Abstract
Magnetic resonance imaging (MRI) is an effective technique for the diagnosis and preoperative staging of deep infiltrative endometriosis (DIE). The usefulness of MRI sequences susceptible to chronic blood degradation products, such as T2*-weighted imaging, remains uncertain. The present study aims to evaluate the diagnostic performance of these sequences in addition to the conventional protocol for DIE assessment. Forty-four MRI examinations performed for clinical and/or ultrasound DIE suspicion were evaluated by three readers with variable experience in female imaging. The inter-observer agreement between the reader who analysed only the conventional protocol and the one who also considered T2*-weighted sequences was excellent. The less experienced reader diagnosed a significantly higher number of endometriosis foci on the T2*-weighted sequences compared with the most experienced observer. T2*-weighted sequences do not seem to provide significant added value in the evaluation of DIE, especially in less experienced readers. Furthermore, artifacts caused by undesirable sources of magnetic inhomogeneity may lead to overdiagnosis.
1. Introduction
Endometriosis is a chronic gynaecological disorder characterized by the presence of ectopic functional endometrial tissue outside the uterine cavity, which responds to ovarian hormones with cyclic haemorrhages, similar to the normal endometrium [1,2]. This condition affects approximately 10% of women of reproductive age and up to 30–50% of women suffering from symptoms such as dysmenorrhea, dyspareunia, chronic pelvic pain, urinary tract symptoms, and infertility [2,3]. Endometriosis typically manifests in three ways: disease limited to ovaries (endometriomas), superficial peritoneal endometriosis, and deep infiltrative endometriosis (DIE), which is defined as subperitoneal invasion by endometriotic lesions that exceed 5 mm in depth [3,4]. The most common sites affected by DIE are the uterosacral ligaments, torus uterinus, posterior vaginal fornix, rectovaginal septum, rectum, and the urinary tract [4,5,6].
Laparoscopy has been conventionally considered the gold standard for diagnosing endometriosis. However, recent guidelines redefined this indication, underlying that imaging methods have achieved high diagnostic accuracy and recommending invasive diagnostic procedures only in patients with negative imaging findings and/or where empirical treatment was unsuccessful [7].
Transvaginal ultrasonography (TVUS) is usually the first-line imaging technique to assess pelvic endometriosis. Nevertheless, the accuracy of TVUS is limited by the restricted field of view and dependence on operator experience [8,9]. Magnetic resonance imaging (MRI) is recognized as the most accurate noninvasive tool for assessing deep endometriosis and defining pre-surgical planning [3,4,10]. T1-weighted (T1W) fat-suppressed MRI images allow DIE assessment when it manifests as lesions containing hyperintense haemorrhagic/proteinaceous foci. At the same time, non-haemorrhagic fibrotic implants are effectively detected on T2-weighted (T2W) images as hypointense irregular plaques [4,6]. However, MRI diagnosis can be challenging because T1W images are sensitive only to subacute blood degradation products, such as methemoglobin, while chronic blood products may not show typical T1W hyperintensity. Conversely, MRI T2*-weighted (T2*W) sequences are susceptible to chronic blood degradation products such as haemosiderin which are detected as signal void artefacts. For this reason, these sequences are widely used in neuroimaging studies to assess cerebrovascular diseases, haemorrhagic infiltrating lesions, neurodegenerative diseases, cerebral amyloid angiopathy, and vascular malformations. [11,12].
A number of publications have recently suggested that T2*W and susceptibility-weighted MRI can provide added value to detecting ectopic endometrium by identifying haemosiderin deposited during cyclic bleeding [13,14]. Nevertheless, these sequences are currently not included in conventional protocols suggested by international guidelines and are still considered under evaluation or optional [10,15].
The present study aims to assess the diagnostic performance of T2*W imaging in DIE detection among readers with different pelvic imaging expertise and evaluate if these sequences can provide valuable additional information to conventionally used MRI protocols.
2. Materials and Methods
The study was conducted in accordance with the Declaration of Helsinki. Clinical and radiological data were anonymized during data collection. The local ethics committee’s review of the protocol deemed that formal approval was not required owing to the observational, retrospective, and anonymous nature of this study.
2.1. Study Population
This study included consecutive women who underwent a pelvic MRI for clinical and/or ultrasound (US) suspicion of DIE between December 2020 and August 2021. The inclusion criteria were as follows: (a) women of reproductive age, (b) a previous TVUS examination suggestive of DIE, and/or (c) patients complaining of symptoms compatible with DIE such as dysmenorrhea, dyspareunia, and chronic pelvic pain, especially with a cyclical pattern. Major exclusion criteria were: (a) patients younger than 18 years old, (b) patients with absolute contraindications to MRI (i.e., pacemakers/defibrillator carriers), and (c) poor image quality on the T2*W and/or in the conventional protocol sequences. Data regarding patients’ clinical history (i.e., previous surgery, ongoing therapies, menstrual cycle phase) were collected through a screening questionnaire before the MRI examinations.
2.2. MRI Protocol
Pelvic MRI examinations were performed on a 3T scanner (Discovery MR750w GEM, GE Healthcare, Little Chalfont, UK), with the patient lying supine and using an anterior 16-channel pelvic-array coil (GE Healthcare, Waukesha, WI, USA). Patients were asked to fast for six hours and empty their bladder one hour before the MRI. An anti-peristaltic agent (Hyoscine butylbromide 20 mg/mL, Boehringer Ingelheim, Ingelheim am Rhein, Germany) was administered by intramuscular injection 15 min prior to the examination. Ultrasound gel (Aquasonic 100, Parker Laboratories, Fairfield, NJ, USA) was instilled into the rectum or the vagina (200 and 50 mL, respectively) in patients with prior US and/or clinical suspicion of involvement of these structures. No rectal preparation was required. MRI examinations were performed regardless of the patient’s menstrual cycle phase. The conventional MRI protocol included: (a) an axial single-shot fast spin-echo (SSFSE) 2D T2-weighted sequence, with a 5 mm slice thickness and a large field of view (FOV) extending from the renal hila to the pubic bone; (b) multiplanar fast relaxation fast spin-echo (FRFSE) 2D T2-weighted sequences (orientated in relation to the uterine long axis on axial, sagittal and coronal planes) with a 3 mm slice thickness and a small FOV dedicated to the area of interest; (c) 3D fast spoiled gradient recalled echo (FSPGR) T1-weighted sequences with and without fat saturation obtained using the Dixon technique on axial plane, with a 3 mm slice thickness and a FOV covering the whole pelvic area. The overall duration of the conventional protocol was 20 min. T2*W images were obtained with multi-echo recombined gradient echo (MERGE) technique in the axial plane, with a 4 mm slice thickness and a large FOV, covering the whole pelvis, with a total imaging time of 5 min. No dynamic post-contrast sequences were acquired, according to international guidelines [10,15]. The detailed MRI protocol used in this study is provided in Table 1.

Table 1.
MRI protocol used in the study.
2.3. Image Analysis
The MRI examinations were independently evaluated by three radiologists with varying years of experience in gynaecological imaging. Reader 1, a radiologist highly experienced in gynaecologic imaging (13 years), analysed the conventional MRI sequences with the additional contribution of T2*W sequences. Reader 2, with 3 years of experience in gynaecologic imaging, assessed the presence of endometriotic lesions only on conventional sequences and blinded to T2*W images. Reader 3, who had 1 year of general experience in MRI, evaluated both the conventional and T2*W sequences after a 3-week training period in endometriosis imaging. The three readers assessed the presence or absence of endometriosis using a checklist of pelvic structures (n = 22) typically involved in endometriosis (Table 2) and reported the MRI signal for each detected lesion.

Table 2.
Checklist of anatomical pelvic structures used by Readers to assess endometriosis sites.
Endometriotic lesions were defined using standard criteria published in the literature [4,15]: (a) foci of hyperintensity on T1W sequences and hypointensity on T2W sequences, representing haemorrhagic/proteinaceous active glandular components; (b) linear or spiculated retracting plaques with low signal intensity on all sequences, representing regions of fibrosis and lesions with smooth muscle hypertrophy. Furthermore, punctate or curvilinear signal voids on T2*WI (considered suggestive for endometriotic foci) were assessed by Readers 1 and 3. The results for Reader 1 were considered the gold standard, being the most experienced reader.
2.4. Statistical Analysis
The data were analysed with the open-source statistical software Jamovi v. 2.2.5 (The jamovi project (2021) [Computer Software]. Retrieved from https://www.jamovi.org (accessed on 21 March 2022), sourced from Bergamo, Italy). Qualitative variables are described as frequencies and percentages. Interobserver agreement rates between Reader 1 and Reader 2 on a DIE location-based level were measured through Cohen’s kappa coefficient. According to Fleiss’s equally arbitrary guidelines, a k-value of less than 0.40 was considered a poor agreement; a k-value ranging from 0.40 to 0.75 was a fair to good agreement, and greater than 0.75 was an excellent agreement. Cohen’s kappa scores were also calculated to measure interobserver agreement between Reader 1 and Reader 3 on locations considered positive for DIE on T2*W imaging. Fisher’s exact test was used to evaluate the prevalence of signal voids assessed by Readers 1 and 3 on these sequences. A p-value < 0.05 was considered significant.
3. Results
3.1. Study Population
Among the 45 consecutive women, 1 patient was excluded due to poor image quality of the T2*W sequence. Therefore, the final study population included 44 patients. Patients’ ages ranged from 20 to 49 years, and the mean age was 32.7 ± 7.8 years. Forty-one patients (93.2%) had a previous TVUS examination positive for DIE, while three patients (6.8%) only had a clinical history suggestive of endometriosis. A total of 5 patients had prior surgery: 3 Caesarean sections, 1 myomectomy, and 1 salpingectomy.
3.2. MRI Findings
The locations and signal characteristics of the endometriotic pelvic lesions reported by the most experienced radiologist (Reader 1) are summarized in Table 3. Diagnosis of endometriosis was confirmed in 42 (95.4%) out of the 44 included patients by Reader 1. The torus uterinus, the uterine-sacral ligaments, and the ovarian peritoneal surfaces were the sites most frequently involved with DIE. Most of the locations positive for endometriosis were observed on T2W (n = 279), followed by T1W (n = 57) and T2*W sequences (n = 43). The most common localizations of T2*W signal voids were ovaries (n = 19, 44.18%) and the torus uterinus (n = 6, 13.95%). Examples of signal voids consistent with DIE are shown in Figure 1.

Table 3.
Localizations and MRI signal characteristics of endometriotic lesions detected by the most experienced observer (Reader 1).
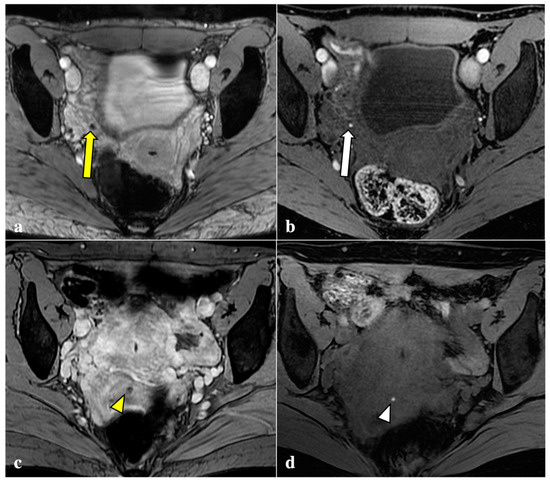
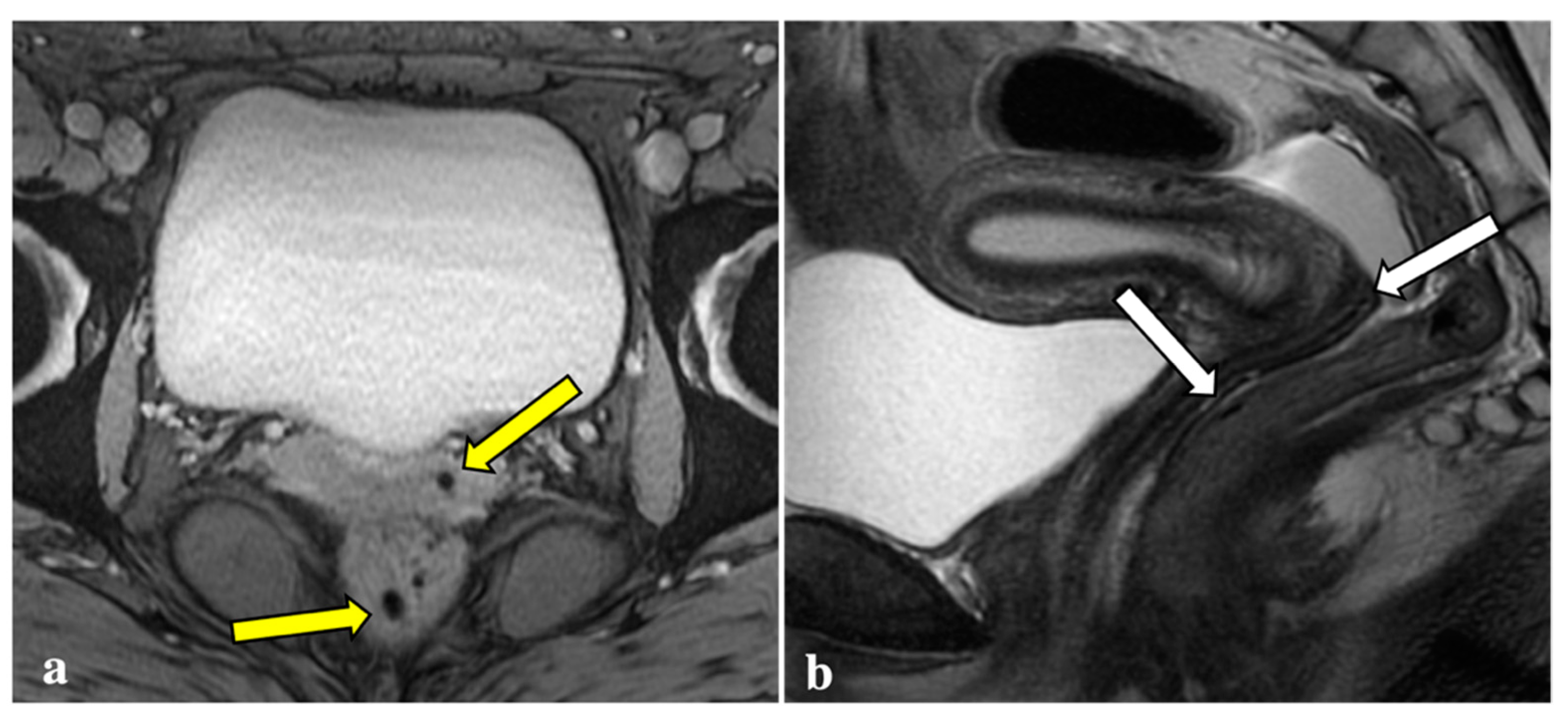
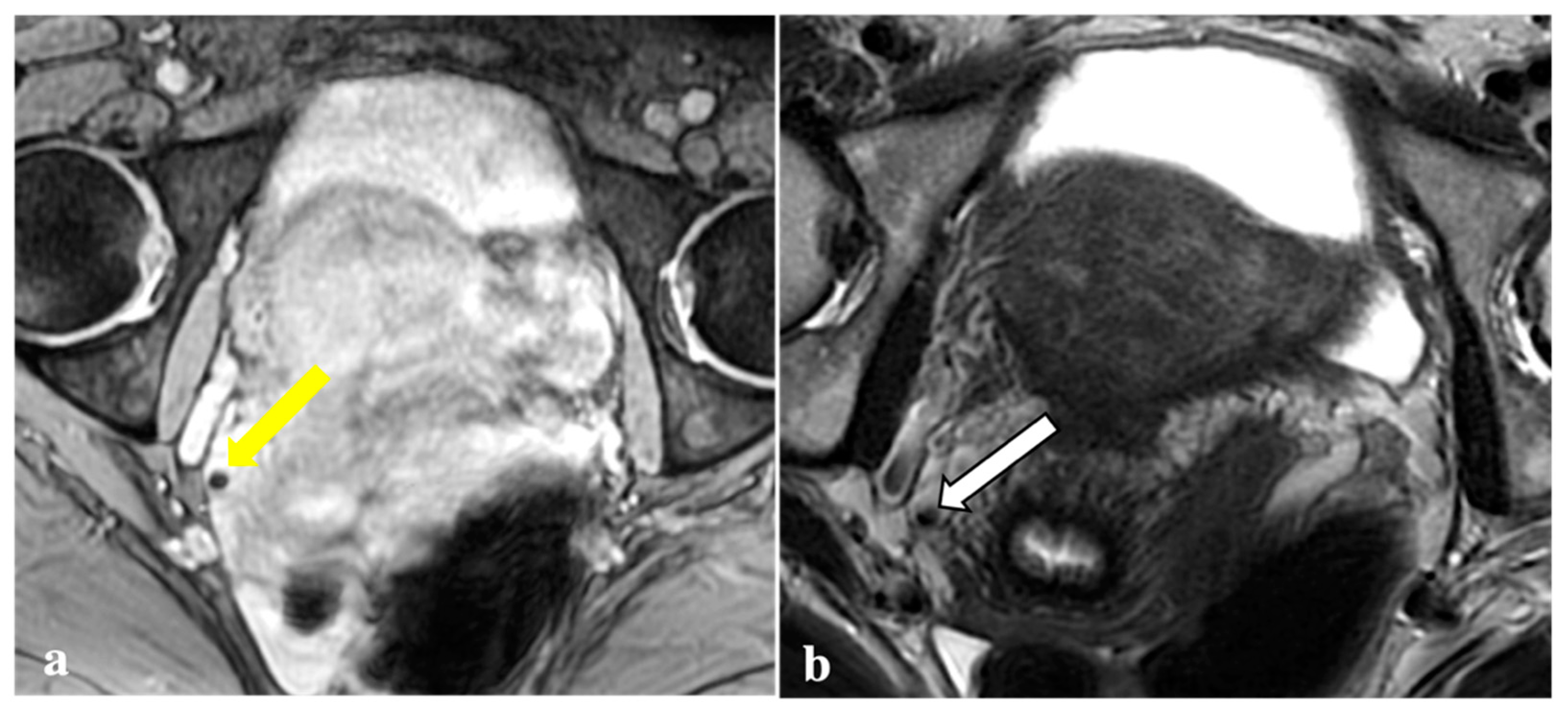
Figure 1.
Signal voids consistent with deep endometriosis foci. (a,b): 27-year-old patient with endometriosis involving the right fallopian tube. T2*W sequence shows a signal void (yellow arrow) seen as a corresponding tiny hyperintense endometriotic haemorrhagic focus on T1W fat sat image (white arrow); (c,d): 38-year-old patient with anterior vaginal fornix endometriosis visible as a T2*W dark lesion (yellow arrowhead) and as a bright spot on the T1W image (white arrowhead), with the same clinical significance.
3.3. Agreement between Readers with Different Experience
A comparison between the MRI findings detected by Reader 1, who analysed the conventional MRI protocol with the addition of T2*W sequences, and Reader 2, who analysed only the conventional protocol, was performed, as reported in Table 4. The overall agreement between the two readers was excellent (95.9%; kappa = 0.891). The inter-observer reliability was almost perfect when only considering the most frequently involved sites: the torus uterinus (97.7%; kappa = 0.933), bilateral utero-sacral ligaments (93.3%; kappa = 0.818), ovaries (97.7%; kappa = 0.953) and ovarian peritoneal surfaces (95.5%; kappa = 0.899).

Table 4.
Comparison between MRI findings detected on the conventional protocol with the addition of T2*W imaging (Reader 1) and only on the conventional protocol (Reader 2).
Table 5 summarizes the number and location of T2*W signal voids assessed by the most experienced reader (Reader 1) and the reader with less experience (Reader 3). The number of T2*W lesions observed by Reader 3 (n = 77) was significantly higher compared with Reader 1 (n = 43) (p-value < 0.001). The inter-observer agreement was poor (54.5%; kappa = 0.360). The sites most frequently misdiagnosed as DIE by Reader 3 were the vaginal fornix and rectum due to intraluminal air artefacts and post-operative scars due to susceptibility artefacts caused by surgical sutures (i.e., Caesarean scars).

Table 5.
Comparison between regions described as positive for signal voids on T2*-weighted sequences by Readers 1 and 3.
4. Discussion
Endometriosis is one of the most common gynaecological diseases, affecting one out of ten women of childbearing age. This condition leads to many nonspecific symptoms, such as chronic pelvic pain, menstrual abnormalities, dysuria, and infertility, that may overlap with other gynaecologic, urologic, and gastrointestinal disorders [1,2]. Consequently, many patients experience long delays between the onset of symptoms and the diagnosis of endometriosis, causing a significant worsening of quality of life, increasing physical and psychological morbidity, and use of health care resources [16,17]. Even if laparoscopy is the gold standard for diagnosis, MRI is considered the best noninvasive modality for the evaluation, staging, and preoperative assessment of pelvic endometriosis. Conventional MRI protocols include T1W sequences that show subacute blood products (i.e., methaemoglobin) as high signal intensity foci, allowing forthe detection of active haemorrhagic lesions. Conversely, T2*W sequences are sensitive to chronic blood by-products (i.e., haemosiderin) that are visualized as signal void artefacts due to their capacity to create localized inhomogeneity of the magnetic field. Endometriotic implants can result in haemosiderin-laden deposits caused by repeated bleeding [4,18].
For this reason, it has recently been proposed in the literature that T2*W imaging can provide added diagnostic value to conventional MRI protocols in endometriosis detection [13,14]. Some authors suggested that susceptibility-weighted imaging (SWI) can contribute to the diagnosis of endometriomas by revealing T2*W signal voids in the cyst wall [18,19]. Further studies confirmed these results, demonstrating that SWI can be effective in the differential diagnosis between endometriomas and haemorrhagic cysts [20]. Recent literature has also shown promising results for T2*W imaging in extra-ovarian endometriosis [13,21]. Pin et al. found that susceptibility-weighted sequences might improve the diagnostic performance of conventional MRI protocol in DIE assessment, with an increase in sensitivity (from 88.2% to 94.1%) and specificity (from 68.8% to 73.3%) [22]. In a recent publication, the addition of T2*W sequences to MRI protocols revealed even more favourable results: the sensitivity improved from 65.4% to 96.15%, while the specificity improved from 71.4% to 85.7% [14]. Nevertheless, in this study both readers were highly experienced radiologists, which may represent a limitation to the generalizability of the results. Moreover, no studies to date analysed how susceptibility artefacts may be a source of confusion and diagnostic overestimation for the interpreting radiologist.
Thus, T2*W sequences currently are not included in MRI conventional protocols suggested by international guidelines and their usefulness as an additional sequence for DIE detection needs to be further assessed [10,15].
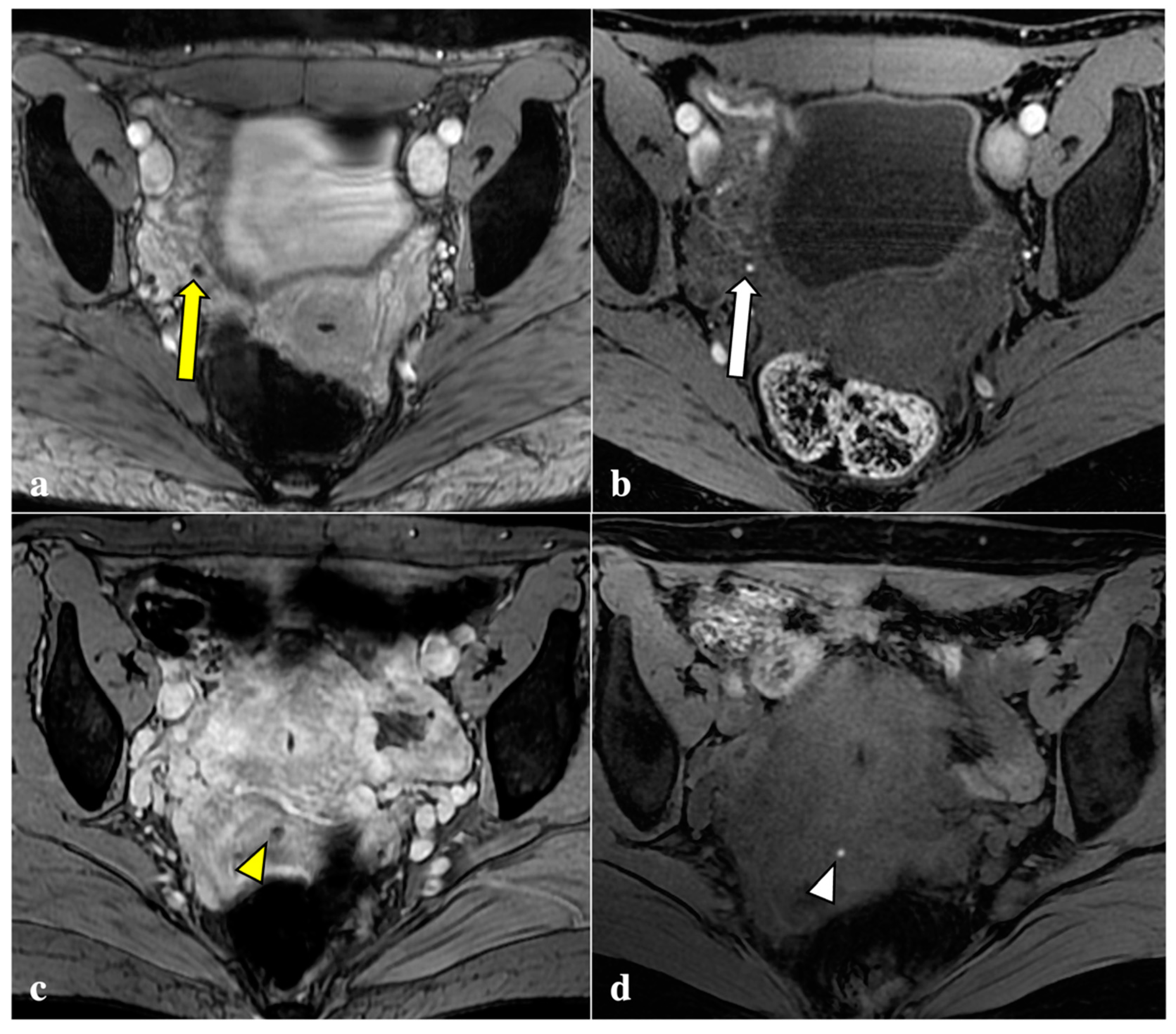
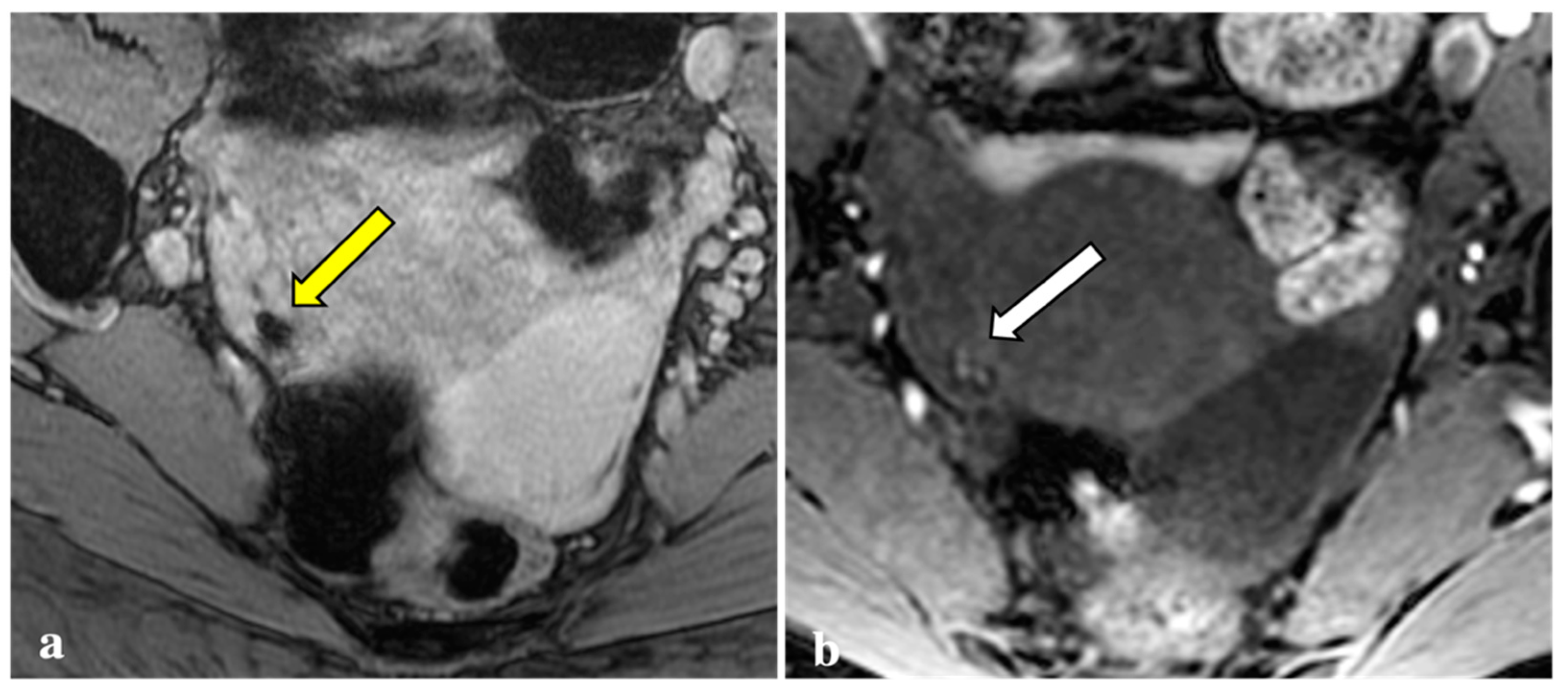
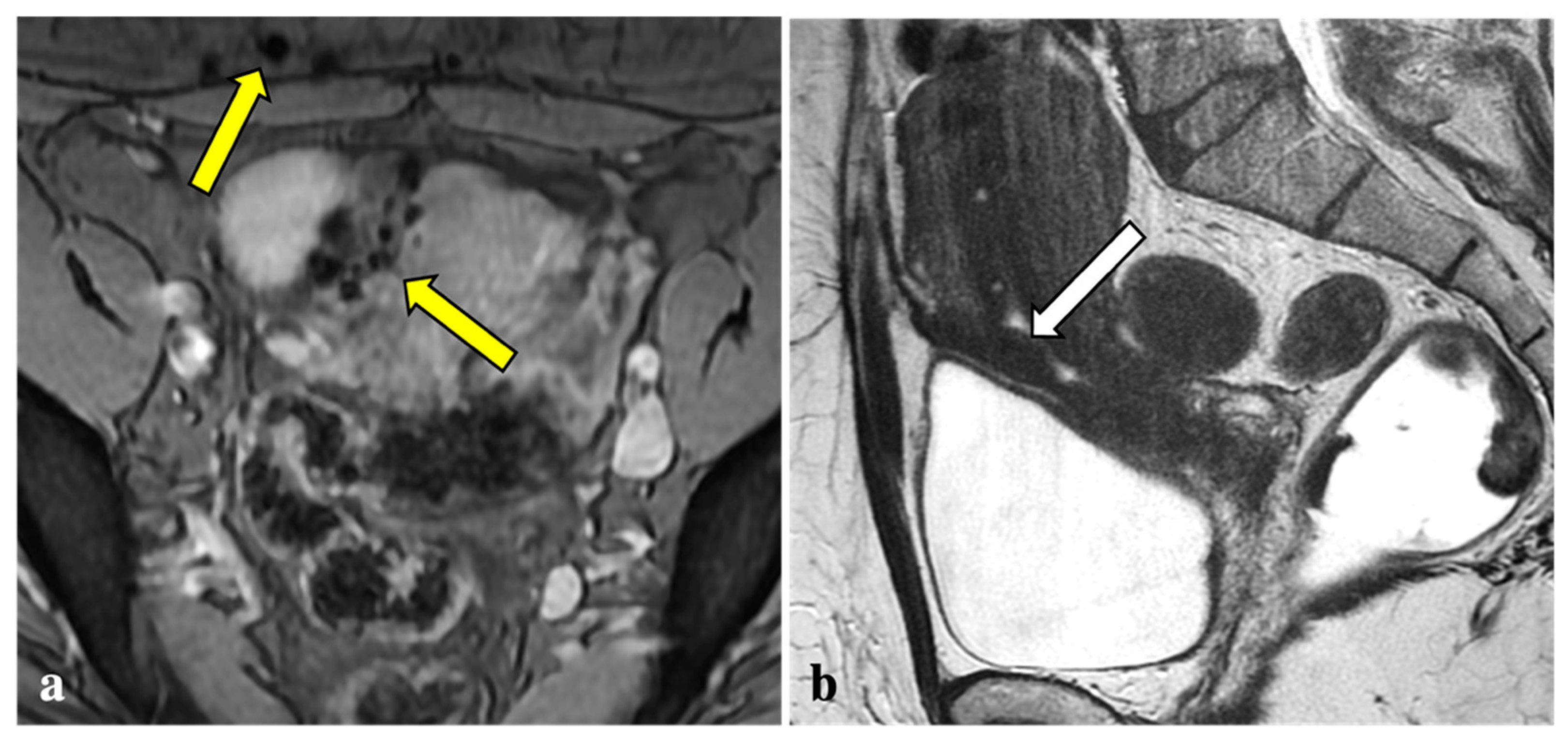
Contrary to reports in the literature, our study revealed that T2*W imaging did not increase the diagnostic accuracy significantly when compared with conventional MRI protocols in assessing DIE. Firstly, artefacts caused by intestinal gas, haemorrhagic foci not linked to endometriosis (i.e., haemorrhagic corpus luteum), phleboliths, and other undesirable sources of magnetic field inhomogeneity are often indistinguishable from signal voids caused by blood by-products (Figure 2, Figure 3, Figure 4 and Figure 5). These artefactual foci of signal loss may lead to overestimating the number and size of endometriotic implants. Moreover, the majority of haemorrhagic endometriotic lesions that demonstrate T2*W signal loss also show typical high signal on the T1W fat sat images and therefore are readily detectable on conventional MRI sequences. At the same time, predominantly fibrotic implants without haemorrhagic components are easily visible on classical T2W images and do not contain areas of T2*W signal void.
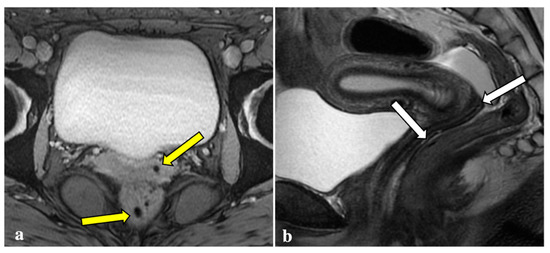
Figure 2.
T2*-weighted imaging pitfall. Signal voids (yellow arrows) on T2*-weighted imaging (a) of a 24-year-old patient were subsequently related to artefacts caused by air within the vaginal fornix and the rectal lumen (white arrows), as shown in a sagittal T2W image (b).
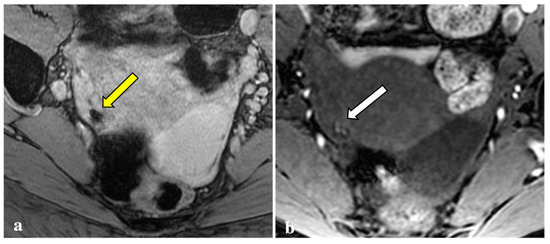
Figure 3.
T2*-weighted imaging pitfall. An oval signal void (yellow arrow) localized in the right ovary was detected on T2*-weighted imaging in a 23-year-old patient (a). This lesion corresponded to a thick-walled haemorrhagic corpus luteal cyst (white arrow) with an internal high signal on T1W (b), consistent with blood content.
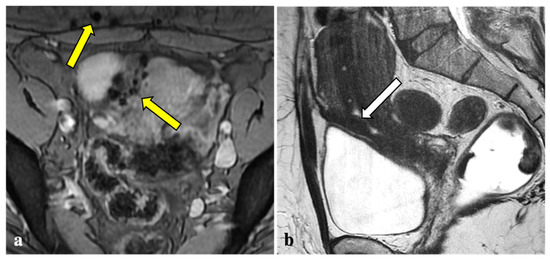
Figure 4.
T2*-weighted imaging pitfall. Multiple T2*W signal voids along the uterine surface and abdominal wall (yellow arrows) (a) of a 43-year-old patient caused by surgical artefacts and caesarean scar (white arrow), as shown in a sagittal T1W image (b).
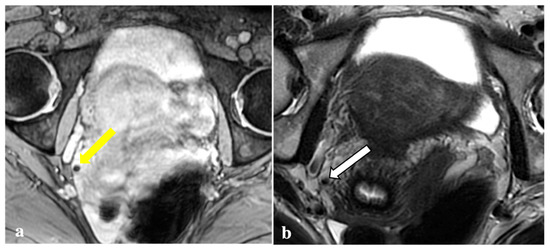
Figure 5.
T2*-weighted imaging pitfall. A punctate signal void on T2*W (yellow arrow) (a) observed in a 44-year-old patient showed low signal in T2W (white arrow) (b) and in the other sequences (not shown). It was reported as a phlebolith by the expert reader.
As previously reported, our data confirmed that the torus uterinus, the utero-sacral ligaments, and the peritoneal surface of the ovarian fossa are the pelvic sites most frequently involved with DIE [23]. Endometriosis implants involving these structures appeared with a signal void on T2*W sequences with a relatively low frequency (17.1%, 7.5%, and 19.2%, respectively) and were easily detected on conventional sequences. For these reasons, the addition of T2* W sequences to conventional endometriosis protocols—which prolongs the exam by approximately 5 min—does not seem to be justifiable.
The present study has several limitations. First, the sample size was relatively small, and studies with larger groups are needed to support our findings. Second, the number of readers was limited, and among them, only one was highly experienced in gynaecological imaging. Third, at the time of writing this manuscript, only a few patients had undergone laparoscopic surgery after the MRI examinations. Therefore, these findings are not discussed in the study, and a correlation with the standard of reference in further analysis will strengthen the imaging results.
5. Conclusions
Due to its capacity to detect chronic blood degradation products, T2*-weighted imaging has many relevant clinical applications, particularly in the neuroimaging field. Moreover, this MRI technique has recently been also applied to pelvic and gynaecologic imaging. Although T2*W sequences can effectively detect haemosiderin deposits in endometriotic foci, they do not appear to add a significant contribution to either the detection or the staging of endometriosis. Furthermore, artefacts caused by additional sources of magnetic signal voids on T2*W sequences may lead to diagnostic overestimation, especially for readers with less experience.
Author Contributions
Conceptualization, P.N.F. and S.A.; methodology, P.N.F., S.A. and P.A.B.; validation, P.N.F. and P.M.; formal analysis, P.N.F.; investigation, P.N.F., S.V. and C.C.; resources, P.N.F., S.A. and C.M.; data curation, P.N.F. and S.V.; writing—original draft preparation, P.N.F.; writing—review and editing, P.N.F., S.A., P.A.B., A.S. and C.R.; visualization, P.N.F. and M.M.O.G.; supervision, M.M.O.G., C.R. and S.S.; project administration, S.A., P.A.B. and S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. The local ethics committee’s review of the protocol deemed that formal approval was not required owing to the observational, retrospective, and anonymous nature of this study.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Giudice, L.C.; Kao, L.C. Endometriosis. Lancet 2004, 364, 1789–1799. [Google Scholar] [CrossRef]
- Burney, R.O.; Giudice, L.C. Pathogenesis and pathophysiology of endometriosis. Fertil. Steril. 2012, 98, 511–519. [Google Scholar] [CrossRef] [Green Version]
- Coutinho, A.; Bittencourt, L.K.; Pires, C.E.; Junqueira, F.; de Oliveira Lima, C.M.A.; Coutinho, E.; Domingues, M.A.; Domingues, R.C.; Marchiori, E. MR Imaging in Deep Pelvic Endometriosis: A Pictorial Essay. Radiographics 2011, 31, 549–567. [Google Scholar] [CrossRef]
- Jha, P.A.; Sakala, M.; Chamie, L.P.; Feldman, M.; Hindman, N.; Huang, C.; Kilcoyne, A.; Laifer-Narin, S.; Nicola, R.; Poder, L.; et al. Endometriosis MRI Lexicon: Consensus Statement from the Society of Abdominal Radiology Endometriosis Disease-Focused Panel. Abdom. Radiol. 2020, 45, 1552–1568. [Google Scholar] [CrossRef]
- Audebert, A.; Petousis, S.; Margioula-Siarkou, C.; Ravanos, K.; Prapas, N.; Prapas, Y. Anatomic Distribution of Endometriosis: A Reappraisal Based on Series of 1101 Patients. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 230, 36–40. [Google Scholar] [CrossRef]
- Foti, P.V.; Farina, R.; Palmucci, S.; Vizzini, I.A.A.; Libertini, N.; Coronella, M.; Spadola, S.; Caltabiano, R.; Iraci, M.; Basile, A.; et al. Endometriosis: Clinical Features, MR Imaging Findings and Pathologic Correlation. Insights Imaging 2018, 9, 149–172. [Google Scholar] [CrossRef] [Green Version]
- The Members of the Endometriosis Guideline Core Group; Becker, C.M.; Bokor, A.; Heikinheimo, O.; Horne, A.; Jansen, F.; Kiesel, L.; King, K.; Kvaskoff, M.; Nap, A.; et al. ESHRE Guideline: Endometriosis. Hum. Reprod. Open 2022, 2022, hoac009. [Google Scholar] [CrossRef]
- Tavcar, J.; Loring, M.; Movilla, P.R.; Clark, N.V. Diagnosing Endometriosis before Laparoscopy: Radiologic Tools to Evaluate the Disease. Curr. Opin. Obstet. Gynecol. 2020, 32, 292–297. [Google Scholar] [CrossRef]
- Bazot, M.; Daraï, E. Diagnosis of Deep Endometriosis: Clinical Examination, Ultrasonography, Magnetic Resonance Imaging, and Other Techniques. Fertil. Steril. 2017, 108, 886–894. [Google Scholar] [CrossRef] [Green Version]
- Bazot, M.; Bharwani, N.; Huchon, C.; Kinkel, K.; Cunha, T.M.; Guerra, A.; Manganaro, L.; Buñesch, L.; Kido, A.; Togashi, K.; et al. European Society of Urogenital Radiology (ESUR) Guidelines: MR Imaging of Pelvic Endometriosis. Eur. Radiol. 2017, 27, 2765–2775. [Google Scholar] [CrossRef] [Green Version]
- Kinoshita, T.; Okudera, T.; Tamura, H.; Ogawa, T.; Hatazawa, J. Assessment of Lacunar Hemorrhage Associated with Hypertensive Stroke by Echo-Planar Gradient-Echo T2*-Weighted MRI. Stroke 2000, 31, 1646–1650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gasparotti, R.; Pinelli, L.; Liserre, R. New MR Sequences in Daily Practice: Susceptibility Weighted Imaging. A Pictorial Essay. Insights Imaging 2011, 2, 335–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cimsit, C.; Yoldemir, T.; Guclu, M.; Akpinar, I.N. Susceptibility-Weighted Magnetic Resonance Imaging for the Evaluation of Deep Infiltrating Endometriosis: Preliminary Results. Acta Radiol. 2016, 57, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Raafat, M.; Talaat, S.H.; Abdelghaffar, S.M.; Ali, E.A. Can Diffusion and T2 Star-Weighted Magnetic Resonance Imaging Aid in the Diagnosis of Ectopic Endometrium? Egypt J. Radiol. Nucl. Med. 2021, 52, 137. [Google Scholar] [CrossRef]
- ESUR Quick Guide to Female Pelvis Imaging. Available online: https://www.esur.org/fileadmin/content/2019/ESUR_2019_-_ESUR_Quick_Guide_to_Female_Pelvis_Imaging.pdf (accessed on 13 May 2022).
- Nnoaham, K.E.; Hummelshoj, L.; Webster, P.; d’Hooghe, T.; de Cicco Nardone, F.; de Cicco Nardone, C.; Jenkinson, C.; Kennedy, S.H.; Zondervan, K.T. Impact of Endometriosis on Quality of Life and Work Productivity: A Multicenter Study across Ten Countries. Fertil. Steril. 2011, 96, 366–373.e8. [Google Scholar] [CrossRef] [Green Version]
- Surrey, E.; Soliman, A.M.; Trenz, H.; Blauer-Peterson, C.; Sluis, A. Impact of Endometriosis Diagnostic Delays on Healthcare Resource Utilization and Costs. Adv. Ther. 2020, 37, 1087–1099. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Wang, G.; Wang, X.; Gao, Y.; Liang, J.; Wang, J. Diagnostic Value of Susceptibility-Weighted Imaging for Endometrioma: Preliminary Results from a Retrospective Analysis. Acta Radiol. 2021, 63, 028418512110224. [Google Scholar] [CrossRef]
- Takeuchi, M.; Matsuzaki, K.; Nishitani, H. Susceptibility-Weighted MRI of Endometrioma: Preliminary Results. Am. J. Roentgenol. 2008, 191, 1366–1370. [Google Scholar] [CrossRef]
- Bulut, E.; Peker, M.; Kupeli, A.; Danisan, G.; Bulut, A.C. The Efficiency of Susceptibility-Weighted MRI in the Differentiation of Endometriomas from Haemorrhagic Ovarian Cysts. Abdom. Radiol. 2021, 46, 5337–5343. [Google Scholar] [CrossRef]
- Takeuchi, M.; Matsuzaki, K.; Harada, M. Susceptibility-Weighted MRI of Extra-Ovarian Endometriosis: Preliminary Results. Abdom. Imaging 2015, 40, 2512–2516. [Google Scholar] [CrossRef]
- Pin, L.; Monseau-Thiburce, A.-C.; Ziade-Coularis, C.; Benjamin, A.; Menut, F.; Brun, J.-L.; Merlot, B.; Chateil, J.-F. Exploratory Study of the Interest of MR Susceptibility-Weighted Imaging for the Preoperative Assessment of Pelvic Endometriosis Extent. Eur. J. Radiol. 2019, 118, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Bazot, M.; Lafont, C.; Rouzier, R.; Roseau, G.; Thomassin-Naggara, I.; Daraï, E. Diagnostic Accuracy of Physical Examination, Transvaginal Sonography, Rectal Endoscopic Sonography, and Magnetic Resonance Imaging to Diagnose Deep Infiltrating Endometriosis. Fertil. Steril. 2009, 92, 1825–1833. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).