Ultra-High-Resolution Photon-Counting Detector CT Arthrography of the Ankle: A Feasibility Study
Abstract
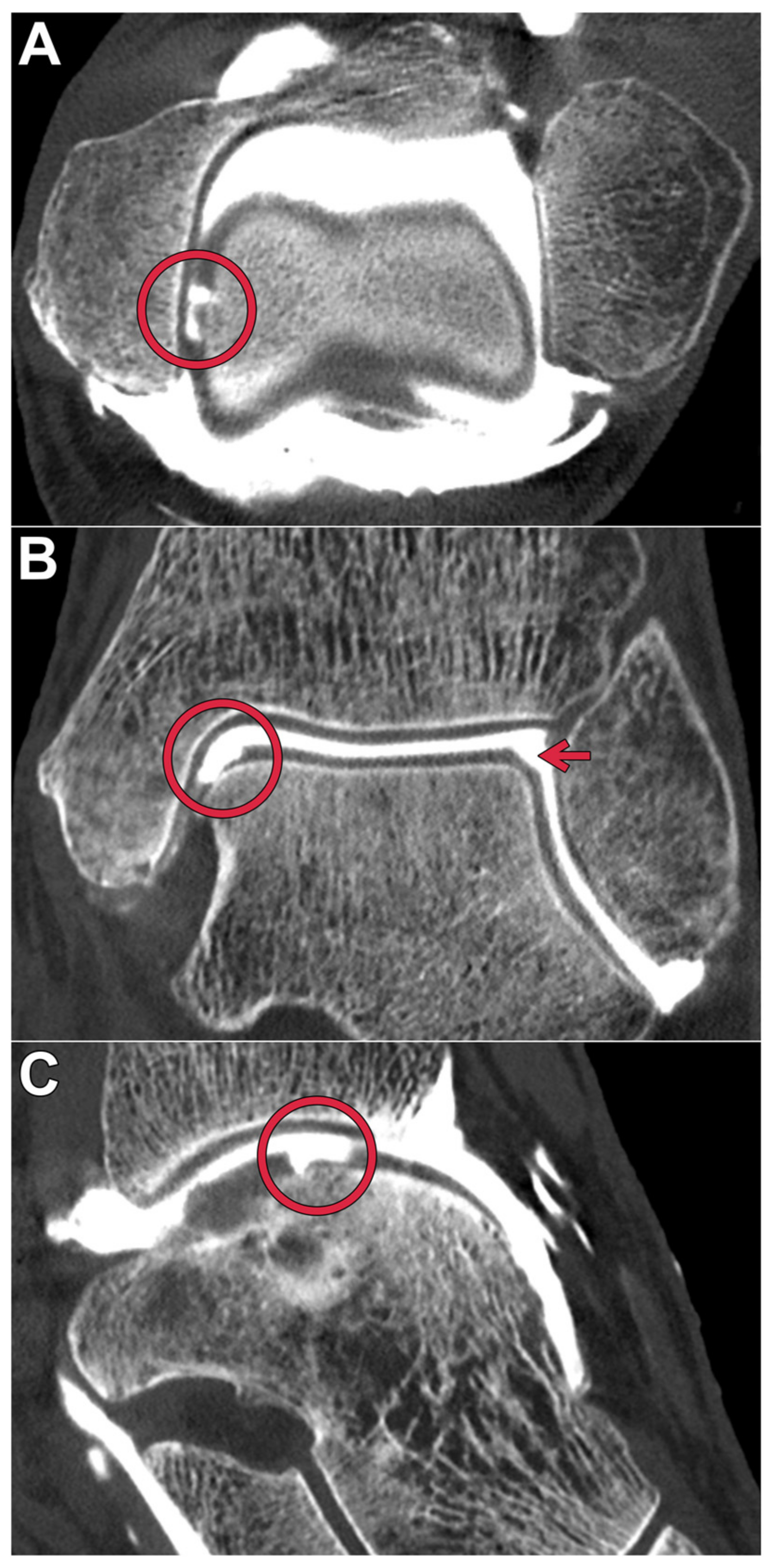
:1. Introduction
2. Materials and Methods
2.1. Cadaveric Specimens
2.2. Arthrography Procedure
2.3. Image Acquisition and Reconstruction Parameters
2.4. Subjective Image Evaluation
2.5. Objective Image Evaluation
2.6. Statistical Analysis
3. Results
3.1. Subjective Image Quality Assessment
3.2. Objective Image Quality Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CNR | contrast-to-noise ratio |
| CTDIvol | volume computed tomography dose index |
| PCD | photon-counting detector |
| UHR | ultra-high-resolution |
References
- Rodgers, M.M. Dynamic foot biomechanics. J. Orthop. Sports Phys. Ther. 1995, 21, 306–316. [Google Scholar] [CrossRef] [Green Version]
- Egloff, C.; Hügle, T.; Valderrabano, V. Biomechanics and pathomechanisms of osteoarthritis. Swiss. Med. Wkly. 2012, 142, w13583. [Google Scholar] [CrossRef]
- Huch, K.; Kuettner, K.E.; Dieppe, P. Osteoarthritis in ankle and knee joints. Semin. Arthritis Rheum. 1997, 26, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Johnson, V.L.; Giuffre, B.M.; Hunter, D.J. Osteoarthritis: What Does Imaging Tell Us about Its Etiology? Semin. Musculoskelet Radiol. 2012, 16, 410–418. [Google Scholar] [CrossRef] [Green Version]
- Van Buecken, K.; Barrack, R.L.; Alexander, A.H.; Ertl, J.P. Arthroscopic treatment of transchondral talar dome frac-tures. Am. J. Sports Med. 1989, 17, 350–355, discussion 355–356. [Google Scholar] [CrossRef]
- Grambart, S.T. Arthroscopic Management of Osteochondral Lesions of the Talus. Clin. Podiatr. Med. Surg. 2016, 33, 521–530. [Google Scholar] [CrossRef]
- O’Loughlin, P.F.; Heyworth, B.E.; Kennedy, J.G. Current concepts in the diagnosis and treatment of osteochondral lesions of the ankle. Am. J. Sports Med. 2010, 38, 392–404. [Google Scholar] [CrossRef] [PubMed]
- Verhagen, R.A.W.; Maas, M.; Dijkgraaf, M.G.W.; Tol, J.L.; Krips, R.; van Dijk, C.N. Prospective study on diagnostic strat-egies in osteochondral lesions of the talus. Is MRI superior to helical CT? J. Bone Jt. Surg. Br. 2005, 87, 41–46. [Google Scholar] [CrossRef] [Green Version]
- Naran, K.N.; Zoga, A.C. Osteochondral lesions about the ankle. Radiol. Clin. N. Am. 2008, 46, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Schmid, M.R.; Pfirrmann, C.; Hodler, J.; Vienne, P.; Zanetti, M. Cartilage lesions in the ankle joint: Comparison of MR arthrography and CT arthrography. Skelet. Radiol. 2003, 32, 259–265. [Google Scholar] [CrossRef]
- Ba-Ssalamah, A.; Schibany, N.; Puig, S.; Herneth, A.M.; Noebauer-Huhmann, I.M.; Trattnig, S. Imaging articular carti-lage defects in the ankle joint with 3D fat-suppressed echo planar imaging: Comparison with conventional 3D fat-suppressed gradient echo imaging. J. Magn. Reson. Imaging 2002, 16, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Barr, C.; Bauer, J.S.; Malfair, D.; Ma, B.; Henning, T.D.; Steinbach, L.; Link, T.M. MR imaging of the ankle at 3 Tesla and 1.5 Tesla: Protocol optimization and application to cartilage, ligament and tendon pathology in cadaver specimens. Eur. Radiol. 2006, 17, 1518–1528. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, D.E.T.; Seedhom, B.B. Thickness of human articular cartilage in joints of the lower limb. Ann. Rheum. Dis. 1999, 58, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Do Cha, S.; Kim, H.S.; Chung, S.T.; Yoo, J.H.; Park, J.H.; Kim, J.H.; Hyung, J.W. Intra-articular lesions in chronic lateral ankle instability: Comparison of ar-throscopy with magnetic resonance imaging findings. Clin. Orthop. Surg. 2012, 4, 293–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waldt, S.; Bruegel, M.; Ganter, K.; Kuhn, V.; Link, T.M.; Rummeny, E.J.; Woertler, K. Comparison of multislice CT arthrography and MR arthrography for the detection of articular cartilage lesions of the elbow. Eur. Radiol. 2005, 15, 784–791. [Google Scholar] [CrossRef]
- Pöhler, G.H.; Sonnow, L.; Ettinger, S.; Rahn, A.; Klimes, F.; Becher, C.; von Falck, C.; Wacker, F.K.; Plaass, C. High resolution flat-panel CT arthrography vs. MR arthrography of artificially created osteochondral defects in ex vivo upper ankle joints. PLoS ONE 2021, 16, e0255616. [Google Scholar] [CrossRef]
- Bette, S.J.; Braun, F.M.; Haerting, M.; Decker, J.A.; Luitjens, J.H.; Scheurig-Muenkler, C.; Kroencke, T.J.; Schwarz, F. Visualization of bone details in a novel photon-counting dual-source CT scanner—Comparison with energy-integrating CT. Eur. Radiol. 2021, 32, 2930–2936. [Google Scholar] [CrossRef]
- Pourmorteza, A.; Symons, R.; Henning, A.; Ulzheimer, S.; Bluemke, D.A. Dose Efficiency of Quarter-Millimeter Pho-ton-Counting Computed Tomography: First-in-Human Results. Investig. Radiol. 2018, 53, 365–372. [Google Scholar] [CrossRef]
- Tortora, M.; Gemini, L.; D’Iglio, I.; Ugga, L.; Spadarella, G.; Cuocolo, R. Spectral Photon-Counting Computed Tomog-raphy: A Review on Technical Principles and Clinical Applications. J. Imaging Sci. Technol. 2022, 8, 4. [Google Scholar]
- McCollough, C.H.; Boedeker, K.; Cody, D.; Duan, X.; Flohr, T.; Halliburton, S.S.; Hsieh, J.; Layman, R.R.; Pelc, N.J. Principles and applications of multienergy CT: Report of AAPM Task Group 291. Med. Phys. 2020, 47, 14157. [Google Scholar] [CrossRef] [Green Version]
- Leng, S.; Bruesewitz, M.; Tao, S.; Rajendran, K.; Halaweish, A.F.; Campeau, N.G.; Fletcher, J.G.; McCollough, C.H. Photon-counting Detector CT: System Design and Clinical Applications of an Emerging Technology. RadioGraphics 2019, 39, 729–743. [Google Scholar] [CrossRef] [PubMed]
- Flohr, T.; Petersilka, M.; Henning, A.; Ulzheimer, S.; Ferda, J.; Schmidt, B. Photon-counting CT review. Phys. Med. 2020, 79, 126–136. [Google Scholar] [CrossRef]
- Klein, L.; Dorn, S.; Amato, C.; Heinze, S.; Uhrig, M.; Schlemmer, H.-P.; Kachelrieß, M.; Sawall, S. Effects of Detector Sampling on Noise Reduction in Clinical Photon-Counting Whole-Body Computed Tomography. Investig. Radiol. 2020, 55, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Baffour, F.I.; Rajendran, K.; Glazebrook, K.N.; Thorne, J.E.; Larson, N.B.; Leng, S.; McCollough, C.H.; Fletcher, J.G. Ultra-high-resolution imaging of the shoulder and pelvis using photon-counting-detector CT: A feasibility study in patients. Eur. Radiol. 2022, 32, 7079–7086. [Google Scholar] [CrossRef] [PubMed]
- Grunz, J.-P.; Huflage, H.; Heidenreich, J.F.; Ergün, S.; Petersilka, M.; Allmendinger, T.; Bley, T.A.; Petritsch, B. Image Quality Assessment for Clinical Cadmium Telluride-Based Photon-Counting Computed Tomography Detector in Cadaveric Wrist Imaging. Investig. Radiol. 2021, 56, 785–790. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Re-search. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [Green Version]
- Benson, J.; Rajendran, K.; Lane, J.; Diehn, F.; Weber, N.; Thorne, J.; Larson, N.; Fletcher, J.; McCollough, C.; Leng, S. A New Frontier in Temporal Bone Imaging: Photon-Counting Detector CT Demonstrates Superior Visualization of Critical Anatomic Structures at Reduced Radiation Dose. Am. J. Neuroradiol. 2022, 43, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Baffour, F.I.; Glazebrook, K.N.; Ferrero, A.; Leng, S.; McCollough, C.H.; Fletcher, J.G.; Rajendran, K. Photon-Counting Detector CT for Musculoskeletal Imaging: A Clinical Perspective. Am. J. Roentgenol. 2023, 220, 551–560. [Google Scholar] [CrossRef]
- Theumann, N.; Favarger, N.; Schnyder, P.; Meuli, R. Wrist ligament injuries: Value of post-arthrography computed tomography. Skelet. Radiol. 2001, 30, 88–93. [Google Scholar] [CrossRef]
- Schmid, M.R.; Schertler, T.; Pfirrmann, C.; Saupe, N.; Manestar, M.; Wildermuth, S.; Weishaupt, D. Interosseous ligament tears of the wrist: Comparison of multi–detector row CT arthrography and MR imaging. Radiology 2005, 237, 1008–1013. [Google Scholar] [CrossRef]
- Moser, T.; Dosch, J.-C.; Moussaoui, A.; Dietemann, J.-L. Wrist ligament tears: Evaluation of MRI and combined MDCT and MR arthrography. Am. J. Roentgenol. 2007, 188, 1278–1286. [Google Scholar] [CrossRef] [PubMed]
- Alam, F.; Schweitzer, M.E.; Li, X.X.; Malat, J.; Hussain, S.M. Frequency and spectrum of abnormalities in the bone marrow of the wrist: MR imaging findings. Skeletal. Radiol. 1999, 28, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Saupe, N.; Pfirrmann, C.W.A.; Schmid, M.R.; Schertler, T.; Manestar, M.; Weishaupt, D. MR imaging of cartilage in cadaveric wrists: Comparison between imaging at 1.5 and 3.0 T and gross pathologic inspection. Radiology 2007, 243, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, K.; Murthy, N.S.; Frick, M.A.; Tao, S.; Unger, M.D.; LaVallee, K.T.; Larson, N.B.; Leng, S.; Maus, T.P.; McCollough, C.H. Quantitative Knee Arthrography in a Large Animal Model of Osteo-arthritis Using Photon-Counting Detector CT. Investig. Radiol. 2020, 55, 349–356. [Google Scholar] [CrossRef]
- Kokkonen, H.T.; Aula, A.S.; Kröger, H.; Suomalainen, J.S.; Lammentausta, E.; Mervaala, E.; Jurvelin, J.S.; Töyräs, J. Delayed Computed Tomography Arthrography of Human Knee Carti-lage In Vivo. Cartilage 2012, 3, 334–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luetkens, K.S.; Grunz, J.P.; Paul, M.M.; Huflage, H.; Conrads, N.; Patzer, T.S.; Gruschwitz, P.; Ergün, S.; Bley, T.A.; Kunz, A.S. One-stop-shop CT arthrography of the wrist without subject reposi-tioning by means of gantry-free cone-beam CT. Sci. Rep. 2022, 12, 14422. [Google Scholar] [CrossRef]


| Scan Protocol | Full-Dose Protocol | Low-Dose Protocol |
|---|---|---|
| Tube voltage [kVp] | 120 | 120 |
| Tube current-time product [eff. mAs] | 125 | 38 |
| Detector collimation [mm] | 120 × 0.2 | 120 × 0.2 |
| Pitch factor | 0.5 | 0.5 |
| Rotation time [sec] | 1.0 | 1.0 |
| CTDIvol [mGy] | 10 | 3 |
| Spatial Frequency | At the 50% Value of the MTF (ρ50) [Line Pairs/cm] | At the 10% Value of the MTF (ρ10) [Line Pairs/cm] | At the Maximum of the MTF (ρmax) [Line Pairs/cm] |
|---|---|---|---|
| Br98 | 39.0 | 42.9 | 20.4 |
| Br84 | 22.6 | 27.9 | 10.5 |
| Br76 | 16.5 | 21.0 | 7.8 |
| Scan Protocol | Full-Dose Protocol | Low-Dose Protocol | ICC | ||||
|---|---|---|---|---|---|---|---|
| Convolution Kernel | Br98 | Br84 | Br76 | Br98 | Br84 | Br76 | |
| Bone | 7 (6–7) | 6 (5–7) | 5 (5–6) | 4 (3–5) | 5 (4–6) | 5 (4–6) | 0.938 |
| Cartilage | 6 (4–6.5) | 6 (5–7) | 7 (6–7) | 4 (3–5) | 5 (4–6) | 5 (5–6) | 0.887 |
| Ligaments | 6 (4.5–7) | 6 (5–7) | 6 (5–7) | 4 (3–5) | 5 (4–6) | 5 (4–7) | 0.805 |
| Percentage of diagnostic examinations | 100% | 100% | 100% | 100% | 100% | 100% | |
| Bone/Cartilage/Ligaments | Full-Dose | Low-Dose | |||||
|---|---|---|---|---|---|---|---|
| Br98 | Br84 | Br76 | Br98 | Br84 | Br76 | ||
| Br98 | +/–/= | +/–/= | +/+/+ | +/=/= | +/=/= | ||
| Full-dose | Br84 | –/+/= | +/=/= | +/+/+ | +/+/+ | +/+/+ | |
| Br76 | –/+/= | –/=/= | +/+/+ | +/+/+ | +/+/+ | ||
| Br98 | –/–/– | –/–/– | –/–/– | –/–/– | –/–/– | ||
| Low-dose | Br84 | –/=/= | –/–/– | –/–/– | +/+/+ | =/=/= | |
| Br76 | –/=/= | –/–/– | –/–/– | +/+/+ | =/=/= | ||
| Scan Protocol | Full-Dose Protocol | Low-Dose Protocol | ||||
|---|---|---|---|---|---|---|
| Convolution Kernel | Br98 | Br84 | Br76 | Br98 | Br84 | Br76 |
| NoiseFat [HU] | 149.5 ± 23.4 | 54.6 ± 10.6 | 38.9 ± 9.3 | 240.6 ± 56.2 | 78.4 ± 10.3 | 52.2 ± 7.5 |
| CNRBone | 3.8 ± 0.9 | 9.8 ± 3.0 | 13.5 ± 4.3 | 3.0 ± 3.8 | 6.8 ± 1.6 | 10.2 ± 2.6 |
| CNRCartilage | 3.0 ± 0.9 | 7.4 ± 3.5 | 10.2 ± 4.5 | 2.9 ± 4.7 | 5.5 ± 2.2 | 7.8 ± 2.3 |
| CNRLigaments | 1.4 ± 3.3 | 3.2 ± 2.4 | 4.3 ± 3.4 | 1.2 ± 1.0 | 2.2 ± 1.6 | 2.9 ± 2.2 |
| CNRBone/CNRCartilage/CNRLigaments | Full-Dose | Low-Dose | |||||
|---|---|---|---|---|---|---|---|
| Br98 | Br84 | Br76 | Br98 | Br84 | Br76 | ||
| Br98 | –/–/– | –/–/– | =/=/= | –/–/– | –/–/– | ||
| Full-dose | Br84 | +/+/+ | –/–/– | +/+/= | +/+/+ | =/=/= | |
| Br76 | +/+/+ | +/+/+ | +/+/+ | +/+/+ | +/+/+ | ||
| Br98 | =/=/= | –/–/= | –/–/– | –/=/= | –/–/= | ||
| Low-dose | Br84 | +/+/+ | –/–/– | –/–/– | +/=/= | –/–/= | |
| Br76 | +/+/+ | =/=/= | –/–/– | +/+/= | +/+/= | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luetkens, K.S.; Grunz, J.-P.; Kunz, A.S.; Huflage, H.; Weißenberger, M.; Hartung, V.; Patzer, T.S.; Gruschwitz, P.; Ergün, S.; Bley, T.A.; et al. Ultra-High-Resolution Photon-Counting Detector CT Arthrography of the Ankle: A Feasibility Study. Diagnostics 2023, 13, 2201. https://doi.org/10.3390/diagnostics13132201
Luetkens KS, Grunz J-P, Kunz AS, Huflage H, Weißenberger M, Hartung V, Patzer TS, Gruschwitz P, Ergün S, Bley TA, et al. Ultra-High-Resolution Photon-Counting Detector CT Arthrography of the Ankle: A Feasibility Study. Diagnostics. 2023; 13(13):2201. https://doi.org/10.3390/diagnostics13132201
Chicago/Turabian StyleLuetkens, Karsten Sebastian, Jan-Peter Grunz, Andreas Steven Kunz, Henner Huflage, Manuel Weißenberger, Viktor Hartung, Theresa Sophie Patzer, Philipp Gruschwitz, Süleyman Ergün, Thorsten Alexander Bley, and et al. 2023. "Ultra-High-Resolution Photon-Counting Detector CT Arthrography of the Ankle: A Feasibility Study" Diagnostics 13, no. 13: 2201. https://doi.org/10.3390/diagnostics13132201
APA StyleLuetkens, K. S., Grunz, J.-P., Kunz, A. S., Huflage, H., Weißenberger, M., Hartung, V., Patzer, T. S., Gruschwitz, P., Ergün, S., Bley, T. A., & Feldle, P. (2023). Ultra-High-Resolution Photon-Counting Detector CT Arthrography of the Ankle: A Feasibility Study. Diagnostics, 13(13), 2201. https://doi.org/10.3390/diagnostics13132201






