plaque Volume, Composition, and Ulceration: A Retrospective Assessment with MDCT. Am. J. Roentgenol. 2012, 199, 151–156. [Google Scholar] [CrossRef]
- Araki, T.; Ikeda, N.; Shukla, D.; Jain, P.K.; Londhe, N.D.; Shrivastava, V.K.; Banchhor, S.K.; Saba, L.; Nicolaides, A.; Shafique, S. PCA-based polling strategy in machine learning framework for coronary artery disease risk assessment in intravascular ultrasound: A link between carotid and coronary grayscale plaque morphology. Comput. Methods Programs Biomed. 2016, 128, 137–158. [Google Scholar] [CrossRef]
- Barman, H.A.; Atici, A.; Sahin, I.; Alici, G.; Tekin, E.A.; Baycan, F.; Ozturk, F.; Oflar, E.; Tugrul, S.; Yavuz, M.B.; et al. Prognostic significance of cardiac injury in COVID-19 patients with and without coronary artery disease. Coron. Artery Dis. 2020. [Google Scholar] [CrossRef]
- Cao, C.; Li, D.; Zhan, S.; Zhang, C.; Sun, B.; Litvak, V. L-dopa treatment increases oscillatory power in the motor cortex of Parkinson’s disease patients. NeuroImage Clin. 2020, 26, 102–116. [Google Scholar] [CrossRef]
- Blesa, J.; Trigo-Damas, I.; Quiroga-Varela, A.; Jackson-Lewis, V.R. Oxidative stress and Parkinson’s disease. Parkinson’s Dis. Cell Vulnerability Dis. Prog. 2016. [Google Scholar] [CrossRef] [Green Version]
- Raees, P.C.M.; Thomas, V. Automated detection of Alzheimer’s Disease using Deep Learning in MRI. J. Phys. Conf. Ser. 2021, 1921, 012024. [Google Scholar] [CrossRef]
- Priya, S.J.; Rani, A.J.; Subathra, M.; Mohammed, M.A.; Damaševičius, R.; Ubendran, N. local pattern transformation based feature extraction for recognition of Parkinson’s disease based on gait signals. Diagnostics 2021, 11, 1395. [Google Scholar] [CrossRef]
- Chen, R.; Kumar, S.; Garg, R.R.; Lang, A.E. Impairment of motor cortex activation and deactivation in Parkinson’s disease. Clin. Neurophysiol. 2001, 112, 600–607. [Google Scholar] [CrossRef]
- Chen, T.; Mirzadeh, Z.; Chapple, K.M.; Lambert, M.; Shill, H.A.; Moguel-Cobos, G.; Tröster, A.I.; Dhall, R.; Ponce, F.A. Clinical outcomes following awake and asleep deep brain stimulation for Parkinson disease. J. Neurosurg. 2018, 130, 109–120. [Google Scholar] [CrossRef] [Green Version]
- Gill, S.; Mouches, P.; Hu, S.; Rajashekar, D.; MacMaster, F.P.; Smith, E.E.; Forkert, N.D.; Ismail, Z. Using Machine Learning to Predict Dementia from Neuropsychiatric Symptom and Neuroimaging Data. J. Alzheimer’s Dis. 2020, 75, 277–288. [Google Scholar] [CrossRef] [Green Version]
- Zappia, M.; Annesi, G.; Nicoletti, G.; Arabia, G.; Annesi, F.; Messina, D.; Pugliese, P.; Spadafora, P.; Tarantino, P.; Carrideo, S. Sex differences in clinical and genetic determinants of levodopa peak-dose dyskinesias in Parkinson disease: An exploratory study. Arch. Neurol. 2005, 62, 601–605. [Google Scholar] [CrossRef]
- Khan, S.; Gill, S.; Mooney, L.; White, P.; Whone, A.; Brooks, D.; Pavese, N. Combined pedunculopontine-subthalamic stimulation in Parkinson disease. Neurology 2012, 78, 1090–1095. [Google Scholar] [CrossRef] [PubMed]
- Değirmenci, H.; Bakirci, E.M.; Hamur, H. Cardiac Effects of Parkinson’s Disease. Open J. Parkinson’s Dis. Treat. 2020, 3, 006–007. [Google Scholar]
- Scherder, E.; Herr, K.; Pickering, G.; Gibson, S.; Benedetti, F.; Lautenbacher, S. Pain in dementia. Pain 2009, 145, 276–278. [Google Scholar] [CrossRef] [PubMed]
- Alzubaidi, M.; Shah, U.; Zubaydi, H.D.; Dolaat, K.; Abd-Alrazaq, A.; Ahmed, A.; Househ, M. The Role of Neural Network for the Detection of Parkinson’s Disease: A Scoping Review. Healthcare 2021, 9, 740. [Google Scholar] [CrossRef] [PubMed]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.-E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Primers 2017, 3, 17013. [Google Scholar] [CrossRef]
- Olanow, C.W.; Stern, M.B.; Sethi, K. The scientific and clinical basis for the treatment of Parkinson disease (2009). Neurology 2009, 72, S1–S136. [Google Scholar] [CrossRef] [Green Version]
- Çubukçu, H.C.; Yurtdaş, M.; Durak, Z.E.; Aytaç, B.; Güneş, H.N.; Çokal, B.G.; Yoldaş, T.K.; Durak, İ. Oxidative and nitrosative stress in serum of patients with Parkinson’s disease. Neurol. Sci. 2016, 37, 1793–1798. [Google Scholar] [CrossRef]
- Yan, L.-Y.; He, Q.-F.; Lu, M.-Y.; Wang, S.-L.; Qi, Z.-Q.; Dong, H.-R. Association between carotid plaque and Parkinson’s disease. Ann. Transl. Med. 2019, 7, 94. [Google Scholar] [CrossRef]
- Guo, T.; Fan, Y.; Chen, M.; Wu, X.; Zhang, L.; He, T.; Wang, H.; Wan, J.; Wang, X.; Lu, Z. Cardiovascular Implications of Fatal Outcomes of Patients with Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 811–818. [Google Scholar] [CrossRef] [Green Version]
- Ruan, Q.; Yang, K.; Wang, W.; Jiang, L.; Song, J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020, 46, 846–848. [Google Scholar] [CrossRef] [Green Version]
- Metzler, B.; Lechner, I.; Reindl, M.; Reinstadler, S.J. Cardiac injury after COVID-19: Primary cardiac and primary non-cardiac etiology makes a difference. Int. J. Cardiol. 2021, 350, 17–18. [Google Scholar] [CrossRef]
- Sisto, T.; Isola, J. Incidence of atherosclerosis in the internal mammary artery. Ann. Thorac. Surg. 1989, 47, 884–886. [Google Scholar] [CrossRef]
- Buob, A.; Winter, H.; Kindermann, M.; Becker, G.; Möller, J.C.; Oertel, W.H.; Böhm, M. Parasympathetic but not sympathetic cardiac dysfunction at early stages of Parkinson’s disease. Clin. Res. Cardiol. 2010, 99, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Van Bilsen, M.; Patel, H.C.; Bauersachs, J.; Böhm, M.; Borggrefe, M.; Brutsaert, D.; Coats, A.J.; De Boer, R.A.; De Keulenaer, G.W.; Filippatos, G.S.; et al. The autonomic nervous system as a therapeutic target in heart failure: A scientific position statement from the Translational Research Committee of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2017, 19, 1361–1378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walter, B.L. Cardiovascular autonomic dysfunction in patients with movement disorders. Clevel. Clin. J. Med. 2008, 75, S54. [Google Scholar] [CrossRef] [Green Version]
- Choi, D.-Y.; Choi, H. Natural products from marine organisms with neuroprotective activity in the experimental models of Alzheimer’s disease, Parkinson’s disease and ischemic brain stroke: Their molecular targets and action mechanisms. Arch. Pharm. Res. 2015, 38, 139–170. [Google Scholar] [CrossRef]
- Kulkantrakorn, K.; Jirapramukpitak, T. A prospective study in one year cumulative incidence of depression after ischemic stroke and Parkinson’s disease: A preliminary study. J. Neurol. Sci. 2007, 263, 165–168. [Google Scholar] [CrossRef]
- Kuan, C.-Y.; Burke, R.E. Targeting the JNK signaling pathway for stroke and Parkinson’s diseases therapy. Curr. Drug Targets-CNS Neurol. Disord. 2005, 4, 63–67. [Google Scholar] [CrossRef]
- Levine, J.; Greenwald, B.D. Fatigue in Parkinson disease, stroke, and traumatic brain injury. Phys. Med. Rehabil. Clin. 2009, 20, 347–361. [Google Scholar] [CrossRef]
- Levine, R.L.; Jones, J.C.; Bee, N. Stroke and Parkinson’s disease. Stroke 1992, 23, 839–842. [Google Scholar] [CrossRef] [Green Version]
- Kitamura, Y.; Watanabe, S.; Taguchi, M.; Takagi, K.; Kawata, T.; Takahashi-Niki, K.; Yasui, H.; Maita, H.; Iguchi-Ariga, S.M.; Ariga, H. Neuroprotective effect of a new DJ-1-binding compound against neurodegeneration in Parkinson’s disease and stroke model rats. Mol. Neurodegener. 2011, 6, 48. [Google Scholar] [CrossRef] [Green Version]
- Spillantini, M.G.; Crowther, R.A.; Jakes, R.; Hasegawa, M.; Goedert, M. α-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with Lewy bodies. Proc. Natl. Acad. Sci. USA 1998, 95, 6469–6473. [Google Scholar] [CrossRef] [Green Version]
- Malek, N.; Lawton, M.A.; Swallow, D.M.; Grosset, K.A.; Marrinan, S.L.; Bajaj, N.; Barker, R.A.; Burn, D.J.; Hardy, J.; Morris, H.R. Vascular disease and vascular risk factors in relation to motor features and cognition in early Parkinson’s disease. Mov. Disord. 2016, 31, 1518–1526. [Google Scholar] [CrossRef] [Green Version]
- Wehrwein, E.A.; Joyner, M.J. Regulation of blood pressure by the arterial baroreflex and autonomic nervous system. Neuroepidemiology 2013, 117, 89–102. [Google Scholar] [CrossRef]
- Hirshoren, N.; Tzoran, I.; Makrienko, I.; Edoute, Y.; Plawner, M.M.; Itskovitz-Eldor, J.; Jacob, G. Menstrual cycle effects on the neurohumoral and autonomic nervous systems regulating the cardiovascular system. J. Clin. Endocrinol. Metab. 2002, 87, 1569–1575. [Google Scholar] [CrossRef]
- Perry, B.D.; Pollard, R. Homeostasis, stress, trauma, and adaptation: A neurodevelopmental view of childhood trauma. Child Adolesc. Psychiatr. Clin. 1998, 7, 33–51. [Google Scholar] [CrossRef]
- Wong, K.K.; Raffel, D.M.; Koeppe, R.A.; Frey, K.A.; Bohnen, N.I.; Gilman, S. Pattern of Cardiac Sympathetic Denervation in Idiopathic Parkinson Disease Studied with 11C Hydroxyephedrine PET. Radiology 2012, 265, 240–247. [Google Scholar] [CrossRef] [Green Version]
- Most, A.S.; Ruocco, N.A.; Gewirtz, H. Effect of a reduction in blood viscosity on maximal myocardial oxygen delivery distal to a moderate coronary stenosis. Circulation 1986, 74, 1085–1092. [Google Scholar] [CrossRef] [Green Version]
- Mansour, M.; Nassef, Y.E.; Abu Shady, M.; Aziz, A.A.; El Malt, H.A. Metabolic Syndrome and Cardiovascular Risk Factors in Obese Adolescent. Open Access Maced. J. Med. Sci. 2016, 4, 118–121. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Wang, C.; Tang, H.; Chen, S.; Ma, J. Stroke and Coronary Artery Disease Are Associated with Parkinson’s Disease. Can. J. Neurol. Sci. J. Can. Sci. Neurol. 2018, 45, 559–565. [Google Scholar] [CrossRef]
- Liu, Y.; Xue, L.; Zhang, Y.; Xie, A. Association Between Stroke and Parkinson’s Disease: A Meta-analysis. J. Mol. Neurosci. 2020, 70, 1169–1176. [Google Scholar] [CrossRef]
- Wiberg, B.; Lind, L.; Kilander, L.; Zethelius, B.; Sundelof, J.E.; Sundstrom, J. Cognitive function and risk of stroke in elderly men. Neurology 2010, 74, 379–385. [Google Scholar] [CrossRef]
- Hartmann, A.; Mast, H.; Mohr, J.P.; Koennecke, H.-C.; Osipov, A.; Pile-Spellman, J.; Duong, D.H.; Young, W.L. Morbidity of intracranial hemorrhage in patients with cerebral arteriovenous malformation. Stroke 1998, 29, 931–934. [Google Scholar] [CrossRef] [Green Version]
- Zaman, A.; Helft, G.; Worthley, S.; Badimon, J. The role of plaque rupture and thrombosis in coronary artery disease. Atherosclerosis 2000, 149, 251–266. [Google Scholar] [CrossRef]
- Respondek, G.; Roeber, S.; Kretzschmar, H.; Troakes, C.; Al-Sarraj, S.; Gelpi, E.; Gaig, C.; Chiu, W.Z.; van Swieten, J.; Oertel, W.H.; et al. Accuracy of the national institute for neurological disorders and stroke/society for progressive supranuclear palsy and neuroprotection and natural history in Parkinson plus syndromes criteria for the diagnosis of progressive supranuclear palsy. Mov. Disord. 2013, 28, 504–509. [Google Scholar] [CrossRef]
- Stolze, H.; Klebe, S.; Zechlin, C.; Baecker, C.; Friege, L. Falls in frequent neurological diseases. J. Neurol. 2004, 251, 79–84. [Google Scholar] [CrossRef]
- Mercuri, N.B.; Bernardi, G. The ‘magic’of L-dopa: Why is it the gold standard Parkinson’s disease therapy? Trends Pharmacol. Sci. 2005, 26, 341–344. [Google Scholar] [CrossRef]
- Goldstein, D.S. Dysautonomia in Parkinson’s disease: Neurocardiological abnormalities. Lancet Neurol. 2003, 2, 669–676. [Google Scholar] [CrossRef]
- Nahimi, A.; Kinnerup, M.B.; Sommerauer, M.; Gjedde, A.; Borghammer, P. Molecular imaging of the noradrenergic system in idiopathic Parkinson’s disease. Int. Rev. Neurobiol. 2018, 141, 251–274. [Google Scholar]
- Cenci, M.A.; Crossman, A.R. Animal models of l-dopa-induced dyskinesia in Parkinson’s disease. Mov. Disord. 2018, 33, 889–899. [Google Scholar] [CrossRef]
- Chagraoui, A.; Boulain, M.; Juvin, L.; Anouar, Y.; Barrière, G.; Deurwaerdère, P.D. L-dopa in parkinson’s disease: Looking at the false neurotransmitters and their meaning. Int. J. Mol. Sci. 2020, 21, 294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blackwell, D.J.; Faggioni, M.; Wleklinski, M.J.; Gomez-Hurtado, N.; Venkataraman, R.; Gibbs, C.E.; Baudenbacher, F.J.; Gong, S.; Fishman, G.I.; Boyle, P.M.; et al. The Purkinje–myocardial junction is the anatomic origin of ventricular arrhythmia in CPVT. JCI Insight 2022, 7, e151893. [Google Scholar] [CrossRef] [PubMed]
- Acharya, U.R.; Joseph, K.P.; Kannathal, N.; Min, L.C.; Suri, J.S. Heart rate variability. Adv. Card. Signal Process. 2007, 121–165. [Google Scholar]
- Ferini-Strambi, L.; Salsone, M. COVID-19 and neurological disorders: Are neurodegenerative or neuroimmunological diseases more vulnerable? J. Neurol. 2020, 268, 409–419. [Google Scholar] [CrossRef]
- Sorbera, C.; Brigandì, A.; Cimino, V.; Bonanno, L.; Ciurleo, R.; Bramanti, P.; Di Lorenzo, G.; Marino, S. The impact of SARS-CoV2 infection on people in residential care with Parkinson Disease or parkinsonisms: Clinical case series study. PLoS ONE 2021, 16, e0251313. [Google Scholar] [CrossRef]
- Ishiyama, H.; Ishii, J.; Yoshimura, H.; Tsunogae, M.; Fujiwara, S.; Hiya, S.; Inui, R.; Shiomi, Y.; Nakazawa, S.; Kimura, M.; et al. Neurological Manifestations and Long-term Sequelae in Hospitalized Patients with COVID-19. Intern. Med. 2022. [Google Scholar] [CrossRef]
- Cau, R.; Pacielli, A.; Fatemeh, H.; Vaudano, P.; Arru, C.; Crivelli, P.; Stranieri, G.; Suri, J.S.; Mannelli, L.; Conti, M. Complications in COVID-19 patients: Characteristics of pulmonary embolism. Clin. Imaging 2021, 77, 244–249. [Google Scholar] [CrossRef]
- Studer, V.; Rocchi, C.; Motta, C.; Lauretti, B.; Perugini, J.; Brambilla, L.; Pareja-Gutierrez, L.; Camera, G.; Barbieri, F.R.; Marfia, G.A.; et al. Heart rate variability is differentially altered in multiple sclerosis: Implications for acute, worsening and progressive disability. Mult. Scler. J. Exp. Transl. Clin. 2017, 3. [Google Scholar] [CrossRef]
- Vascellari, S.; Palmas, V.; Melis, M.; Pisanu, S.; Cusano, R.; Uva, P.; Perra, D.; Madau, V.; Sarchioto, M.; Oppo, V.; et al. Gut microbiota and metabolome alterations associated with Parkinson’s disease. Msystems 2020, 5, e00561-20. [Google Scholar] [CrossRef]
- Rickards, H. Depression in neurological disorders: Parkinson’s disease, multiple sclerosis, and stroke. J. Neurol. Neurosurg. Psychiatry 2005, 76 (Suppl. 1), i48–i52. [Google Scholar] [CrossRef] [Green Version]
- Mastaglia, F.L.; Johnsen, R.D.; Kakulas, B.A. Prevalence of stroke in Parkinson’s disease: A postmortem study. Mov. Disord. 2002, 17, 772–774. [Google Scholar] [CrossRef]
- Adams, W.R. High-accuracy detection of early Parkinson’s Disease using multiple characteristics of finger movement while typing. PLoS ONE 2017, 12, e0188226. [Google Scholar] [CrossRef] [Green Version]
- Cau, R.; Bassareo, P.P.; Mannelli, L.; Suri, J.S.; Saba, L. Imaging in COVID-19-related myocardial injury. Int. J. Cardiovasc. Imaging 2021, 37, 1349–1360. [Google Scholar] [CrossRef]
- Maniruzzaman; Rahman, J.; Hasan, A.M.; Suri, H.S.; Abedin, M.; El-Baz, A.; Suri, J.S. Accurate Diabetes Risk Stratification Using Machine Learning: Role of Missing Value and Outliers. J. Med. Syst. 2018, 42, 92. [Google Scholar] [CrossRef] [Green Version]
- Banchhor, S.K.; Londhe, N.D.; Araki, T.; Saba, L.; Radeva, P.; Laird, J.R.; Suri, J.S. Wall-based measurement features provides an improved IVUS coronary artery risk assessment when fused with plaque texture-based features during machine learning paradigm. Comput. Biol. Med. 2017, 91, 198–212. [Google Scholar] [CrossRef]
- Acharya, U.R.; Faust, O.; Sree, S.V.; Molinari, F.; Suri, J.S. ThyroScreen system: High resolution ultrasound thyroid image characterization into benign and malignant classes using novel combination of texture and discrete wavelet transform. Comput. Methods Programs Biomed. 2011, 107, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Pareek, G.; Acharya, U.R.; Sree, S.V.; Swapna, G.; Yantri, R.; Martis, R.J.; Saba, L.; Krishnamurthi, G.; Mallarini, G.; El-Baz, A.; et al. Prostate Tissue Characterization/Classification in 144 Patient Population Using Wavelet and Higher Order Spectra Features from Transrectal Ultrasound Images. Technol. Cancer Res. Treat. 2013, 12, 545–557. [Google Scholar] [CrossRef]
- McClure, P.; Elnakib, A.; El-Ghar, M.A.; Khalifa, F.; Soliman, A.; El-Diasty, T.; Suri, J.S.; Elmaghraby, A.; El-Baz, A. In-Vitro and In-Vivo Diagnostic Techniques for Prostate Cancer: A Review. J. Biomed. Nanotechnol. 2014, 10, 2747–2777. [Google Scholar] [CrossRef]
- Acharya, U.R.; Saba, L.; Molinari, F.; Guerriero, S.; Suri, J.S. Ovarian tumor characterization and classification: A class of GyneScan™ systems. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; pp. 4446–4449. [Google Scholar]
- Fasano, A.; Cereda, E.; Barichella, M.; Cassani, E.; Ferri, V.; Zecchinelli, A.L.; Pezzoli, G. COVID-19 in Parkinson’s disease patients living in Lombardy, Italy. Mov. Disord. 2020, 35, 1089–1093. [Google Scholar] [CrossRef]
- Mitchell, F. Vitamin-D and COVID-19: Do deficient risk a poorer outcome? Lancet Diabetes Endocrinol. 2020, 8, 570. [Google Scholar] [CrossRef]
- De Siqueira, J.V.V.; Almeida, L.G.; Zica, B.O.; Brum, I.B.; Barceló, A.; Galil, A.G.d.S. Impact of obesity on hospitalizations and mortality, due to COVID-19: A systematic review. Obes. Res. Clin. Pract. 2020, 14, 398–403. [Google Scholar] [CrossRef]
- Ding, H.; Dhima, K.; Lockhart, K.C.; Locascio, J.J.; Hoesing, A.N.; Duong, K.; Trisini-Lipsanopoulos, A.; Hayes, M.T.; Sohur, U.S.; Wills, A.-M.; et al. Unrecognized vitamin D3 deficiency is common in Parkinson disease: Harvard Biomarker Study. Neurology 2013, 81, 1531–1537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hribar, C.A.; Cobbold, P.H.; Church, F.C. Potential role of vitamin D in the elderly to resist COVID-19 and to slow progression of Parkinson’s disease. Brain Sci. 2020, 10, 284. [Google Scholar] [CrossRef] [PubMed]
- Saba, L.; Agarwal, M.; Patrick, A.; Puvvula, A.; Gupta, S.K.; Carriero, A.; Laird, J.R.; Kitas, G.D.; Johri, A.M.; Balestrieri, A.; et al. Six artificial intelligence paradigms for tissue characterisation and classification of non-COVID-19 pneumonia against COVID-19 pneumonia in computed tomography lungs. Int. J. Comput. Assist. Radiol. Surg. 2021, 16, 423–434. [Google Scholar] [CrossRef] [PubMed]
- De Velasco Oriol, J.; Vallejo, E.E.; Estrada, K.; Taméz Peña, J.G. Disease Neuroimaging Initiative. Benchmarking machine learning models for late-onset alzheimer’s disease prediction from genomic data. BMC Bioinform. 2019, 20, 709. [Google Scholar] [CrossRef]
- Da Silva, R.M.; Santos, V.L.d.; Silva, T.V.d.A.; Lins, C.C.d.S.A. Prevalence of temporomandibular joint disorder in people with Parkinson’s disease in a public university hospital. Rev. CEFAC 2019, 21. [Google Scholar] [CrossRef]
- Choi, H.-G.; Yoon, J.-H.; Chung, T.-H.; Min, C.; Yoo, D.-M.; Wee, J.-H.; Kang, S.-Y.; Choi, Y.; Hong, S.-J.; Byun, S.-H. Association between Temporomandibular Joint Disorder and Parkinson’s Disease. Brain Sci. 2021, 11, 747. [Google Scholar] [CrossRef]
- Moccia, S.; Nucci, L.; Spagnuolo, C.; D’Apuzzo, F.; Piancino, M.G.; Minervini, G. Polyphenols as Potential Agents in the Management of Temporomandibular Disorders. Appl. Sci. 2020, 10, 5305. [Google Scholar] [CrossRef]
- Manfredini, D. A better definition of counselling strategies is needed to define effectiveness in temporomandibular disorders management. Evid. Based Dent. 2013, 14, 118–119. [Google Scholar] [CrossRef]
- Baba, Y.; Higuchi, M.A.; Fukuyama, K.; Abe, H.; Uehara, Y.; Inoue, T.; Yamada, T. Effect of chronic kidney disease on excessive daytime sleepiness in Parkinson disease. Eur. J. Neurol. 2011, 18, 1299–1303. [Google Scholar] [CrossRef]
- Moore, S.T.; MacDougall, H.G.; Ondo, W.G. Ambulatory monitoring of freezing of gait in Parkinson’s disease. Neurosci. Methods 2008, 167, 340–348. [Google Scholar] [CrossRef]
- Kummer, B.R.; Diaz, I.; Wu, X.; Aaroe, A.E.; Chen, M.L.; Iadecola, C.; Kamel, H.; Navi, B.B. Associations between cerebrovascular risk factors and Parkinson disease. Ann. Neurol. 2019, 86, 572–581. [Google Scholar] [CrossRef]
- Shahwar, T.; Zafar, J.; Almogren, A.; Zafar, H.; Rehman, A.U.; Shafiq, M.; Hamam, H. Automated detection of Alzheimer’s via hybrid classical quantum neural networks. Electronics 2022, 11, 721. [Google Scholar] [CrossRef]
- Raglione, L.M.; Sorbi, S.; Nacmias, B. Osteoporosis and Parkinson’s disease. Clin. Cases Miner. Bone Metab. 2011, 8, 16. [Google Scholar]
- Invernizzi, M.; Carda, S.; Viscontini, G.S.; Cisari, C. Osteoporosis in Parkinson’s disease. Parkinsonism Relat. Disord. 2009, 15, 339–346. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, F.; Jin, W.-S.; Zhu, C.; Wang, Q.-H.; Bu, X.-L.; Luo, H.-B.; Zou, H.-Q.; Pu, J.; Zhou, Z.-H. Comorbidity burden of patients with Parkinson’s disease and Parkinsonism between 2003 and 2012: A multicentre, nationwide, retrospective study in China. Sci. Rep. 2017, 7, 1671. [Google Scholar] [CrossRef] [Green Version]
- Brown, E.G.; Chahine, L.M.; Goldman, S.M.; Korell, M.; Mann, E.; Kinel, D.R.; Arnedo, V.; Marek, K.L.; Tanner, C.M. The Effect of the COVID-19 Pandemic on People with Parkinson’s Disease. J. Parkinson’s Dis. 2020, 10, 1365–1377. [Google Scholar] [CrossRef]
- Sattar, Y.; Ullah, W.; Rauf, H.; Yadav, S.; Chowdhury, M.; Connerney, M.; Mamtani, S.; Pahuja, M.; Patel, R.D.; Mir, T. COVID-19 cardiovascular epidemiology, cellular pathogenesis, clinical manifestations and management. IJC Heart Vasc. 2020, 29, 100589. [Google Scholar] [CrossRef]
- Orayj, K.; Lacey, A.; Akbari, A.; Smith, M.; Pickrell, O.; Lane, E. Association between levodopa and ischemic heart disease. Int. J. Popul. Data Sci. 2019, 4. [Google Scholar] [CrossRef]
- Suri, J.S.; Agarwal, S.; Gupta, S.K.; Puvvula, A.; Biswas, M.; Saba, L.; Bit, A.; Tandel, G.S.; Agarwal, M.; Patrick, A.; et al. A narrative review on characterization of acute respiratory distress syndrome in COVID-19-infected lungs using artificial intelligence. Comput. Biol. Med. 2021, 130, 104210. [Google Scholar] [CrossRef]
- Zheng, K.S.; Dorfman, B.; Christos, P.J.; Khadem, N.R.; Henchcliffe, C.; Piboolnurak, P.; Nirenberg, M.J. Clinical Characteristics of Exacerbations in Parkinson Disease. Neurologist 2012, 18, 120–124. [Google Scholar] [CrossRef] [Green Version]
- Rocchi, C.; Pierantozzi, M.; Galati, S.; Chiaravalloti, A.; Pisani, V.; Prosperetti, C.; Lauretti, B.; Bassi, M.S.; Olivola, E.; Schillaci, O. Autonomic function tests and MIBG in Parkinson’s disease: Correlation to disease duration and motor symptoms. CNS Neurosci. Ther. 2015, 21, 727–732. [Google Scholar] [CrossRef]
- Hardy, J. Genetic Analysis of Pathways to Parkinson Disease. Neuron 2010, 68, 201–206. [Google Scholar] [CrossRef] [Green Version]
- Su, C.; Tong, J.; Wang, F. Mining genetic and transcriptomic data using machine learning approaches in Parkinson’s disease. npj Parkinson’s Dis. 2020, 6, 24. [Google Scholar] [CrossRef]
- Shukla, V.; Mishra, S.K.; Pant, H.C. Oxidative stress in neurodegeneration. Adv. Pharmacol. Sci. 2011, 2011, 572634. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Zhang, C.; Xing, Z.; Ahmad, Z.; Li, J.-S.; Chang, M.-W. Pharmacological effects of natural Ganoderma and its extracts on neurological diseases: A comprehensive review. Int. J. Biol. Macromol. 2018, 121, 1160–1178. [Google Scholar] [CrossRef]
- Yu, J.; Park, S.; Kwon, S.-H.; Ho, C.M.B.; Pyo, C.-S.; Lee, H. AI-based Stroke Disease Prediction System Using Real-Time Electromyography Signals. Appl. Sci. 2020, 10, 6791. [Google Scholar] [CrossRef]
- Emma, P.; Bennett, M.R. The role of mitochondrial DNA damage in the development of atherosclerosis. Free Radic. Biol. Med. 2016, 100, 223–230. [Google Scholar]
- Wang, X.; Cao, G.; Ding, D.; Li, F.; Zhao, X.; Wang, J.; Yang, Y. Ferruginol prevents degeneration of dopaminergic neurons by enhancing clearance of α-synuclein in neuronal cells. Fitoterapia 2021, 156, 105066. [Google Scholar] [CrossRef] [PubMed]
- Sampedro, F.; Martínez-Horta, S.; Horta-Barba, A.; Grothe, M.J.; Labrador-Espinosa, M.A.; Jesús, S.; Adarmes-Gómez, A.; Carrillo, F.; Puig-Davi, A.; Lora, F.R. Increased homocysteine levels correlate with cortical structural damage in Parkinson’s disease. J. Neurol. Sci. 2022, 434, 120148. [Google Scholar] [CrossRef] [PubMed]
- Madamanchi, N.R.; Runge, M.S. Mitochondrial dysfunction in atherosclerosis. Circ. Res. 2007, 100, 460–473. [Google Scholar] [CrossRef] [Green Version]
- Frostegard, J.; Haegerstrand, A.; Gidlund, M.; Nilsson, J. Biologically modified LDL increases the adhesive properties of endothelial cells. Atherosclerosis 1991, 90, 119–126. [Google Scholar] [CrossRef]
- Chirkov, Y.Y.; Nguyen, T.H.; Horowitz, J.D. Impairment of Anti-Aggregatory Responses to Nitric Oxide and Prostacyclin: Mechanisms and Clinical Implications in Cardiovascular Disease. Int. J. Mol. Sci. 2022, 23, 1042. [Google Scholar] [CrossRef]
- Kavanagh, T.; Mertens, D.J.; Hamm, L.F.; Beyene, J.; Kennedy, J.; Corey, P.; Shephard, R.J. Prediction of Long-Term Prognosis in 12 169 Men Referred for Cardiac Rehabilitation. Circulation 2002, 106, 666–671. [Google Scholar] [CrossRef]
- Kavanagh, T.; Mertens, D.J.; Hamm, L.F.; Beyene, J.; Kennedy, J.; Corey, P.; Shephard, R.J. Peak oxygen intake and cardiac mortality in women referred for cardiac rehabilitation. J. Am. Coll. Cardiol. 2003, 42, 2139–2143. [Google Scholar] [CrossRef] [Green Version]
- Kamal, R.M.; Razis, A.F.A.; Sukri, N.S.M.; Perimal, E.K.; Ahmad, H.; Patrick, R.; Djedaini-Pilard, F.; Mazzon, E.; Rigaud, S. Beneficial Health Effects of Glucosinolates-Derived Isothiocyanates on Cardiovascular and Neurodegenerative Diseases. Molecules 2022, 27, 624. [Google Scholar] [CrossRef]
- Qorchi, S.; Vray, D.; Orkisz, M. Estimating Arterial Wall Deformations from Automatic Key-Point Detection and Matching. Ultrasound Med. Biol. 2021, 47, 1367–1376. [Google Scholar] [CrossRef]
- Sarraju, A.; Ward, A.; Chung, S.; Li, J.; Scheinker, D.; Rodríguez, F. Machine learning approaches improve risk stratification for secondary cardiovascular disease prevention in multiethnic patients. Open Heart 2021, 8, e001802. [Google Scholar] [CrossRef] [PubMed]
- Jamthikar, A.; Gupta, D.; Khanna, N.N.; Saba, L.; Araki, T.; Viskovic, K.; Suri, H.S.; Gupta, A.; Mavrogeni, S.; Turk, M.; et al. A low-cost machine learning-based cardiovascular/stroke risk assessment system: Integration of conventional factors with image phenotypes. Cardiovasc. Diagn. Ther. 2019, 9, 420–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeo, M.; Tahayori, B.; Kok, H.K.; Maingard, J.; Kutaiba, N.; Russell, J.; Thijs, V.; Jhamb, A.; Chandra, R.V.; Brooks, M.; et al. Review of deep learning algorithms for the automatic detection of intracranial hemorrhages on computed tomography head imaging. J. Neurointerv. Surg. 2021, 13, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Kemp, K.; Griffiths, J.; Campbell, S.; Lovell, K. An exploration of the follow-up up needs of patients with inflammatory bowel disease. J. Crohn’s Colitis 2013, 7, e386–e395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, M.M.; Rahman, M.J.; Roy, D.C.; Maniruzzaman, M. Automated detection and classification of diabetes disease based on Bangladesh demographic and health survey data, 2011 using machine learning approach. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 217–219. [Google Scholar] [CrossRef]
- Maniruzzaman, M.; Suri, H.S.; Kumar, N.; Abedin, M.; Rahman, J.; El-Baz, A.; Bhoot, M.; Teji, J.S.; Suri, J.S. Risk factors of neonatal mortality and child mortality in Bangladesh. J. Glob. Health 2018, 8, 010417. [Google Scholar] [CrossRef]
- Johnson, K.B.; Wei, W.; Weeraratne, D.; Frisse, M.E.; Misulis, K.; Rhee, K.; Zhao, J.; Snowdon, J.L. Precision Medicine, AI, and the Future of Personalized Health Care. Clin. Transl. Sci. 2020, 14, 86–93. [Google Scholar] [CrossRef]
- Hurvitz, N.; Azmanov, H.; Kesler, A.; Ilan, Y. Establishing a second-generation artificial intelligence-based system for improving diagnosis, treatment, and monitoring of patients with rare diseases. Eur. J. Hum. Genet. 2021, 29, 1485–1490. [Google Scholar] [CrossRef]
- Yeh, C.-H.; Chou, Y.-J.; Tsai, T.-H.; Hsu, P.W.-C.; Li, C.-H.; Chan, Y.-H.; Tsai, S.-F.; Ng, S.-C.; Chou, K.-M.; Lin, Y.-C. Artificial-Intelligence-Assisted Discovery of Genetic Factors for Precision Medicine of Antiplatelet Therapy in Diabetic Peripheral Artery Disease. Biomedicines 2022, 10, 116. [Google Scholar] [CrossRef]
- Saba, L.; Sanagala, S.S.; Gupta, S.K.; Koppula, V.K.; Johri, A.M.; Khanna, N.N.; Mavrogeni, S.; Laird, J.R.; Pareek, G.; Miner, M.; et al. Multimodality carotid plaque tissue characterization and classification in the artificial intelligence paradigm: A narrative review for stroke application. Ann. Transl. Med. 2021, 9, 1206. [Google Scholar] [CrossRef]
- Kadhel, P.; Revaux, A.; Carbonnel, M.; Naoura, I.; Asmar, J.; Ayoubi, J.M. An update on preoperative assessment of the resectability of advanced ovarian cancer. Horm. Mol. Biol. Clin. Investig. 2020, 41, 331. [Google Scholar] [CrossRef]
- Acharya, U.R.; Faust, O.; Sree, S.V.; Molinari, F.; Garberoglio, R.; Suri, J.S. Cost-effective and non-invasive automated benign & malignant thyroid lesion classification in 3D contrast-enhanced ultrasound using combination of wavelets and textures: A class of ThyroScan™ algorithms. Technol. Cancer Res. Treat. 2011, 10, 371–380. [Google Scholar]
- Huang, S.-F.; Chang, R.-F.; Moon, W.K.; Lee, Y.-H.; Chen, D.-R.; Suri, J.S. Analysis of tumor vascularity using three-dimensional power Doppler ultrasound images. IEEE Trans. Med. Imaging 2008, 27, 320–330. [Google Scholar] [CrossRef]
- Luo, D.; Wang, X.; Zeng, S.; Ramamurthy, G.; Burda, C.; Basilion, J.P. Targeted gold nanocluster-enhanced radiotherapy of prostate cancer. Small 2019, 34, 1900968. [Google Scholar] [CrossRef]
- Acharya, U.R.; Sree, S.V.; Kulshreshtha, S.; Molinari, F.; Koh, J.E.W.; Saba, L.; Suri, J.S. GyneScan: An improved online paradigm for screening of ovarian cancer via tissue characterization. Technol. Cancer Res. Treat. 2014, 13, 529–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winston, P.H. Artificial Intelligence; Addison-Wesley Longman Publishing Co., Inc.: Boston, MA, USA, 1992. [Google Scholar]
- Ramesh, A.; Kambhampati, C.; Monson, J.R.; Drew, P. Artificial intelligence in medicine. Ann. R. Coll. Surg. Engl. 2004, 86, 334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hesamian, M.H.; Jia, W.; He, X.; Kennedy, P. Deep learning techniques for medical image segmentation: Achievements and challenges. J. Digit. Imaging 2019, 32, 582–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fourcade, A.; Khonsari, R. Deep learning in medical image analysis: A third eye for doctors. J. Stomatol. Oral Maxillofac. Surg. 2019, 120, 279–288. [Google Scholar] [CrossRef]
- Mandal, I.; Sairam, N. New machine-learning algorithms for prediction of Parkinson’s disease. Int. J. Syst. Sci. 2014, 45, 647–666. [Google Scholar] [CrossRef]
- Sriram, T.V.; Rao, M.V.; Narayana, G.S.; Kaladhar, D.; Vital, T.P.R. Intelligent Parkinson disease prediction using machine learning algorithms. Int. J. Eng. Innov. Technol. 2013, 3, 1568–1572. [Google Scholar]
- Pereira, C.R.; Pereira, D.R.; da Silva, F.A.; Hook, C.; Weber, S.A.; Pereira, L.A.; Papa, J.P. A step towards the automated diagnosis of parkinson’s disease: Analyzing handwriting movements. In Proceedings of the 2015 IEEE 28th International Symposium on Computer-Based Medical Systems, Sao Carlos, Brazil, 22–25 June 2015; pp. 171–176. [Google Scholar]
- Halder, A.; Datta, B. COVID-19 detection from lung CT-scan images using transfer learning approach. Mach. Learn. Sci. Technol. 2021, 2, 045013. [Google Scholar] [CrossRef]
- Jamthikar, A.; Gupta, D.; Khanna, N.N.; Saba, L.; Laird, J.R.; Suri, J.S. Cardiovascular/stroke risk prevention: A new machine learning framework integrating carotid ultrasound image-based phenotypes and its harmonics with conventional risk factors. Indian Heart J. 2020, 72, 258–264. [Google Scholar] [CrossRef]
- Jamthikar, A.D.; Gupta, D.; Puvvula, A.; Johri, A.M.; Khanna, N.N.; Saba, L.; Mavrogeni, S.; Laird, J.R.; Pareek, G.; Miner, M.; et al. Cardiovascular risk assessment in patients with rheumatoid arthritis using carotid ultrasound B-mode imaging. Rheumatol. Int. 2020, 40, 1921–1939. [Google Scholar] [CrossRef]
- Jamthikar, A.; Gupta, D.; Saba, L.; Khanna, N.N.; Viskovic, K.; Mavrogeni, S.; Laird, J.R.; Sattar, N.; Johri, A.M.; Pareek, G. Artificial intelligence framework for predictive cardiovascular and stroke risk assessment models: A narrative review of integrated approaches using carotid ultrasound. Comput. Biol. Med. 2020, 126, 104043. [Google Scholar] [CrossRef]
- Murgia, A.; Balestrieri, A.; Crivelli, P.; Suri, J.S.; Conti, M.; Cademartiri, F.; Saba, L. Cardiac computed tomography radiomics: An emerging tool for the non-invasive assessment of coronary atherosclerosis. Cardiovasc. Diagn. Ther. 2020, 10, 2005–2017. [Google Scholar] [CrossRef]
- Shrivastava, V.K.; Londhe, N.D.; Sonawane, R.S.; Suri, J.S. A novel and robust Bayesian approach for segmentation of psoriasis lesions and its risk stratification. Comput. Methods Programs Biomed. 2017, 150, 9–22. [Google Scholar] [CrossRef]
- Saba, L.; Sanfilippo, R.; Porcu, M.; Lucatelli, P.; Montisci, R.; Zaccagna, F.; Suri, J.S.; Anzidei, M.; Wintermark, M. Relationship between white matter hyperintensities volume and the circle of Willis configurations in patients with carotid artery pathology. Eur. J. Radiol. 2017, 89, 111–116. [Google Scholar] [CrossRef]
- Araki, T.; Ikeda, N.; Dey, N.; Chakraborty, S.; Saba, L.; Kumar, D.; Godia, E.C.; Jiang, X.; Gupta, A.; Radeva, P.; et al. A comparative approach of four different image registration techniques for quantitative assessment of coronary artery calcium lesions using intravascular ultrasound. Comput. Methods Programs Biomed. 2015, 118, 158–172. [Google Scholar] [CrossRef]
- Jain, P.K.; Sharma, N.; Saba, L.; Paraskevas, K.I.; Kalra, M.K.; Johri, A.; Nicolaides, A.N.; Suri, J.S. Automated deep learning-based paradigm for high-risk plaque detection in B-mode common carotid ultrasound scans: An asymptomatic Japanese cohort study. Int. Angiol. 2021, 41, 9–23. [Google Scholar] [CrossRef]
- Shrivastava, V.K.; Londhe, N.D.; Sonawane, R.S.; Suri, J.S. Reliable and accurate psoriasis disease classification in dermatology images using comprehensive feature space in machine learning paradigm. Expert Syst. Appl. 2015, 42, 6184–6195. [Google Scholar] [CrossRef]
- Little, B.P. Disease Severity Scoring for COVID-19: A Welcome Semiquantitative Role for Chest Radiography. Radiology 2022, 2, 470. [Google Scholar] [CrossRef]
- Suri, J.S. Imaging Based Symptomatic Classification and Cardiovascular Stroke Risk Score Estimation. U.S. Patent 13/053,971, 20 October 2011. [Google Scholar]
- Khan, A.A.; Shafiq, S.; Kumar, R.; Kumar, J.; Haq, A.U. H3DNN: 3D deep learning based detection of COVID-19 virus using lungs computed tomography. In Proceedings of the 2020 17th International Computer Conference on Wavelet Active Media Technology and Information Processing (ICCWAMTIP), Chengdu, China, 18–21 December 2020; pp. 183–186. [Google Scholar]
- Saba, L.; Agarwal, M.; Sanagala, S.; Gupta, S.; Sinha, G.; Johri, A.; Khanna, N.; Mavrogeni, S.; Laird, J.; Pareek, G.; et al. Brain MRI-based Wilson disease tissue classification: An optimised deep transfer learning approach. Electron. Lett. 2020, 56, 1395–1398. [Google Scholar] [CrossRef]
- Suri, J.S.; Puvvula, A.; Majhail, M.; Biswas, M.; Jamthikar, A.D.; Saba, L.; Faa, G.; Singh, I.M.; Oberleitner, R.; Turk, M. Integration of cardiovascular risk assessment with COVID-19 using artificial intelligence. Rev. Cardiovasc. Med. 2020, 21, 541–560. [Google Scholar]
- Jamthikar, A.; Gupta, D.; Saba, L.; Khanna, N.N.; Araki, T.; Viskovic, K.; Mavrogeni, S.; Laird, J.R.; Pareek, G.; Miner, M.; et al. Cardiovascular/stroke risk predictive calculators: A comparison between statistical and machine learning models. Cardiovasc. Diagn. Ther. 2020, 10, 919–938. [Google Scholar] [CrossRef]
- Jamthikar, A.D.; Gupta, D.; Johri, A.M.; Mantella, L.E.; Saba, L.; Kolluri, R.; Sharma, A.M.; Viswanathan, V.; Nicolaides, A.; Suri, J.S. Low-Cost Office-Based Cardiovascular Risk Stratification Using Machine Learning and Focused Carotid Ultrasound in an Asian-Indian Cohort. J. Med. Syst. 2020, 44, 208. [Google Scholar] [CrossRef] [PubMed]
- Molinari, F.; Liboni, W.; Giustetto, P.; Badalamenti, S.; Suri, J.S. Automatic computer-based tracings (act) in longitudinal 2-d ultrasound images using different scanners. J. Mech. Med. Biol. 2009, 9, 481–505. [Google Scholar] [CrossRef]
- Sudeep, P.; Palanisamy, P.; Rajan, J.; Baradaran, H.; Saba, L.; Gupta, A.; Suri, J.S. Speckle reduction in medical ultrasound images using an unbiased non-local means method. Biomed. Signal Process. Control 2016, 28, 1–8. [Google Scholar] [CrossRef]
- Pewowaruk, R.J.; Tedla, Y.; Korcarz, C.E.; Tattersall, M.C.; Stein, J.H.; Chesler, N.C.; Gepner, A.D. Carotid Artery Stiffening with Aging: Structural Versus Load-Dependent Mechanisms in MESA (the Multi-Ethnic Study of Atherosclerosis). Hypertension 2022, 79, 150–158. [Google Scholar] [CrossRef]
- Alqahtani, E.J.; Alshamrani, F.H.; Syed, H.F.; Olatunji, S.O. Classification of Parkinson’s Disease Using NNge Classification Algorithm. In Proceedings of the 2018 21st Saudi Computer Society National Computer Conference (NCC), Riyadh, Saudi Arabia, 25–26 April 2018; pp. 1–7. [Google Scholar]
- Naghsh, E.; Sabahi, M.F.; Beheshti, S. Spatial analysis of EEG signals for Parkinson’s disease stage detection. Signal Image Video Process. 2019, 14, 397–405. [Google Scholar] [CrossRef]
- Khatamino, P.; Cantürk, İ.; Özyılmaz, L. A deep learning-CNN based system for medical diagnosis: An application on Parkinson’s disease handwriting drawings. In Proceedings of the 2018 6th International Conference on Control Engineering & Information Technology (CEIT), Istanbul, Turkey, 25–27 October 2018. [Google Scholar]
- Hoq, M.; Uddin, M.N.; Park, S. Vocal Feature Extraction-Based Artificial Intelligent Model for Parkinson’s Disease Detection. Diagnostics 2021, 11, 1076. [Google Scholar] [CrossRef]
- Kamble, M.; Shrivastava, P.; Jain, M. Digitized spiral drawing classification for Parkinson’s disease diagnosis. Meas. Sens. 2021, 16, 100047. [Google Scholar] [CrossRef]
- Akyol, K. Comparing of deep neural networks and extreme learning machines based on growing and pruning approach. Expert Syst. Appl. 2020, 140, 112875. [Google Scholar] [CrossRef]
- Khedr, E.M.; el Fetoh, N.A.; Khalifa, H.; Ahmed, M.A.; el Beh, K.M. Prevalence of non motor features in a cohort of Parkinson’s disease patients. Clin. Neurol. Neurosurg. 2013, 115, 673–677. [Google Scholar] [CrossRef]
- Mathew, M.J.; Baiju, J. Machine learning technique based parkinson’s disease detection from spiral and voice inputs. Eur. J. Mol. Clin. Med. 2020, 7, 2815–2819. [Google Scholar]
- Jo, T.; Nho, K.; Saykin, A.J. Deep Learning in Alzheimer’s Disease: Diagnostic Classification and Prognostic Prediction Using Neuroimaging Data. Front. Aging Neurosci. 2019, 11, 220. [Google Scholar] [CrossRef] [Green Version]
- Maitín, A.M.; García-Tejedor, A.J.; Muñoz, J.P.R. Machine Learning Approaches for Detecting Parkinson’s Disease from EEG Analysis: A Systematic Review. Appl. Sci. 2020, 10, 8662. [Google Scholar] [CrossRef]
- Poorjam, A.H.; Kavalekalam, M.S.; Shi, L.; Raykov, J.P.; Jensen, J.R.; Little, M.A.; Christensen, M.G. Automatic quality control and enhancement for voice-based remote Parkinson’s disease detection. Speech Commun. 2020, 127, 1–16. [Google Scholar] [CrossRef]
- Naseer, A.; Rani, M.; Naz, S.; Razzak, M.I.; Imran, M.; Xu, G. Refining Parkinson’s neurological disorder identification through deep transfer learning. Neural Comput. Appl. 2019, 32, 839–854. [Google Scholar] [CrossRef] [Green Version]
- Amin, J.; Sharif, M.; Raza, M.; Saba, T.; Sial, R.; Shad, S.A. Brain tumor detection: A long short-term memory (LSTM)-based learning model. Neural Comput. Appl. 2019, 32, 15965–15973. [Google Scholar] [CrossRef]
- An, Y.; Tang, K.; Wang, J. Time-Aware Multi-Type Data Fusion Representation Learning Framework for Risk Prediction of Cardiovascular Diseases. IEEE ACM Trans. Comput. Biol. Bioinform. 2021. [Google Scholar] [CrossRef]
- Tan, L.; Yu, K.; Bashir, A.K.; Cheng, X.; Ming, F.; Zhao, L.; Zhou, X. Toward real-time and efficient cardiovascular monitoring for COVID-19 patients by 5G-enabled wearable medical devices: A deep learning approach. Neural Comput. Appl. 2021, 1–14. [Google Scholar] [CrossRef]
- Priyanga, P.; Pattankar, V.V.; Sridevi, S. A hybrid recurrent neural network—Logistic chaos-based whale optimization framework for heart disease prediction with electronic health records. Comput. Intell. 2020, 37, 315–343. [Google Scholar] [CrossRef]
- Khanna, N.N.; Maindarkar, M.; Saxena, A.; Ahluwalia, P.; Paul, S.; Srivastava, S.K.; Cuadrado-Godia, E.; Sharma, A.; Omerzu, T.; Saba, L.; et al. Cardiovascular/Stroke Risk Assessment in Patients with Erectile Dysfunction—A Role of Carotid Wall Arterial Imaging and Plaque Tissue Characterization Using Artificial Intelligence Paradigm: A Narrative Review. Diagnostics 2022, 12, 1249. [Google Scholar] [CrossRef]
- Kakadiaris, I.A.; Vrigkas, M.; Yen, A.A.; Kuznetsova, T.; Budoff, M.; Naghavi, M. Machine Learning Outperforms ACC/AHA CVD Risk Calculator in MESA. J. Am. Heart Assoc. 2018, 7, e009476. [Google Scholar] [CrossRef] [Green Version]
- Rava, R.A.; Seymour, S.E.; Snyder, K.V.; Waqas, M.; Davies, J.M.; Levy, E.I.; Siddiqui, A.H.; Ionita, C.N. Automated Collateral Flow Assessment in Patients with Acute Ischemic Stroke Using Computed Tomography with Artificial Intelligence Algorithms. World Neurosurg. 2021, 155, e748–e760. [Google Scholar] [CrossRef]
- Unnikrishnan, P.; Kumar, D.K.; Arjunan, S.P.; Kumar, H.; Mitchell, P.; Kawasaki, R. Development of health parameter model for risk prediction of CVD using SVM. Comput. Math. Methods Med. 2016, 2016, 3016245. [Google Scholar] [CrossRef] [Green Version]
- Saba, L.; Sanagala, S.S.; Gupta, S.K.; Koppula, V.K.; Laird, J.R.; Viswanathan, V.; Sanches, M.J.; Kitas, G.D.; Johri, A.M.; Sharma, N. A Multicenter Study on Carotid Ultrasound Plaque Tissue Characterization and Classification Using Six Deep Artificial Intelligence Models: A Stroke Application. IEEE Trans. Instrum. Meas. 2021, 70, 2505312. [Google Scholar] [CrossRef]
- Jain, P.K.; Sharma, N.; Giannopoulos, A.A.; Saba, L.; Nicolaides, A.; Suri, J.S. Hybrid deep learning segmentation models for atherosclerotic plaque in internal carotid artery B-mode ultrasound. Comput. Biol. Med. 2021, 136, 104721. [Google Scholar] [CrossRef]
- Suri, J.S.; Paul, S.; Maindarkar, M.A.; Puvvula, A.; Saxena, S.; Saba, L.; Turk, M.; Laird, J.R.; Khanna, N.N.; Viskovic, K.; et al. Cardiovascular/Stroke Risk Stratification in Parkinson’s Disease Patients Using Atherosclerosis Pathway and Artificial Intelligence Paradigm: A Systematic Review. Metabolites 2022, 12, 312. [Google Scholar] [CrossRef]
- Park, E.; Chang, H.-J.; Nam, H.S. Use of machine learning classifiers and sensor data to detect neurological deficit in stroke patients. J. Med. Internet Res. 2017, 19, e7092. [Google Scholar] [CrossRef]
- Munjral, S.; Maindarkar, M.; Ahluwalia, P.; Puvvula, A.; Jamthikar, A.; Jujaray, T.; Suri, N.; Paul, S.; Pathak, R.; Saba, L. Cardiovascular Risk Stratification in Diabetic Retinopathy via Atherosclerotic Pathway in COVID-19/Non-COVID-19 Frameworks Using Artificial Intelligence Paradigm: A Narrative Review. Diagnostics 2022, 12, 1234. [Google Scholar] [CrossRef]
- El-Baz, A.; Gimel’farb, G.; Suri, J.S. Stochastic Modeling for Medical Image Analysis; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Zimmerman, A.; Kalra, D. Usefulness of machine learning in COVID-19 for the detection and prognosis of cardiovascular complications. Rev. Cardiovasc. Med. 2020, 21, 345–352. [Google Scholar] [CrossRef]
- Handy, A.; Wood, A.; Sudlow, C.; Tomlinson, C.; Kee, F.; Thygesen, J.H.; Mamouei, M.; Sofat, R.; Dobson, R.; Ip, H.Y.S. A nationwide deep learning pipeline to predict stroke and COVID-19 death in atrial fibrillation. medRxiv 2021. [Google Scholar] [CrossRef]
- Bergamaschi, L.; D’Angelo, E.C.; Paolisso, P.; Toniolo, S.; Fabrizio, M.; Angeli, F.; Donati, F.; Magnani, I.; Rinaldi, A.; Bartoli, L.; et al. The value of ECG changes in risk stratification of COVID-19 patients. Ann. Noninvasive Electrocardiol. 2021, 26, e12815. [Google Scholar] [CrossRef]
- Wodzinski, M.; Skalski, A.; Hemmerling, D.; Orozco-Arroyave, J.R.; Nöth, E. Deep learning approach to Parkinson’s disease detection using voice recordings and convolutional neural network dedicated to image classification. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 717–720. [Google Scholar]
- Konstantonis, G.; Singh, K.V.; Sfikakis, P.P.; Jamthikar, A.D.; Kitas, G.D.; Gupta, S.K.; Saba, L.; Verrou, K.; Khanna, N.N.; Ruzsa, Z. Cardiovascular disease detection using machine learning and carotid/femoral arterial imaging frameworks in rheumatoid arthritis patients. Rheumatol. Int. 2022, 42, 215–239. [Google Scholar] [CrossRef] [PubMed]
- Jamthikar, A.; Gupta, D.; Khanna, N.N.; Araki, T.; Saba, L.; Nicolaides, A.; Sharma, A.; Omerzu, T.; Suri, H.S.; Gupta, A. A special report on changing trends in preventive stroke/cardiovascular risk assessment via B-mode ultrasonography. Cogn. Inform. Comput. Model. Cogn. Sci. 2020, 2, 291–318. [Google Scholar]
- Kotsis, V.; Jamthikar, A.D.; Araki, T.; Gupta, D.; Laird, J.R.; Giannopoulos, A.A.; Saba, L.; Suri, H.S.; Mavrogeni, S.; Kitas, G.D. Echolucency-based phenotype in carotid atherosclerosis disease for risk stratification of diabetes patients. Diabetes Res. Clin. Pract. 2018, 143, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Khanna, N.N.; Jamthikar, A.D.; Araki, T.; Gupta, D.; Piga, M.; Saba, L.; Carcassi, C.; Nicolaides, A.; Laird, J.R.; Suri, H.S. Nonlinear model for the carotid artery disease 10-year risk prediction by fusing conventional cardiovascular factors to carotid ultrasound image phenotypes: A Japanese diabetes cohort study. Echocardiography 2019, 36, 345–361. [Google Scholar] [CrossRef] [PubMed]
- Khanna, N.N.; Jamthikar, A.; Gupta, D.; Nicolaides, A.; Araki, T.; Saba, L.; Cuadrado-Godia, E.; Sharma, A.; Omerzu, T.; Suri, H.S.; et al. Performance evaluation of 10-year ultrasound image-based stroke/cardiovascular (CV) risk calculator by comparing against ten conventional CV risk calculators: A diabetic study. Comput. Biol. Med. 2019, 105, 125–143. [Google Scholar] [CrossRef]
- Khanna, N.N.; Jamthikar, A.D.; Gupta, D.; Araki, T.; Piga, M.; Saba, L.; Carcassi, C.; Nicolaides, A.; Laird, J.R.; Suri, H.S.; et al. Effect of carotid image-based phenotypes on cardiovascular risk calculator: AECRS1.0. Med. Biol. Eng. Comput. 2019, 57, 1553–1566. [Google Scholar] [CrossRef]
- Cuadrado-Godia, E.; Jamthikar, A.; Gupta, D.; Khanna, N.N.; Araki, T.; Maniruzzaman; Saba, L.; Nicolaides, A.; Sharma, A.; Omerzu, T.; et al. Ranking of stroke and cardiovascular risk factors for an optimal risk calculator design: Logistic regression approach. Comput. Biol. Med. 2019, 108, 182–195. [Google Scholar] [CrossRef]
- Venetsanopoulou, A.I.; Voulgari, P.V.; Drosos, A.A. Hyperlipidemia and rheumatoid arthritis. In Cholesterol; Academic Press: Cambridge, MA, USA, 2022; pp. 969–997. [Google Scholar]
- Jamthikar, A.; Gupta, D.; Cuadrado-Godia, E.; Puvvula, A.; Khanna, N.N.; Saba, L.; Viskovic, K.; Mavrogeni, S.; Turk, M.; Laird, J.R. Ultrasound-based stroke/cardiovascular risk stratification using Framingham Risk Score and ASCVD Risk Score based on Integrated Vascular Age instead of Chronological Age: A multi-ethnic study of Asian Indian, Caucasian, and Japanese cohorts. Cardiovasc. Diagn. Ther. 2020, 10, 939. [Google Scholar] [CrossRef]
- Saba, L.; Jamthikar, A.; Gupta, D.; Khanna, N.N.; Viskovic, K.; Suri, H.S.; Gupta, A.; Mavrogeni, S.; Turk, M.; Laird, J.R. Global perspective on carotid intima-media thickness and plaque: Should the current measurement guidelines be revisited? Int. Angiol. 2020, 38, 451–465. [Google Scholar] [CrossRef]
- Puvvula, A.; Jamthikar, A.D.; Gupta, D.; Khanna, N.N.; Porcu, M.; Saba, L.; Viskovic, K.; Ajuluchukwu, J.N.; Gupta, A.; Mavrogeni, S. Morphological carotid plaque area is associated with glomerular filtration rate: A study of south asian indian patients with diabetes and chronic kidney disease. Angiology 2020, 71, 520–535. [Google Scholar] [CrossRef]
- Suri, J.S.; Viswanathan, V.; Jamthikar, A.D.; Gupta, D.; Shanu, N.; Puvvula, A.; Khanna, N.N.; Saba, L.; Omerzum, T.; Viskovic, K.; et al. Low-cost preventive screening using carotid ultrasound in patients with diabetes. Front. Biosci. 2020, 25, 1132–1171. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, J.-W.; Zang, G.-Y.; Pu, J. The primary use of artificial intelligence in cardiovascular diseases: What kind of potential role does artificial intelligence play in future medicine? J. Geriatr. Cardiol. 2019, 16, 585–591. [Google Scholar] [CrossRef]
- Aljameel, S.S.; Khan, I.U.; Aslam, N.; Aljabri, M.; Alsulmi, E.S. Machine Learning-Based Model to Predict the Disease Severity and Outcome in COVID-19 Patients. Sci. Program. 2021, 2021, 5587188. [Google Scholar] [CrossRef]
- Mouridsen, K.; Thurner, P.; Zaharchuk, G. Artificial Intelligence Applications in Stroke. Stroke 2020, 51, 2573–2579. [Google Scholar] [CrossRef]
- Paul, S.; Maindarkar, M.; Saxena, S.; Saba, L.; Turk, M.; Kalra, M.; Krishnan, P.R.; Suri, J.S. Bias Investigation in Artificial Intelligence Systems for Early Detection of Parkinson’sDisease: A Narrative Review. Diagnostics 2022, 12, 166. [Google Scholar] [CrossRef]
- Suri, J.S.; Bhagawati, M.; Paul, S.; Protogeron, A.; Sfikakis, P.P.; Kitas, G.D.; Khanna, N.N.; Ruzsa, Z.; Sharma, A.M.; Saxena, S.; et al. Understanding the bias in machine learning systems for cardiovascular disease risk assessment: The first of its kind review. Comput. Biol. Med. 2022, 142, 105204. [Google Scholar] [CrossRef]
- Suri, J.S.; Agarwal, S.; Jena, B.; Saxena, S.; El-Baz, A.; Agarwal, V.; Kalra, M.K.; Saba, L.; Viskovic, K.; Fatemi, M.; et al. Five Strategies for Bias Estimation in Artificial Intelligence-based Hybrid Deep Learning for Acute Respiratory Distress Syndrome COVID-19 Lung Infected Patients using AP(ai)Bias 2.0: A Systematic Review. IEEE Trans. Instrum. Meas. 2022. [Google Scholar] [CrossRef]
- El-Baz, A.; Suri, J.S. Big Data in Multimodal Medical Imaging; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Murray, C.J.; Lopez, A.D. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet 1997, 349, 1498–1504. [Google Scholar] [CrossRef]
- Tandel, G.S.; Biswas, M.; Kakde, O.G.; Tiwari, A.; Suri, H.S.; Turk, M.; Laird, J.R.; Asare, C.K.; Ankrah, A.A.; Khanna, N.N.; et al. A Review on a Deep Learning Perspective in Brain Cancer Classification. Cancers 2019, 11, 111. [Google Scholar] [CrossRef] [Green Version]
- Willmen, T.; Völkel, L.; Ronicke, S.; Hirsch, M.C.; Kaufeld, J.; Rychlik, R.P.; Wagner, A.D. Health economic benefits through the use of diagnostic support systems and expert knowledge. BMC Health Serv. Res. 2021, 21, 947. [Google Scholar] [CrossRef]
- Mital, S.; Nguyen, H.V. Cost-effectiveness of using artificial intelligence versus polygenic risk score to guide breast cancer screening. BMC Cancer 2022, 22, 501. [Google Scholar] [CrossRef]
- Areia, M.; Mori, Y.; Correale, L.; Repici, A.; Bretthauer, M.; Sharma, P.; Taveira, F.; Spadaccini, M.; Antonelli, G.; Ebigbo, A.; et al. Cost-effectiveness of artificial intelligence for screening colonoscopy: A modelling study. Lancet Digit. Health 2022, 4, e436–e444. [Google Scholar] [CrossRef]
- Morrison, S.L.; Dukhovny, D.; Chan, R.P.; Chiang, M.F.; Campbell, J.P. Cost-effectiveness of Artificial Intelligence–Based Retinopathy of Prematurity Screening. JAMA Ophthalmol. 2022, 140, 401. [Google Scholar] [CrossRef]
- Bao, H.; Zhang, X.; Bi, H.; Zhao, Y.; Fang, L.; Wang, H.; Wang, L. How Can a High-Performance Screening Strategy Be Determined for Cervical Cancer Prevention? Evidence From a Hierarchical Clustering Analysis of a Multicentric Clinical Study. Front. Oncol. 2022, 12, 816789. [Google Scholar] [CrossRef]
- Hoshida, Y.; Fuchs, B.C.; Tanabe, K.K. Prevention of hepatocellular carcinoma: Potential targets, experimental models, and clinical challenges. Curr. Cancer Drug Targets 2012, 12, 1129–1159. [Google Scholar]
- Lee, J.; Choi, W.; Kim, J. A Cost-Effective CNN-LSTM-Based Solution for Predicting Faulty Remote Water Meter Reading Devices in AMI Systems. Sensors 2021, 21, 6229. [Google Scholar] [CrossRef]
- World Health Organization. Cardiovascular Diseases (CVDs). 2017. Available online: http://www.who.int/mediacentre/factsheets/fs317/en.ed (accessed on 11 May 2022).
- Suri, J.S.; Agarwal, S.; Chabert, G.L.; Carriero, A.; Paschè, A.; Danna, P.S.C.; Saba, L.; Mehmedović, A.; Faa, G.; Singh, I.M.; et al. COVLIAS 1.0Lesion vs. MedSeg: An Artificial Intelligence Framework for Automated Lesion Segmentation in COVID-19 Lung Computed Tomography Scans. Diagnostics 2022, 12, 1283. [Google Scholar] [CrossRef]
- Agarwal, M.; Agarwal, S.; Saba, L.; Chabert, G.L.; Gupta, S.; Carriero, A.; Pasche, A.; Danna, P.; Mehmedovic, A.; Faa, G.; et al. Eight pruning deep learning models for low storage and high-speed COVID-19 computed tomography lung segmentation and heatmap-based lesion localization: A multicenter study using COVLIAS 2.0. Comput. Biol. Med. 2022, 146. [Google Scholar] [CrossRef]
- Hallows, R.; Glazier, L.; Katz, M.; Aznar, M.; Williams, M. Safe and Ethical Artificial Intelligence in Radiotherapy—Lessons Learned From the Aviation Industry. Clin. Oncol. 2021, 34, 99–101. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Nayak, G.; Saba, L.; Kalra, M.; Suri, J.S.; Saxena, S. An artificial intelligence framework and its bias for brain tumor segmentation: A narrative review. Comput. Biol. Med. 2022, 143, 105273. [Google Scholar] [CrossRef] [PubMed]
- Pablo, R.-G.J.; Roberto, D.-P.; Victor, S.-U.; Isabel, G.-R.; Paul, C.; Elizabeth, O.-R. Big data in the healthcare system: A synergy with artificial intelligence and blockchain technology. J. Integr. Bioinform. 2021, 19. [Google Scholar] [CrossRef] [PubMed]
- Munir, K.; Elahi, H.; Farooq, M.U.; Ahmed, S.; Frezza, F.; Rizzi, A. Detection and screening of COVID-19 through chest computed tomography radiographs using deep neural networks. In Data Science for COVID-19; Academic Press: Cambridge, MA, USA, 2021; pp. 63–73. [Google Scholar]
- Acharya, U.R.; Mookiah, M.R.K.; Sree, S.V.; Yanti, R.; Martis, R.J.; Saba, L.; Molinari, F.; Guerriero, S.; Suri, J.S. Evolutionary Algorithm-Based Classifier Parameter Tuning for Automatic Ovarian Cancer Tissue Characterization and Classification. Ultraschall Med. Eur. J. Ultrasound 2012, 35, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Sanagala, S.S.; Nicolaides, A.; Gupta, S.K.; Koppula, V.K.; Saba, L.; Agarwal, S.; Johri, A.M.; Kalra, M.S.; Suri, J.S. Ten Fast Transfer Learning Models for Carotid Ultrasound Plaque Tissue Characterization in Augmentation Framework Embedded with Heatmaps for Stroke Risk Stratification. Diagnostics 2021, 11, 2109. [Google Scholar] [CrossRef] [PubMed]











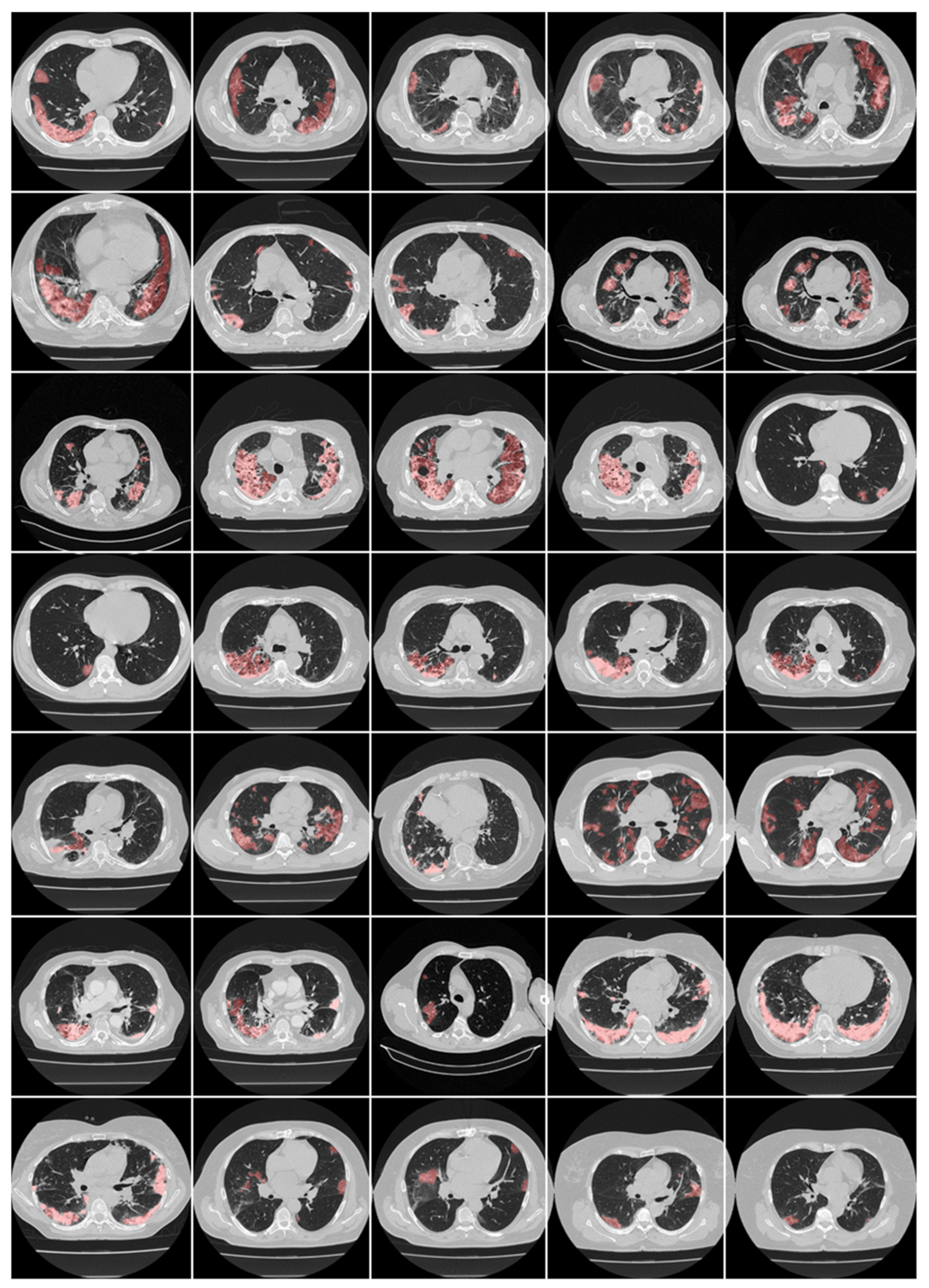


| SN | Citations | PS | ME | Relation * | Outcome | Treatment |
|---|---|---|---|---|---|---|
| 1 | Huang et al. [83] (2015) | 156 | LBBM | Plasma cholesterol risk in PD | Total high cholesterol levels have been linked to a lower risk of developing Parkinson’s disease, but statin use has been linked to an increased risk. | Statins |
| 2 | Yan et al. [72] (2019) | 68 | LBBM | Carotid plaque in PD | As Parkinson’s disease advances, the thickness of carotid plaques rises. | NR |
| 3 | Potashkin et al. [83] (2020) | 47 | LBBM | CVD and PD | Both CV and PD share inflammation, insulin resistance, lipid metabolism, and oxidative stress. Moderate coffee consumption and physical activity reduce the risk of heart disease and PD. | NR |
| 4 | Park et al. [35] (2020) | NR | Population-based cohort study | PD with risk of CVD | CVD is linked to PD. Patients with PD should be monitored for CVD. | NR |
| 5 | Değirmenci et al. [64] | NR | LBBM | Cardiac effect in PD | Cardiac problems are prevalent among Parkinson’s disease sufferers. | Levodopa, MOBI, COMT, anticholinergic drugs, deep brain simulations |
| 6 | Scorza et al. [84] (2018) | NR | LBBM | Cardiac abnormalities in PD | Cardiomyopathy, coronary heart disease, arrhythmias, conduction anomalies, and sudden cardiac arrest are among the symptoms of PD/PS. | NR |
| 7 | Günaydın et al. [85] (2016) | 65 | LBBM | CVD risk in PD under levodopa treatment | PD patients with L-dopa exhibited increased aortic stiffness and impaired diastolic performance. Homocysteine levels may influence diseases. | NR |
| 8 | Fanciulli et al. [86] (2020) | NR | LBBM | Orthostatic hypertension in PD | Orthostatic hypotension causes tachycardia, uncommon falls, disorientation, mental impairment, vision issues, fatigue, and painful shoulders, neck, or low back. They appear when the patient stands up and leave when the patient lies down. | Droxidopa, fludrocortisone, clonidine, transdermal nitroglycerin, nifedipine |
| 9 | Cuenca-Bermejo et al. [87] (2021) | NR | LBBM | Cardiac changes in PD | Cardiac anomalies have been observed in PD individuals who do not have sufficient sympathetic innervation in the heart. Hypotension after a meal is followed by supine hypertension; rising blood pressure variability, decreased heart rate and blood pressure, and chronotropic incompetence is all indications. | NR |
| 10 | Vikdahl et al. [88] (2015) | 147 | LBBM | CVD risk in PD | Exercise may be beneficial in lowering the risk of cardiovascular disease in some people. High levels of blood cholesterol, tobacco smoking, and a high BMI have all been associated with the progression of PD. | NR |
| SN | Authors and Citations | Total CT Scan Samples | Pretrained Model | Accuracy (%) | |
|---|---|---|---|---|---|
| Positive COVID-19 | Negative COVID-19 | ||||
| 1 | Halder et al. [206] (2021) | 1252 | 1229 | DenseNet 201 | 97.00 |
| ResNet50 V2 | 96.00 | ||||
| Mobile Net | 95.00 | ||||
| VGG-16 | 94.00 | ||||
| 2 | Kumari et al. [189] (2020) | 987 | 921 | VGG-16 | 87.68 |
| 3-layer CNN | 56.16 | ||||
| 3 | Mishra et al. [211] (2021) | 360 | 397 | Deep CNN | 86.00 |
| 4 | Saood et al. [175] (2021) | 287 | 314 | SegNet | 95.00 |
| Unet | 92.00 | ||||
| SN | S0 | COVID-19 Symptoms in PD Patients | PD Motor Symptoms | PD Non-Motor Symptoms | Risk Factors | Gold Standard | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S10 | S11 | S12 | S13 | S14 | S15 | S16 | ||
| 1 | Antonini et al. [56] (2020) | ✓ | ✕ | ✓ | ✓ | ✓ | ✕ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | PD + COVID-19 + Pneumonia |
| 2 | Baschi et al. [7] (2020) | ✓ | ✓ | ✓ | ✓ | ✓ | ✕ | ✓ | ✓ | ✓ | ✓ | ✓ | ✕ | ✓ | ✓ | ✓ | PD + COVID-19 + Respiratory dysfunctions |
| 3 | Brown et al. [163] (2020) | ✓ | ✓ | ✓ | ✓ | ✕ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✕ | ✓ | ✓ | ✓ | PD + COVID-19 + Pneumonia |
| 4 | Cella et al. [2] (2020) | ✓ | ✓ | ✓ | ✕ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✕ | ✓ | ✓ | ✓ | ✓ | PD + COVID-19 + Respiratory dysfunctions |
| 5 | Starmbi et al. [129] (2021) | ✓ | ✕ | ✓ | ✕ | ✓ | ✕ | ✓ | ✕ | ✓ | ✕ | ✓ | ✕ | ✓ | ✓ | ✓ | PD + COVID-19 + Respiratory dysfunctions |
| 6 | Helmich et al. [6] (2020) | ✕ | ✕ | ✓ | ✕ | ✓ | ✕ | ✓ | ✕ | ✓ | ✓ | ✓ | ✕ | ✓ | ✓ | ✕ | PD + COVID-19 + Pneumonia |
| 7 | Khoshnood et al. [5] (2021) | ✕ | ✕ | ✓ | ✕ | ✕ | ✕ | ✓ | ✓ | ✓ | ✕ | ✓ | ✕ | ✓ | ✓ | ✓ | PD + COVID-19 + Respiratory dysfunctions |
| 8 | Lau et al. [16] (2021) | ✓ | ✕ | ✓ | ✓ | ✓ | ✓ | ✕ | ✓ | ✓ | ✓ | ✓ | ✕ | ✓ | ✕ | ✓ | PD + COVID-19 + Pneumonia |
| 9 | Sulzer et al. [4] (2021) | ✓ | ✓ | ✓ | ✕ | ✕ | ✓ | ✕ | ✓ | ✕ | ✓ | ✓ | ✕ | ✓ | ✓ | ✓ | PD + COVID-19 + Respiratory dysfunctions |
| 10 | Tsivgoulis et al. [131] (2021) | ✓ | ✓ | ✓ | ✓ | ✓ | ✕ | ✕ | ✕ | ✕ | ✓ | ✕ | ✕ | ✕ | ✓ | ✓ | PD + COVID-19 + Respiratory dysfunctions |
| 11 | Sorbera et al. [130] (2021) | ✓ | ✓ | ✓ | ✓ | ✓ | ✕ | ✓ | ✓ | ✓ | ✓ | ✓ | ✕ | ✓ | ✓ | ✓ | PD + COVID-19 + Pneumonia |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suri, J.S.; Maindarkar, M.A.; Paul, S.; Ahluwalia, P.; Bhagawati, M.; Saba, L.; Faa, G.; Saxena, S.; Singh, I.M.; Chadha, P.S.; et al. Deep Learning Paradigm for Cardiovascular Disease/Stroke Risk Stratification in Parkinson’s Disease Affected by COVID-19: A Narrative Review. Diagnostics 2022, 12, 1543. https://doi.org/10.3390/diagnostics12071543
Suri JS, Maindarkar MA, Paul S, Ahluwalia P, Bhagawati M, Saba L, Faa G, Saxena S, Singh IM, Chadha PS, et al. Deep Learning Paradigm for Cardiovascular Disease/Stroke Risk Stratification in Parkinson’s Disease Affected by COVID-19: A Narrative Review. Diagnostics. 2022; 12(7):1543. https://doi.org/10.3390/diagnostics12071543
Chicago/Turabian StyleSuri, Jasjit S., Mahesh A. Maindarkar, Sudip Paul, Puneet Ahluwalia, Mrinalini Bhagawati, Luca Saba, Gavino Faa, Sanjay Saxena, Inder M. Singh, Paramjit S. Chadha, and et al. 2022. "Deep Learning Paradigm for Cardiovascular Disease/Stroke Risk Stratification in Parkinson’s Disease Affected by COVID-19: A Narrative Review" Diagnostics 12, no. 7: 1543. https://doi.org/10.3390/diagnostics12071543
APA StyleSuri, J. S., Maindarkar, M. A., Paul, S., Ahluwalia, P., Bhagawati, M., Saba, L., Faa, G., Saxena, S., Singh, I. M., Chadha, P. S., Turk, M., Johri, A., Khanna, N. N., Viskovic, K., Mavrogeni, S., Laird, J. R., Miner, M., Sobel, D. W., Balestrieri, A., ... Fouda, M. M. (2022). Deep Learning Paradigm for Cardiovascular Disease/Stroke Risk Stratification in Parkinson’s Disease Affected by COVID-19: A Narrative Review. Diagnostics, 12(7), 1543. https://doi.org/10.3390/diagnostics12071543

















