Diagnostic Accuracy of ki-67 Labeling Index in Endoscopic Ultrasonography-Fine-Needle Aspiration Cytology and Biopsy of Pancreatic Neuroendocrine Neoplasms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Published Study Search and Selection Criteria
2.2. Data Extraction
2.3. Statistical Analysis
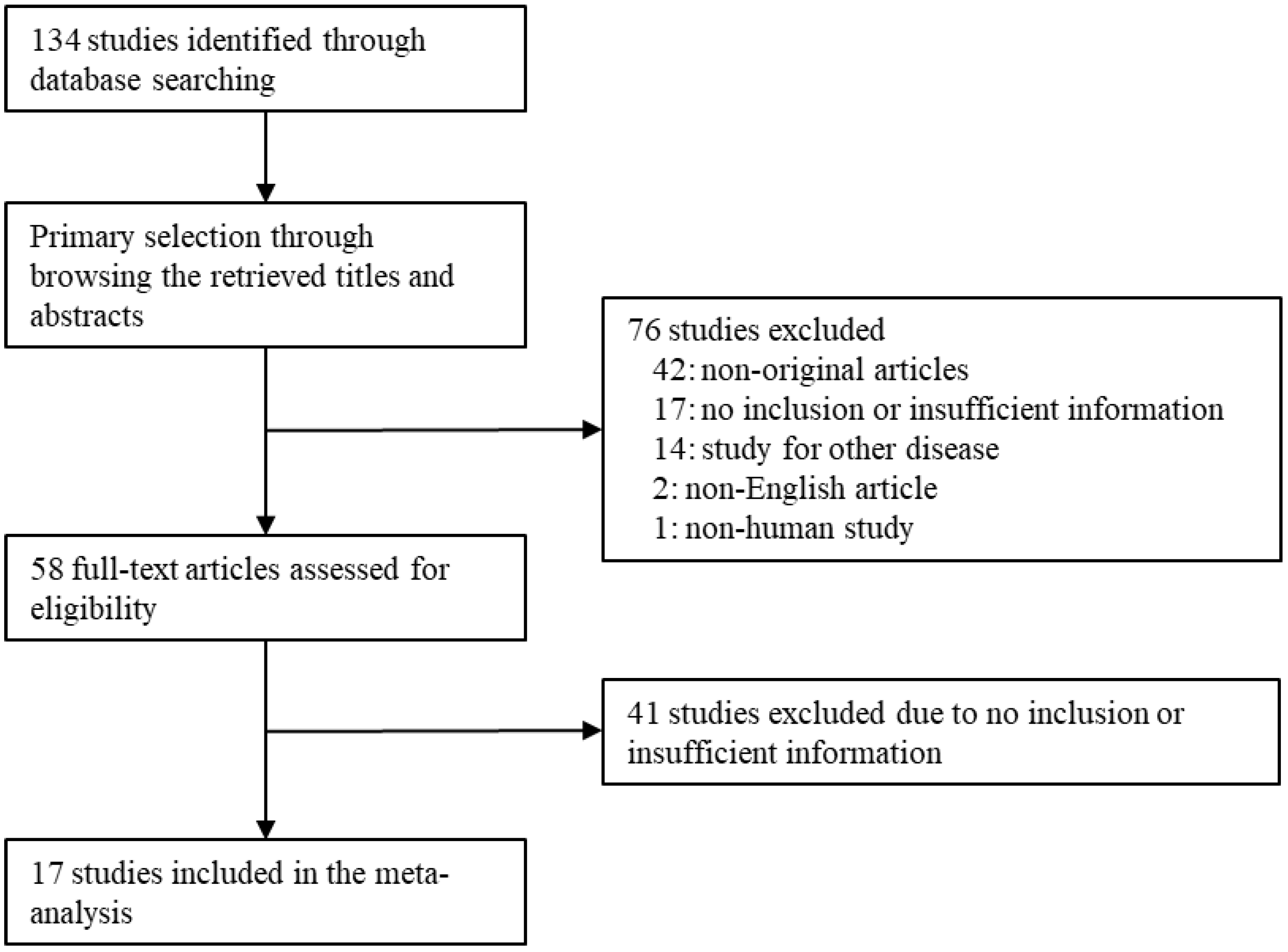
3. Results
3.1. Subsection
Selection and Characteristics
3.2. Concordance Rate of Tumor Grade of Pancreatic Neuroendocrine Neoplasm between EUS-FNAC/FNB and Surgical Specimen
3.3. Diagnostic Test Accuracy Review of Ki-67 in the Grading of Pancreatic Neuroendocrine Neoplasm
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO Classification of Tumours Editorial Board. Digestive System Tumours. Lyon (France): International Agency for Research on Cancer, 5th ed.; WHO classification of tumours series; International Agency for Research on Cancer: Lyon, France, 2019; Volume 1. [Google Scholar]
- Yao, J.C.; Hassan, M.; Phan, A.; Dagohoy, C.; Leary, C.; Mares, J.E.; Abdalla, E.K.; Fleming, J.B.; Vauthey, J.N.; Rashid, A.; et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J. Clin. Oncol. 2008, 26, 3063–3072. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.-H.; Zhang, X.-F.; Lopez-Aguiar, A.G.; Poultsides, G.; Makris, E.; Rocha, F.; Kanji, Z.; Weber, S.; Fisher, A.; Fields, R.; et al. Resection of pancreatic neuroendocrine tumors: Defining patterns and time course of recurrence. HPB 2019, 22, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Xue, F.; Dong, D.H.; Alexandra, L.A.; George, P.; Eleftherios, M.; Flavio, R.; Zaheer, K.; Sharon, W.; Alexander, F.; et al. New nodal staging for primary pancreatic neuroendocrine tumors: A multi-institutional and national data analysis. Ann. Surg 2021, 274, e28–e35. [Google Scholar] [CrossRef] [PubMed]
- Falconi, M.; Eriksson, B.; Kaltsas, G.; Bartsch, D.K.; Capdevila, J.; Caplin, M.; Kos-Kudla, B.; Kwekkeboom, D.; Rindi, G.; Klöppel, G.; et al. Vienna Consensus Conference participants. ENETS consensus guidelines update for the management of patients with functional pancreatic neuroendocrine tumors and non-functional pancreatic neuroendocrine tumors. Neuroendocrinology 2016, 103, 153–171. [Google Scholar] [CrossRef] [PubMed]
- Hosoda, W.; Takagi, T.; Mizuno, N.; Shimizu, Y.; Sano, T.; Yamao, K.; Yatabe, Y. Diagnostic approach to pancreatic tumors with the specimens of endoscopic ultrasound-guided fine needle aspiration. Pathol. Int. 2010, 60, 358–364. [Google Scholar] [CrossRef]
- Abi-Raad, R.; Lavik, J.P.; Barbieri, A.L.; Zhang, X.; Adeniran, A.J.; Cai, G. Grading Pancreatic Neuroendocrine Tumors by Ki-67 Index Evaluated on Fine-Needle Aspiration Cell Block Material. Am. J. Clin. Pathol. 2020, 153, 74–81. [Google Scholar] [CrossRef]
- Boutsen, L.; Jouret-Mourin, A.; Borbath, I.; van Maanen, A.; Weynand, B. Accuracy of Pancreatic Neuroendocrine Tumour Grading by Endoscopic Ultrasound-Guided Fine Needle Aspiration: Analysis of a Large Cohort and Perspectives for Improvement. Neuroendocrinology 2018, 106, 158–166. [Google Scholar] [CrossRef]
- Crinò, S.F.; Ammendola, S.; Meneghetti, A.; Bernardoni, L.; Conti Bellocchi, M.C.; Gabbrielli, A.; Landoni, L.; Paiella, S.; Pin, F.; Parisi, A.; et al. Comparison between EUS-guided fine-needle aspiration cytology and EUS-guided fine-needle biopsy histology for the evaluation of pancreatic neuroendocrine tumors. Pancreatology 2021, 21, 443–450. [Google Scholar] [CrossRef]
- Cui, Y.; Khanna, L.G.; Saqi, A.; Crapanzano, J.P.; Mitchell, J.M.; Sethi, A.; Gonda, T.A.; Kluger, M.D.; Schrope, B.A.; Allendorf, J.; et al. The Role of Endoscopic Ultrasound-Guided Ki67 in the Management of Non-Functioning Pancreatic Neuroendocrine Tumors. Clin. Endosc. 2020, 53, 213–220. [Google Scholar] [CrossRef]
- Arco, C.D.D.; Pérez, J.Á.D.; Medina, L.O.; Valera, J.S.; Aceñero, M.J.F. Reliability of Ki-67 Determination in FNA Samples for Grading Pancreatic Neuroendocrine Tumors. Endocr. Pathol. 2016, 27, 276–283. [Google Scholar] [CrossRef]
- Leo, M.D.; Poliani, L.; Rahal, D.; Auriemma, F.; Anderloni, A.; Ridolfi, C.; Spaggiari, P.; Capretti, G.; Tommaso, L.D.; Preatoni, P.; et al. Pancreatic Neuroendocrine Tumours: The Role of Endoscopic Ultrasound Biopsy in Diagnosis and Grading Based on the WHO 2017 Classification. Dig. Dis. 2019, 37, 325–333. [Google Scholar] [PubMed]
- Farrell, J.M.; Pang, J.C.; Kim, G.E.; Tabatabai, Z.L. Pancreatic neuroendocrine tumors: Accurate grading with Ki-67 index on fine-needle aspiration specimens using the WHO 2010/ENETS criteria. Cancer Cytopathol. 2014, 122, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Grosse, C.; Noack, P.; Silye, R. Accuracy of grading pancreatic neuroendocrine neoplasms with Ki-67 index in fine-needle aspiration cellblock material. Cytopathology 2019, 30, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Heidsma, C.M.; Tsilimigras, D.I.; Rocha, F.; Abbott, D.E.; Fields, R.; Smith, P.M.; Poultsides, G.A.; Cho, C.; van Eijck, C.; van Dijkum, E.N.; et al. US Neuroendocrine Tumor Study Group. Clinical relevance of performing endoscopic ultrasound-guided fine-needle biopsy for pancreatic neuroendocrine tumors less than 2 cm. J. Surg Oncol. 2020, 122, 1393–1400. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.S.; Kim, Y.; An, S.; Kim, S.J.; Kim, J.Y.; Kim, S.Y.; Hwang, D.W.; Park, D.H.; Lee, S.S.; Kim, S.C.; et al. Grading by the Ki-67 Labeling Index of Endoscopic Ultrasound-Guided Fine Needle Aspiration Biopsy Specimens of Pancreatic Neuroendocrine Tumors Can Be Underestimated. Pancreas 2018, 47, 1296–1303. [Google Scholar] [CrossRef] [PubMed]
- Kalantri, S.; Bakshi, P.; Verma, K. Grading of pancreatic neuroendocrine tumors on endoscopic ultrasound-guided fine-needle aspiration using Ki-67 index and 2017 World Health Organization criteria: An analysis of 32 cases. Cytojournal 2020, 17, 21. [Google Scholar] [CrossRef]
- Laskiewicz, L.; Jamshed, S.; Gong, Y.; Ainechi, S.; LaFemina, J.; Wang, X. The diagnostic value of FNA biopsy in grading pancreatic neuroendocrine tumors. Cancer Cytopathol. 2018, 126, 170–178. [Google Scholar] [CrossRef]
- Leeds, J.S.; Nayar, M.K.; Bekkali, N.L.H.; Wilson, C.H.; Johnson, S.J.; Haugk, B.; Darne, A.; Oppong, K.W. Endoscopic ultrasound-guided fine-needle biopsy is superior to fine-needle aspiration in assessing pancreatic neuroendocrine tumors. Endosc. Int. Open 2019, 7, E1281–E1287. [Google Scholar] [CrossRef]
- Paiella, S.; Landoni, L.; Rota, R.; Valenti, M.; Elio, G.; Crinò, S.F.; Manfrin, E.; Parisi, A.; Cingarlini, S.; D’Onofrio, M.; et al. Endoscopic ultrasound-guided fine-needle aspiration for the diagnosis and grading of pancreatic neuroendocrine tumors: A retrospective analysis of 110 cases. Endoscopy 2020, 52, 988–994. [Google Scholar] [CrossRef]
- Piani, C.; Franchi, G.M.; Cappelletti, C.; Scavini, M.; Albarello, L.; Zerbi, A.; Arcidiacono, P.G.; Bosi, E.; Manzoni, M.F. Cytological Ki-67 in pancreatic endocrine tumours: An opportunity for pre-operative grading. Endocr. Relat. Cancer 2008, 15, 175–181. [Google Scholar] [CrossRef]
- Sugimoto, M.; Takagi, T.; Hikichi, T.; Suzuki, R.; Watanabe, K.; Nakamura, J.; Kikuchi, H.; Konno, N.; Waragai, Y.; Asama, H.; et al. Efficacy of endoscopic ultrasonography-guided fine needle aspiration for pancreatic neuroendocrine tumor grading. World J. Gastroenterol. 2015, 21, 8118–8124. [Google Scholar] [CrossRef] [PubMed]
- Tacelli, M.; Petrone, M.C.; Capurso, G.; Muffatti, F.; Andreasi, V.; Partelli, S.; Doglioni, C.; Falconi, M.; Arcidiacono, P.G. Diagnostic accuracy of EUS-FNA in the evaluation of pancreatic neuroendocrine neoplasms grading: Possible clinical impact of misclassification. Endosc. Ultrasound 2021, 10, 372–380. [Google Scholar] [CrossRef]
- Polkowski, M.; Jenssen, C.; Kaye, P.; Carrara, S.; Deprez, P.; Fernández-Esparrach, G.; Eisendrath, P.; Aithal, G.P.; Arcidiacono, P.; Barthet, M.; et al. Technical aspects of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline—March 2017. Endoscopy 2017, 49, 989–1006. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.A.; Brown, L.J.; Hong, S.K.; Draganova-Tacheva, R.A.; Korenblit, J.; Loren, D.E.; Kowalski, T.E.; Solomides, C. Relationship of pancreatic mass size and diagnostic yield of endoscopic ultrasound-guided fine needle aspiration. Dig. Dis. Sci. 2011, 56, 3370–3375. [Google Scholar] [CrossRef] [PubMed]
- Zamora, J.; Abraira, V.; Muriel, A.; Khan, K.; Coomarasamy, A. Meta-DiSc: A software for meta-analysis of test accuracy data. BMC Med. Res. Methodol. 2006, 6, 31. [Google Scholar] [CrossRef]
- Moses, L.E.; Shapiro, D.; Littenberg, B. Combining independent studies of a diagnostic test into a summary ROC curve: Data-analytic approaches and some additional considerations. Stat Med. 1993, 12, 1293–1316. [Google Scholar] [CrossRef]
- Li, H.; Li, W.; Zhou, Q.Y.; Fan, B. Fine needle biopsy is superior to fine needle aspiration in endoscopic ultrasound guided sampling of pancreatic masses: A meta-analysis of randomized controlled trials. Medicine 2018, 97, e0207. [Google Scholar] [CrossRef]
- NANETS treatment guidelines. Well-differentiated neuroendocrine tumors of the stomach and pancreas. Pancreas 2010, 39, 735–752. [Google Scholar] [CrossRef]
- Hasegawa, T.; Yamao, K.; Hijioka, S.; Bhatia, V.; Mizuno, N.; Hara, K.; Imaoka, H.; Niwa, Y.; Tajika, M.; Kondo, S.; et al. Evaluation of Ki- 67 index in EUS-FNA specimens for the assessment of malignancy risk in pancreatic neuroendocrine tumors. Endoscopy 2014, 46, 32–38. [Google Scholar] [CrossRef]
| Author and Publication Year | Location | Specimen | Antibody Clone | Number of Patients | Surgical Specimen | Tumor Size (cm, Mean ± SD) | ||
|---|---|---|---|---|---|---|---|---|
| G1 | G2 | G3 | ||||||
| Abi-Raad 2020 [7] | USA | FNAC with CB | MIB-1 | 49 | 27 | 22 | 0 | 3.00 ± 1.90 |
| Boutsen 2018 [8] | Belgium | FNAC | MIB-1 | 57 | 30 | 23 | 4 | 2.85 ± 2.16 |
| Crinò 2021 [9] | Italy | FNAC with CB | MIB-1 | 69 | 13 | 4 | 0 | 1.98 ± 1.50 |
| FNB | 73 | 46 | 25 | 2 | 2.15 ± 1.33 | |||
| Cui 2020 [10] | USA | FNAC | 30–9 | 37 | 24 | 8 | 5 | ND |
| Díaz Del Arco 2016 [11] | Spain | FNAC | ND | 10 | 7 | 3 | 0 | 3.20 ± 3.28 |
| Di Leo 2019 [12] | Italy | FNB | ND | 25 | 20 | 4 | 1 | 2.10 ± 1.49 |
| Farrell 2014 [13] | USA | FNAC | MIB-1 | 22 | 15 | 5 | 2 | 3.03 ± 1.73 |
| Grosse 2019 [14] | Austria | FNAC with CB | ND | 15 | 2 | 9 | 4 | 3.84 ± 1.8 |
| Heidsma 2020 [15] | USA | FNAC | ND | 63 | 46 | 16 | 1 | ND |
| Hwang 2018 [16] | Korea | FNB | 33 | 20 | 10 | 3 | 3.30 ± 2.20 | |
| Kalantri 2020 [17] | India | FNAC with CB | BGX-297 | 11 | 4 | 4 | 3 | ND |
| Laskiewicz 2018 [18] | USA | FNAC | MIB-1 | 26 | 15 | 11 | 0 | ND |
| Leeds 2019 [19] | UK | FNAC with CB | ND | 23 | 16 | 7 | 0 | 2.57 ± 0.31 |
| FNB | 26 | 12 | 14 | 0 | 3.25 ± 0.36 | |||
| Paiella 2020 [20] | Italy | FNAC | ND | 77 | 48 | 28 | 1 | 2.45 ± 1.34 |
| Piani 2008 [21] | Italy | FNAC | MIB-1 | 18 | 11 | 6 | 1 | 3.05 ± 2.77 |
| Sugimoto 2015 [22] | Japan | FNAC | MIB-1 | 8 | 5 | 3 | 0 | 2.57 ± 1.32 |
| Tacelli 2021 [23] | Italy | FNAC with CB | MIB-1 | 112 | 59 | 50 | 3 | 2.39 ± 0.31 |
| Number of Subsets | Fixed Effect [95% CI] | Heterogeneity Test [p-Value] | Random Effect [95% CI] | Egger’s Test [p-Value] | |
|---|---|---|---|---|---|
| Overall | 19 | 0.754 [0.719, 0.786] | 0.006 | 0.767 [0.713, 0.814] | 0.080 |
| FNAC | 15 | 0.734 [0.694, 0.771] | 0.039 | 0.741 [0.681, 0.794] | 0.136 |
| FNB a | 4 | 0.840 [0.770, 0.892] | 0.140 | 0.839 [0.738, 0.906] | 0.826 |
| Grade 1/2 | 19 | 0.757 [0.722, 0.790] | 0.028 | 0.772 [0.722, 0.816] | 0.024 |
| FNAC | 15 | 0.739 [0.699, 0.776] | 0.194 | 0.745 [0.695, 0.789] | 0.052 |
| FNB b | 4 | 0.840 [0.766, 0.894] | 0.062 | 0.846 [0.722, 0.921] | 0.352 |
| Grade 1 | 19 | 0.756 [0.713, 0.794] | 0.064 | 0.772 [0.712, 0.820] | 0.026 |
| Grade 2 | 17 | 0.732 [0.657, 0.796] | 0.310 | 0.741 [0.655, 0.812] | 0.062 |
| Grade 3 | 6 | 0.743 [0.628, 0.945] | 0.966 | 0.743 [0.628, 0.945] | 0.019 |
| FNAC | 5 | 0.879 [0.660, 0.965] | 0.999 | 0.879 [0.660, 0.965] | <0.001 |
| FNB c | 1 | 0.667 [0.154, 0.957] | 1.000 | 0.667 [0.154, 0.957] | - |
| Tumor size, less than 2 cm | 6 | 0.797 [0.726, 0.853] | 0.777 | 0.797 [0.726, 0.853] | 0.204 |
| Grade 1 d | 5 | 0.877 [0.791, 0.930] | 0.939 | 0.877 [0.791, 0.930] | 0.385 |
| Grade 2 | 3 | 0.665 [0.453, 0.827] | 0.276 | 0.685 [0.414, 0.870] | 0.757 |
| Number of Subsets | Sensitivity (%) [95% CI] | Specificity (%) [95% CI] | Diagnostic OR [95% CI] | AUC on SROC | |
|---|---|---|---|---|---|
| Overall | |||||
| Grade 1 * | 18 | 0.908 [0.876, 0.937] | 0.616 [0.557, 0.674] | 14.467 [8.892, 23.536] | 0.871 |
| Grade 2 * | 17 | 0.599 [0.534, 0.661] | 0.904 [0.872, 0.930] | 13.971 [8.364, 23.335] | 0.859 |
| Grade 3 # | 10 | 0.786 [0.590, 0.917] | 0.998 [0.987, 1.000] | 150.220 [46.145, 489.000] | 0.983 |
| Tumor size, less than 2 cm | |||||
| Grade 1 | 4 | 0.852 [0.771, 0.913] | 0.675 [0.509, 0.814] | 15.319 [5.915, 39.677] | 0.841 |
| Grade 2 | 4 | 0.667 [0.498, 0.809] | 0.844 [0.762, 0.906] | 13.093 [5.143, 33.332] | 0.834 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pyo, J.-S.; Kim, N.Y.; Min, K.-W.; Oh, I.H.; Lim, D.H.; Son, B.K. Diagnostic Accuracy of ki-67 Labeling Index in Endoscopic Ultrasonography-Fine-Needle Aspiration Cytology and Biopsy of Pancreatic Neuroendocrine Neoplasms. Diagnostics 2023, 13, 2756. https://doi.org/10.3390/diagnostics13172756
Pyo J-S, Kim NY, Min K-W, Oh IH, Lim DH, Son BK. Diagnostic Accuracy of ki-67 Labeling Index in Endoscopic Ultrasonography-Fine-Needle Aspiration Cytology and Biopsy of Pancreatic Neuroendocrine Neoplasms. Diagnostics. 2023; 13(17):2756. https://doi.org/10.3390/diagnostics13172756
Chicago/Turabian StylePyo, Jung-Soo, Nae Yu Kim, Kyueng-Whan Min, Il Hwan Oh, Dae Hyun Lim, and Byoung Kwan Son. 2023. "Diagnostic Accuracy of ki-67 Labeling Index in Endoscopic Ultrasonography-Fine-Needle Aspiration Cytology and Biopsy of Pancreatic Neuroendocrine Neoplasms" Diagnostics 13, no. 17: 2756. https://doi.org/10.3390/diagnostics13172756





