With the increasing usage of MRI in the clinical setting, there is a greater need for MRI-guided intervention as well [
1,
2]. The lesions detected on MRI with concerning features may need histological correlation [
3]. If no definite correlate is identified on mammogram or ultrasound, then a biopsy needs to be performed under MRI guidance for such MRI-only lesions [
2].
Several studies describe the feasibility and outcome of MRI-guided breast interventions, but most of them are from American or European countries. There is limited data available on MRI-guided breast biopsies in Asian countries. In this part of the world, there is limited use of MRI-guided intervention [
4] due to its extremely high cost and limited availability [
5] of this procedure and expertise.
An MRI-guided breast biopsy is advantageous as it allows biopsy of non-palpable breast lesions that are not detected on conventional imaging, including mammograms and ultrasound [
6,
7]. However, the procedure has its own unique set of challenges and limitations. Appropriate knowledge and understanding of these issues can enhance the overall success rate of the procedure [
8] and boost the confidence of the radiologist performing it. In this article, we discuss various challenges that one may come across while performing MRI-guided biopsies and share some practical strategies to manage these challenges from our experience. Hopefully, this can help readers to pre-empt, and better equip themselves to solve difficulties they may face during the procedure. This could in turn improve the success rate of MRI-guided breast biopsies.
Of note, one must remember the different appearances of the breast [
9] in its compressed versus non-compressed state and make use of adjacent landmarks to localize the correct target, especially if the lesion is small, or in the case of high background enhancement. It is advisable to check for adequate inclusion of the target within the compressed breast as much as possible before injecting the contrast and adjust the position, if needed, before the contrast injection.
Moreover, it is important to remind readers that though the medially located lesions may be approached from the medial side, it may be technically challenging. The longer lateral route may be opted for if the medial approach is too difficult.
The challenges discussed in-depth in this paper may be grossly divided into patient factors, lesion factors, technical factors, and others. Let us look into these challenges separately, along with case examples and suggested strategies.
1.1. Patient Factors
(a) Anxious patient: Anxiety could come with the patient’s anticipation of pain or discomfort during core biopsy [
10]. Claustrophobia, excessive movement, or other anxiety reactions in the MRI machine can affect the quality of images produced [
11] and hence accurate targeting. Answering the patient’s queries and providing detailed explanations of the procedure, available alternatives, and possible complications can reduce the patient’s anxiety to some degree as the patient knows what to expect during the procedure [
12]. Reassurance, comfort, and a good rapport can improve the patient’s cooperation during the procedure, which is pivotal for success. When needed, anxiolytics [
13] or even sedation can be administered. Sedation can increase patient comfort, reduce motion artifacts, and decrease failure rates of the procedure due to premature termination [
14]. At our practice, nearly 10% of patients opt for intravenous sedation under the care of an anesthetist. MRI-compatible monitors and a few other accessories may be needed for patient care. Minimizing patient anxiety not only improves patient satisfaction and comfort, but also the clinic’s efficiency and diagnostic capabilities [
15].
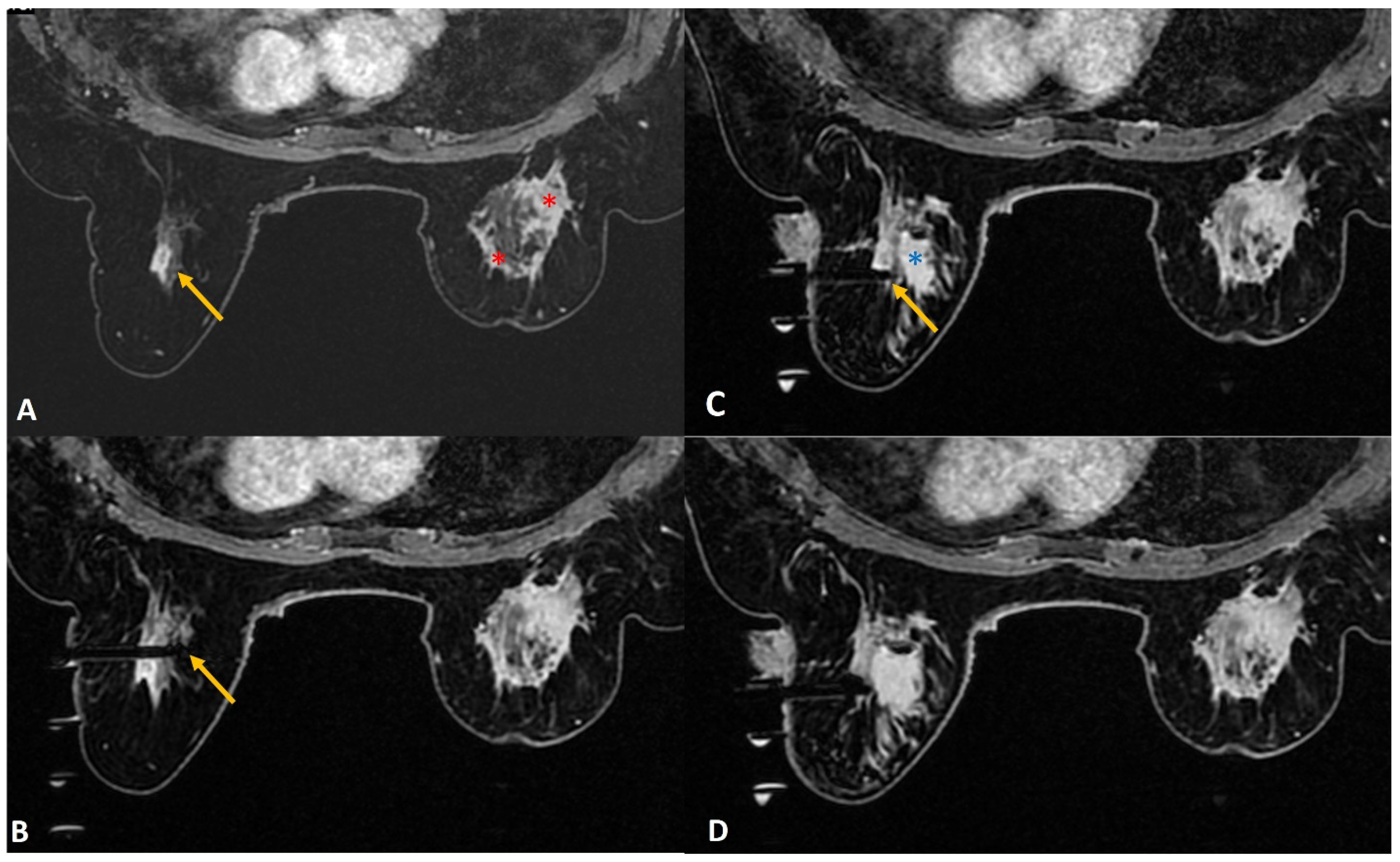
(b) Lactating mothers (
Figure 1): Although MRI carries no radiation risk, contrast-enhanced MRI is generally not recommended as the initial choice of investigation in lactating women. Only 0.04% of the intravenous dose of gadolinium-based contrast medium is being excreted in breast milk, of which less than one percent of the contrast medium excreted is being absorbed by the infant’s gastrointestinal tract [
16]. As such, a decision to proceed with the MRI-guided breast biopsy may be taken if the benefit seems to be significantly higher. However, if the woman is concerned, the ACR suggests using a breast pump to express adequate breast milk before contrast-based procedures. After the procedure, she can be advised to stop breastfeeding and to express and discard the breast milk up to 24 h post-procedure [
16].
In a lactating woman, the background parenchymal enhancement may significantly obscure small lesions. Also, intervention may be associated with a higher risk of infection and the risk of developing a milk fistula. A milk fistula is a rare potential complication of core or excision biopsy in a lactating woman [
17]. Despite these risks, one may proceed with an MRI-guided biopsy when indicated with the utmost care to avoid infection, as a prompt diagnosis is important when suspecting breast cancer in a lactating mother [
18]. The authors suggest optimum sampling in these women to reduce the risk of milk fistula. Tight compression after the procedure to close the tract can also play an important part.
Figure 1.
A 36-year lactating mother diagnosed with left breast inflammatory breast cancer underwent staging MRI. (A). Sagittal post contrast T1w fat-saturated image showed a segmental non-mass enhancement (yellow arrows) in the upper outer quadrant of the right breast. This was also distinctly FDG avid on PET scan (not shown here). (B). Axial staging scan showed diffusely enhancing mass in the left breast correlating with known cancer (red asterisks). Segmental non-mass enhancement was also seen in upper outer quadrant of the right breast (yellow arrow). (C). MRI-guided biopsy shows the tip of the obturator in correct location with surrounding post-biopsy hematoma (yellow arrow). Histology was reported as fibrocystic change.
Figure 1.
A 36-year lactating mother diagnosed with left breast inflammatory breast cancer underwent staging MRI. (A). Sagittal post contrast T1w fat-saturated image showed a segmental non-mass enhancement (yellow arrows) in the upper outer quadrant of the right breast. This was also distinctly FDG avid on PET scan (not shown here). (B). Axial staging scan showed diffusely enhancing mass in the left breast correlating with known cancer (red asterisks). Segmental non-mass enhancement was also seen in upper outer quadrant of the right breast (yellow arrow). (C). MRI-guided biopsy shows the tip of the obturator in correct location with surrounding post-biopsy hematoma (yellow arrow). Histology was reported as fibrocystic change.
(c) Hematoma formation (
Figure 2): MRI-guided biopsy, being a vacuum-assisted biopsy procedure needing a large bore needle size (generally 8-10G), may cause some bleeding and hematoma formation at the site of biopsy. An inquiry should be made in every patient for any known bleeding disorders or history of anticoagulant therapy. In routine cases, the hematoma can be managed with adequate post-procedure local compression. Additional tight breast wrap at the biopsy site can be provided to the patient for continued compression for the next 24–48 h. Aspirating a large hematoma with the use of a vacuum before deploying the marker clip may be helpful in some cases. In the case of an arterial injury leading to significant bleeding, injection of thrombin in the biopsy cavity may help to control active bleeding.
Although some papers suggest that no significant complications were experienced in patients on anticoagulation therapy post-MRI-guided biopsies [
19,
20], there may still be a risk of hematoma formation due to the large sample size and, hence, whenever possible, the anti-coagulant therapy should be withheld during the procedure time. On the other hand, it is important to understand that, for any reason, if anticoagulant therapy cannot be stopped in a patient needing an MRI-guided biopsy, one can proceed with the biopsy with added precautionary measures. This is more so in a patient with an INR within the therapeutic range within two weeks of the procedure [
21]. Therefore, checking the patient’s International Normalized Ratio (INR) prior to the biopsy day may be helpful. Depending on the INR, discussions with clinicians can be made to consider continuing, withholding, or bridging blood thinners if the patient cannot be off anticoagulant therapy [
22]. When possible, stopping anticoagulant medicine for at least 5–7 days before the biopsy reduces the bleeding risk. Overall, these contraindications should be evaluated with the radiologist before the scheduled MRI biopsy day, and one must follow institutional guidelines.
(d) Small Breasts (
Figure 3): This is a common issue in this part of the world, with a cup size of A or AA encountered in nearly a third of patients. Small breasts pose a challenge when the mid to posterior part of the breast does not fall into the grid. Adequate care taken while positioning the patient can contribute significantly to procedural success. Removing the chest pad from the coil allows more posterior breast tissue to fall into the grid. In addition, tilting the patient into an oblique position allows lesions in the posterior breast to fall into a more accessible location for biopsy. Using a Petit needle or half-aperture size needle with extra cores can be used in a thin breast with borderline thickness to avoid injury to the skin or chest wall.
1.2. Lesion Factors
Characteristics of the lesion such as its size, location, and mobility can make the procedure challenging.
(a) Location of Lesion:
(ai) Posterior lesions (
Figure 4): Lesions in the posterior breast that are close to the chest wall or abutting the pectoralis major can be difficult to access. This is even more so in smaller breasts as the target lesion may lie outside of the grid. In addition to the approaches to smaller breasts discussed above, we suggest a few more strategies that readers may find useful. Firstly, the breast lesion may sometimes fall out of the grid due to its extremely posterior location close to the chest wall. Advancing the needle into the breast posterior to the grid, along with adjusting the depth of the needle, can resolve this issue. One must remember that due to the absence of support by the grid in this location, the trocar may be unstable and extra care should be taken to obtain accurate sampling. Secondly, a freehand insertion of the needle can be performed with the breast lying freely in the coil without using the grid and compression. The needle can then be advanced and angled freely. Some compression can be applied to stabilize the breast during the process [
22]. The pillar and post method allows wider accessibility of breast lesions.
(aii) Periareolar lesion (
Figure 5): As the areola is more vascular in nature, a biopsy of the lesions in the peri/retroareolar region poses a significant bleeding risk. Piercing the areola is avoided as much as possible for reasons of cosmesis, reduction of pain, and reduction of bleeding. Rolling the breast such that the nipple-areola complex is away from the point of skin entry and the use of additional padding at the anterior aspect of the breast can increase the thickness of the anterior breast and thus aid access to the periareolar lesion for biopsy.
(aiii) Superficial lesions (
Figure 6): A superficially located breast lesion may pose a challenge when using the regular needle size due to the increased risk of cutting the skin during the biopsy. This may lead to air leaks and loss of vacuum. Firstly, raising a good-sized skin wheal using a local anesthetic agent may allow more depth for the needle to be inserted so as to avoid unwanted trauma to the skin [
22]. Additionally, using a smaller aperture needle may be considered. Another option could be to adjust the depth by a few millimeters beyond the target, allowing the lesion to fall at the periphery of the aperture.
(b) Small-sized lesions obscured by grid (
Figure 7): Occasionally, a small lesion to be biopsied may fall on a “cross” of the grid during the biopsy. In this case, the grid obscures the ideal entry site and offsets the needle. Hence, using two diagonally placed entry sites from the adjacent holes, along with directed sampling, may further enhance the chance of success. Targeted sampling with extra cores directed towards the lesion may help in this situation.
(c) Lesion Movement (
Figure 8): Lesion movement may be due to patient movement, inadequate breast compression, or skin indentation. Firstly, the patient must be comfortable and well-informed of the procedure to avoid startling her. If needed, readjust the patient’s position. Adequate breast compression is needed to immobilize the breast well, and a good skin incision may avoid excessive skin indentation. Occasionally, inserting the needle beyond the target and then withdrawing it to the correct depth may help to get a perfect location of the needle aperture at the targeted site.
Figure 7.
(A). High-risk screening in a BRCA 2 woman showed a new 4-mm enhancing focus in the left breast (yellow arrow). (B). Sagittal image on the biopsy day showed the target focus enhancing well (red circle). (C). During targeting, the entry point was noted to fall on the “cross” of the grid (red circle), which blocked the ideal entry site. (D). In this case, we performed dual-needle targeting though diagonally adjacent holes. Two obturators in these holes (white arrows) are seen adjacent to the target (red circle). Selected directed sampling through both these needles towards the target would increase the chance of getting the tiny lesion biopsied adequately. (E). Post biopsy hematoma cavity (yellow arrow) was seen at the target site with non-visualization of the target suggesting adequate sampling. The histology was proven as 3-mm invasive tubular carcinoma.
Figure 7.
(A). High-risk screening in a BRCA 2 woman showed a new 4-mm enhancing focus in the left breast (yellow arrow). (B). Sagittal image on the biopsy day showed the target focus enhancing well (red circle). (C). During targeting, the entry point was noted to fall on the “cross” of the grid (red circle), which blocked the ideal entry site. (D). In this case, we performed dual-needle targeting though diagonally adjacent holes. Two obturators in these holes (white arrows) are seen adjacent to the target (red circle). Selected directed sampling through both these needles towards the target would increase the chance of getting the tiny lesion biopsied adequately. (E). Post biopsy hematoma cavity (yellow arrow) was seen at the target site with non-visualization of the target suggesting adequate sampling. The histology was proven as 3-mm invasive tubular carcinoma.
Figure 8.
(A). Screening MRI in a high-risk patient showed a new indeterminate focal non-mass enhancement (yellow arrow) in left breast. (B). During the biopsy, the enhancing lesion (yellow arrow) was seen compressed and pushed along posterior aspect. (C). The lesion (yellow arrow) was displaced medially by the obturator of the biopsy guide. (D). The needle was pushed beyond the target and then withdrawn to correct depth (yellow arrow). This strategy helped to place the needle well in relation to the target lesion. (E). Post-biopsy hematoma (yellow arrow) is noted. (F). Follow up MRI after a year confirmed appropriate location of the marker clip with lack of enhancement in this region (yellow arrow). The histology of this lesion was reported as sclerosing adenosis.
Figure 8.
(A). Screening MRI in a high-risk patient showed a new indeterminate focal non-mass enhancement (yellow arrow) in left breast. (B). During the biopsy, the enhancing lesion (yellow arrow) was seen compressed and pushed along posterior aspect. (C). The lesion (yellow arrow) was displaced medially by the obturator of the biopsy guide. (D). The needle was pushed beyond the target and then withdrawn to correct depth (yellow arrow). This strategy helped to place the needle well in relation to the target lesion. (E). Post-biopsy hematoma (yellow arrow) is noted. (F). Follow up MRI after a year confirmed appropriate location of the marker clip with lack of enhancement in this region (yellow arrow). The histology of this lesion was reported as sclerosing adenosis.
1.3. Technical Factors
(a) Non-Enhancement of Lesion: To minimize the incidence of lesion non-enhancement, radiologists can check if contrast was indeed injected. If injected, one must rule out extravasation of contrast into the soft tissues at the injection site. If adequate enhancement of the heart is confirmed, then measures such as taking delayed sequences and reducing the breast compression, allowing greater flow in the breast, may be attempted. If the lesion shows minimal or faint enhancement, then making use of landmarks such as vessels, biopsy clips, or fat planes may be considered to ensure correct targeting. If all these considerations fail to enhance the lesion, it is recommended to withhold the biopsy and request a six-month follow-up MRI [
23]. A cancellation rate of nearly 8–13% is reported in the literature for MRI-guided breast biopsies [
24,
25]. Though it is likely that the majority of these lesions may represent fluctuating physiologic background enhancement, non-enhancement on the day of biopsy does not guarantee benignity. It has been shown that there is approximately a 3.5% chance of cancer in these patients on follow-up [
26]. Hence, as per the ACR recommendations, we must ensure that the patient returns for close follow-up in 3–6 months. If the lesion reappears on follow-up MRI, or if the lesion is seen larger or with suspicious features, then the biopsy should be rearranged.
(b) Improper targeting: The risk of improper targeting is higher with manual methods compared to using commercially available software. Incorrect localization of the target may be due to the selection of the wrong laterality (left versus right localization chart) while calculating the coordinates, or from the improper translation of calculated x and y axes coordinates from the “image” view to the “patient” view. An improper z-axis (depth of target) can result from entering the wrong needle size on computer-aided detection (CAD) software (
Figure 9).
Careful use of correct laterality and approach, as well as accurate translation of calculated target from the “image” view to the “patient” view, are of utmost importance, while using the manual targeting method. Note that the patient views differ for the left and right breasts, as demonstrated in
Figure 10.
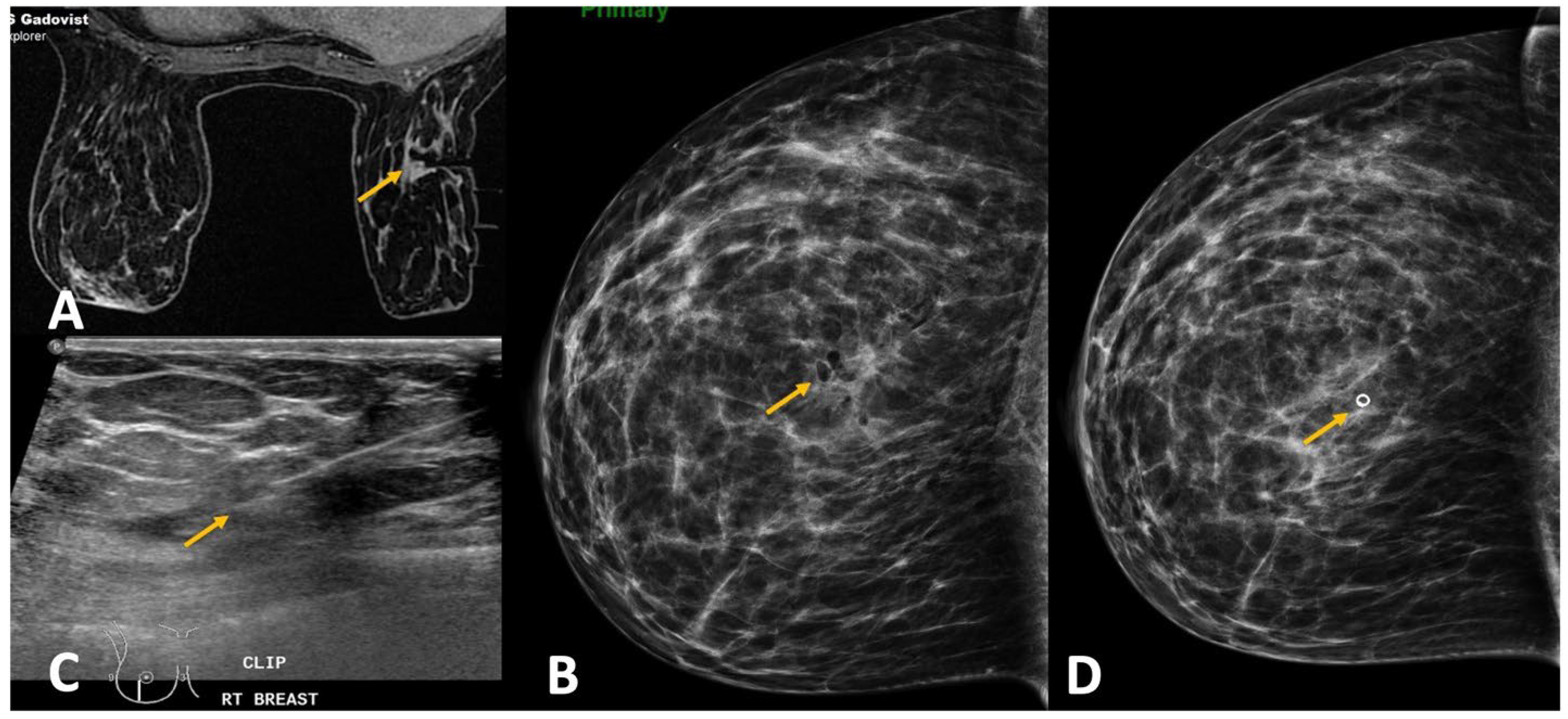
(c) Non-Deployment of Clip (
Figure 11): A rather uncommon challenge is the non-deployment of the clip. This may be due to technical errors, such as using an improper device or the wrong technique. Occasionally, the clip may remain adherent to the tip of the sheath. Upon withdrawing the sheath at the end of the procedure, the adherent clip may then be pulled out together with the sheath. Aspiration of a large hematoma post-biopsy may also be associated with extrusion of the clip. Other times, a large hematoma formed may lead to the displacement of the clip by preventing adherence of the clip to tissue or by mass effect [
27]. To reduce the risk of a large hematoma formation, which can result in clip migration, a few techniques can be used. Firstly, lavaging the biopsy cavity adequately after the sampling before deploying the clip. Secondly, aspirating a large hematoma through the sheath prior to deployment of the clip. Thirdly, the use of a vasoconstriction agent along with lidocaine during biopsy may reduce the bleeding risk. A check mammogram is generally performed after the biopsy procedure to document adequate clip deployment, in addition to post-biopsy hematoma. If it fails to show the clip, this confirms the failure of the deployment. An alternative modality using ultrasound-guided deployment of a new clip may be extremely useful to annotate the site of biopsy [
28].