Automated Detection of Dysplasia: Data Mining from Our Hematology Analyzers
Abstract
:1. Introduction
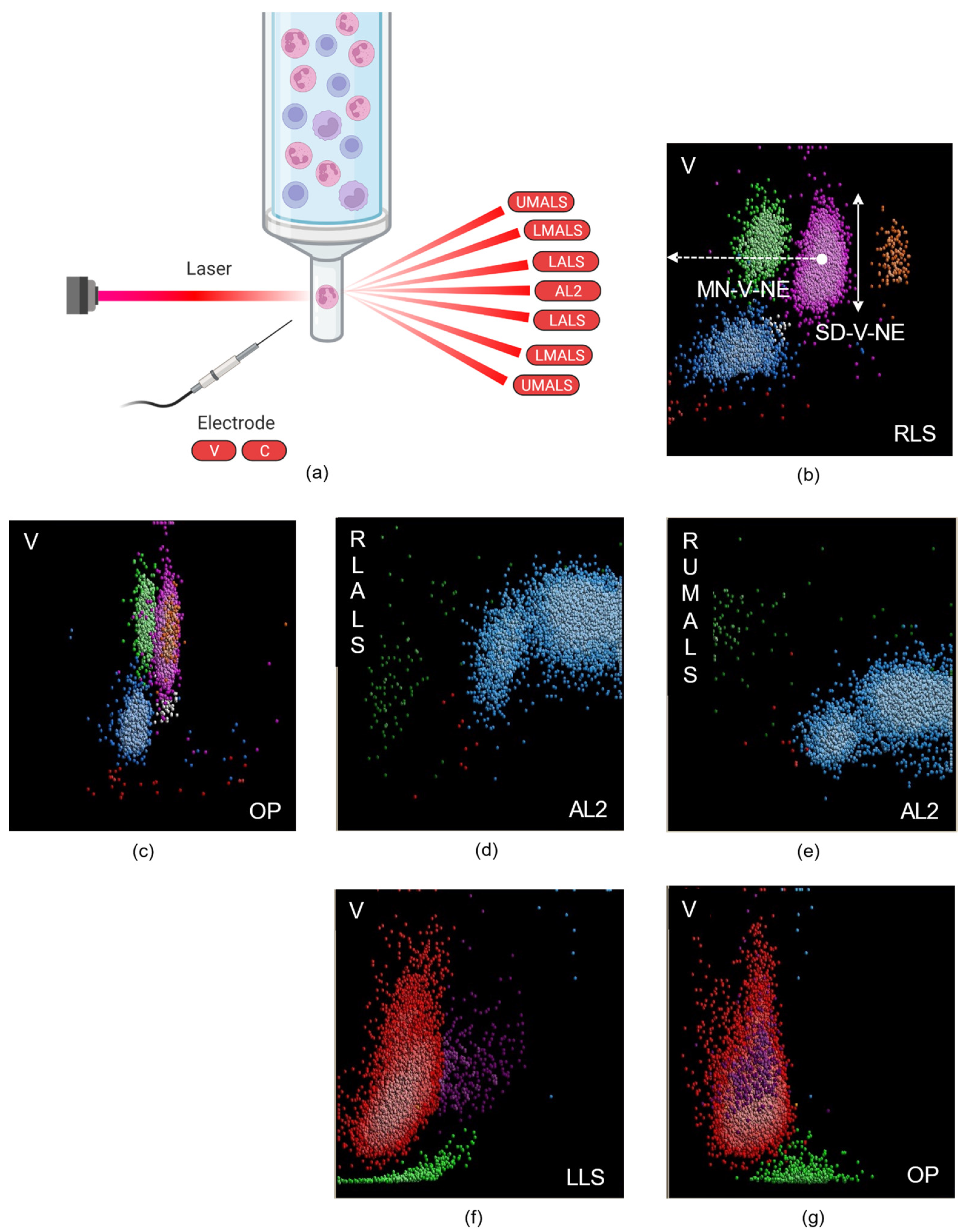
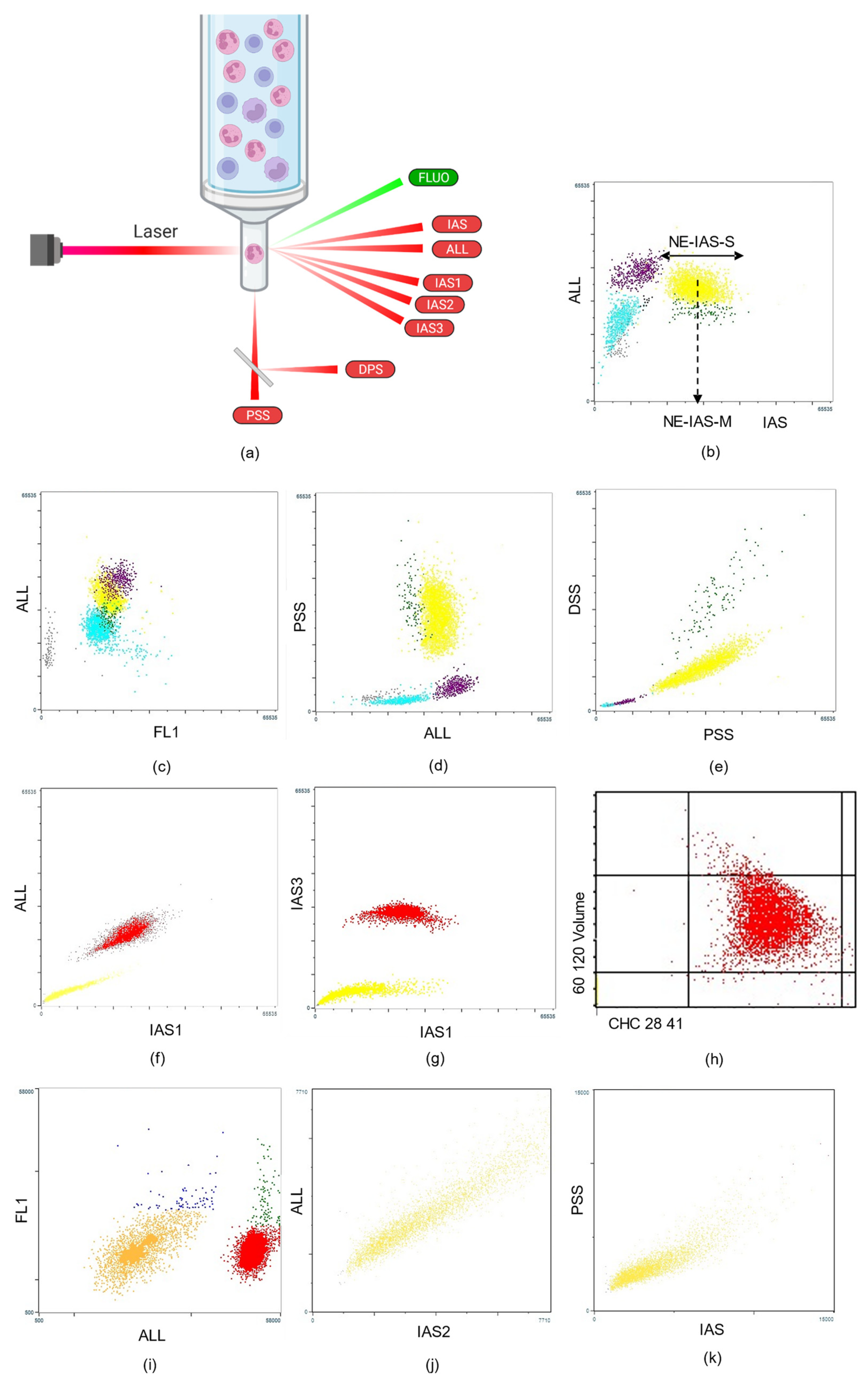
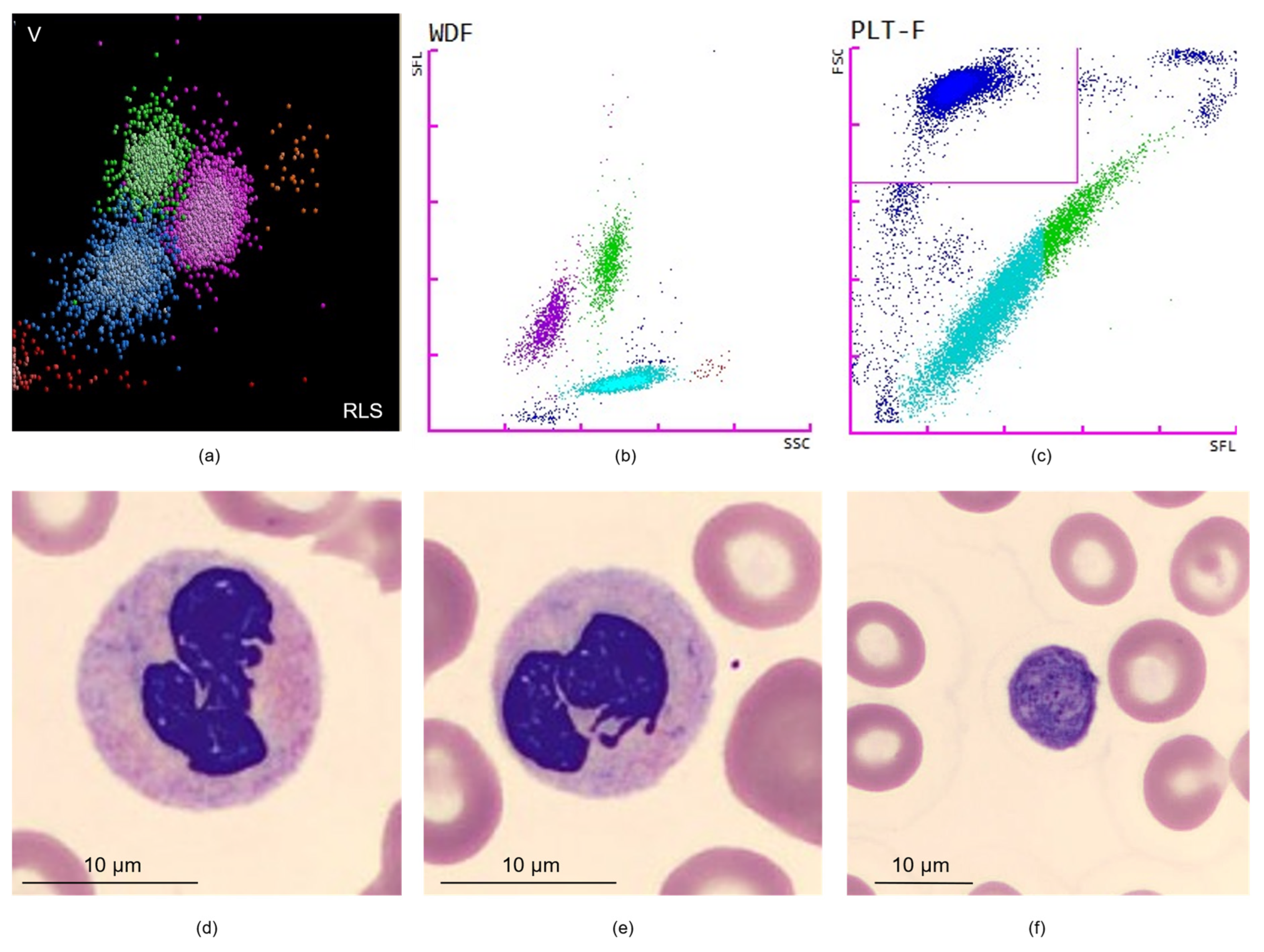
2. Cell Population Data
2.1. Leukocyte-Derived CPD
2.2. Red Blood Cell–Derived CPD
2.3. Platelet-Derived CPD
3. Discussion and Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Swerdlow, S.H.; Campo, E.; Harris, N.L.; Jaffe, E.S.; Pileri, S.A.; Stein, H.; Thiele, J.; Arber, D.A.; Hasserjian, R.P.; le Beau, M.M.; et al. Who Classification of Tumors of Haematopoeitic and Lymphoid Tissues. In Who Classification of Tumors of Haematopoietic and Lymphoid Tissues; Swerdlow, S.H., Campo, E., Harris, N.L., Jaffe, E.S., Pileri, S.A., Stein, H., Thiele, J., Arber, D.A., Hasserjian, R.P., le Beau, M.M., et al., Eds.; IARC: Lyon, France, 2017. [Google Scholar]
- Greenberg, P.; Cox, C.; LeBeau, M.M.; Fenaux, P.; Morel, P.; Sanz, G.; Sanz, M.; Vallespi, T.; Hamblin, T.; Oscier, D.; et al. International Scoring System for Evaluating Prognosis in Myelodysplastic Syndromes. Blood 1997, 89, 2079–2088. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, P.L.; Tuechler, H.; Schanz, J.; Sanz, G.; Garcia-Manero, G.; Sole, F.; Bennett, J.M.; Bowen, D.; Fenaux, P.; Dreyfus, F.; et al. Revised International Prognostic Scoring System for Myelodysplastic Syndromes. Blood 2012, 120, 2454–2465. [Google Scholar] [CrossRef] [PubMed]
- Hasserjian, R.P.; Orazi, A.; Brunning, R.D.; Germing, U.; le Beau, M.M.; Porwit, A.; Baumann, I.; Hellstrom-Lindberg, E.; List, A.F.; Cazzola, M.; et al. Myelodysplastic Syndromes: Overview. In Who Classification of Tumors of Haematopoietic and Lymphoid Tissues; Swerdlow, S.H., Campo, E., Harris, N.L., Jaffe, E.S., Pileri, S.A., Stein, H., Thiele, J., Arber, D.A., Hasserjian, R.P., le Beau, M.M., et al., Eds.; IARC: Lyon, France, 2017; pp. 98–106. [Google Scholar]
- Cogle, C.R.; Craig, B.M.; Rollison, D.E.; List, A.F. Incidence of the Myelodysplastic Syndromes Using a Novel Claims-Based Algorithm: High Number of Uncaptured Cases by Cancer Registries. Blood 2011, 117, 7121–7125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnes, P.W.; McFadden, S.L.; Machin, S.J.; Simson, E.; International Consensus Group for Hematology Review. The International Consensus Group for Hematology Review: Suggested Criteria for Action Following Automated Cbc and Wbc Differential Analysis. Lab. Hematol. 2005, 11, 83–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rappaport, E.S.; Helbert, B.; Ladd, D.J.; Trowbridge, A.A. Myelodysplastic Syndrome: Identification in the Routine Hematology Laboratory. South Med. J. 1987, 80, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Baccini, V.; Genevieve, F.; Jacqmin, H.; Chatelain, B.; Girard, S.; Wuilleme, S.; Vedrenne, A.; Guiheneuf, E.; Toussaint-Hacquard, M.; Everaere, F.; et al. Platelet Counting: Ugly Traps and Good Advice. Proposals from the French-Speaking Cellular Hematology Group (Gfhc). J. Clin. Med. 2020, 9, 808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Luise, D.; Giannotta, J.A.; Ammirabile, M.; de Zordi, V.; Torricelli, S.; Bottalico, S.; Chiaretto, M.L.; Fattizzo, B.; Migliorini, A.C.; Ceriotti, F. Cell Population Data Ne-Wx, Ne-Fsc, Ly-Y of Sysmex Xn-9000 Can Provide Additional Information to Differentiate Macrocytic Anaemia from Myelodysplastic Syndrome: A Preliminary Study. Int. J. Lab. Hematol. 2022, 44, e40–e43. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Park, C.J.; Lee, B.R.; Nam, K.S.; Kim, M.J.; Han, M.Y.; Kim, Y.J.; Cho, Y.U.; Jang, S. Sepsis Affects Most Routine and Cell Population Data (Cpd) Obtained Using the Sysmex Xn-2000 Blood Cell Analyzer: Neutrophil-Related Cpd Ne-Sfl and Ne-Wy Provide Useful Information for Detecting Sepsis. Int. J. Lab. Hematol. 2015, 37, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Boutault, R.; Peterlin, P.; Boubaya, M.; Sockel, K.; Chevallier, P.; Garnier, A.; Guillaume, T.; le Bourgeois, A.; Debord, C.; Godon, C.; et al. A Novel Complete Blood Count-Based Score to Screen for Myelodysplastic Syndrome in Cytopenic Patients. Br. J. Haematol. 2018, 183, 736–746. [Google Scholar] [CrossRef] [PubMed]
- Ravalet, N.; Foucault, A.; Picou, F.; Gombert, M.; Renoult, E.; Lejeune, J.; Vallet, N.; Lachot, S.; Rault, E.; Gyan, E.; et al. Automated Early Detection of Myelodysplastic Syndrome within the General Population Using the Research Parameters of Beckman-Coulter Dxh 800 Hematology Analyzer. Cancers 2021, 13, 389. [Google Scholar] [CrossRef] [PubMed]
- Schillinger, F.; Sourdeau, E.; Boubaya, M.; Baseggio, L.; Clauser, S.; Cornet, E.; Debord, C.; Defour, J.P.; Dubois, F.; Eveillard, M.; et al. A New Approach for Diagnosing Chronic Myelomonocytic Leukemia Using Structural Parameters of Sysmex Xn(Tm) Analyzers in Routine Laboratory Practice. Scand. J. Clin. Lab. Investig. 2018, 78, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Shestakova, A.; Nael, A.; Nora, V.; Rezk, S.; Zhao, X. Automated Leukocyte Parameters Are Useful in the Assessment of Myelodysplastic Syndromes. Cytom. B Clin. Cytom. 2021, 100, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, J.J. Reticulated Platelets: Analytical Aspects and Clinical Utility. Clin. Chem. Lab. Med. 2014, 52, 1107–1117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van De Wyngaert, Z.; Fournier, E.; Bera, E.; Carrette, M.; Soenen, V.; Gauthier, J.; Preudhomme, C.; Boyer, T. Immature Platelet Fraction (Ipf): A Reliable Tool to Predict Peripheral Thrombocytopenia. Curr. Res. Transl. Med. 2020, 68, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Park, Y.; Kim, H.; Kim, J.; Kwon, G.C.; Koo, S.H. Discriminating Myelodysplastic Syndrome and Other Myeloid Malignancies from Non-Clonal Disorders by Multiparametric Analysis of Automated Cell Data. Clin. Chim. Acta 2018, 480, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.M.; Nam, Y. Complete Blood Count and Cell Population Data Parameters from the Abbott Alinity Hq Analyzer Are Useful in Differentiating Myelodysplastic Syndromes from Other Forms of Cytopenia. Int. J. Lab. Hematol. 2021, 44, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Larruzea Ibarra, A.; Marin, L.M.; Duran, G.P.; Puig, M.T. Evaluation of Immature Platelet Fraction in Patients with Myelodysplastic Syndromes. Association with Poor Prognosis Factors. Clin. Chem. Lab. Med. 2019, 57, e128–e130. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Chen, Y.; Zhang, Y.; Zhang, L.; Chen, K.; He, Z.; Wang, C.; Yu, L. Prognostic Impact of Platelet-Large Cell Ratio in Myelodysplastic Syndromes. Front. Oncol. 2022, 12, 846044. [Google Scholar] [CrossRef] [PubMed]
- Buoro, S.; Carobene, A.; Seghezzi, M.; Manenti, B.; Pacioni, A.; Ceriotti, F.; Ottomano, C.; Lippi, G. Short- and Medium-Term Biological Variation Estimates of Leukocytes Extended to Differential Count and Morphology-Structural Parameters (Cell Population Data) in Blood Samples Obtained from Healthy People. Clin. Chim. Acta 2017, 473, 147–156. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Clauser, S.; Freynet, N.; Bardet, V. Automated Detection of Dysplasia: Data Mining from Our Hematology Analyzers. Diagnostics 2022, 12, 1556. https://doi.org/10.3390/diagnostics12071556
Zhu J, Clauser S, Freynet N, Bardet V. Automated Detection of Dysplasia: Data Mining from Our Hematology Analyzers. Diagnostics. 2022; 12(7):1556. https://doi.org/10.3390/diagnostics12071556
Chicago/Turabian StyleZhu, Jaja, Sylvain Clauser, Nicolas Freynet, and Valérie Bardet. 2022. "Automated Detection of Dysplasia: Data Mining from Our Hematology Analyzers" Diagnostics 12, no. 7: 1556. https://doi.org/10.3390/diagnostics12071556





