Delta Cord as a Radiological Localization Sign of Postoperative Adhesive Arachnoiditis: A Case Report and Literature Review
Abstract
:1. Introduction
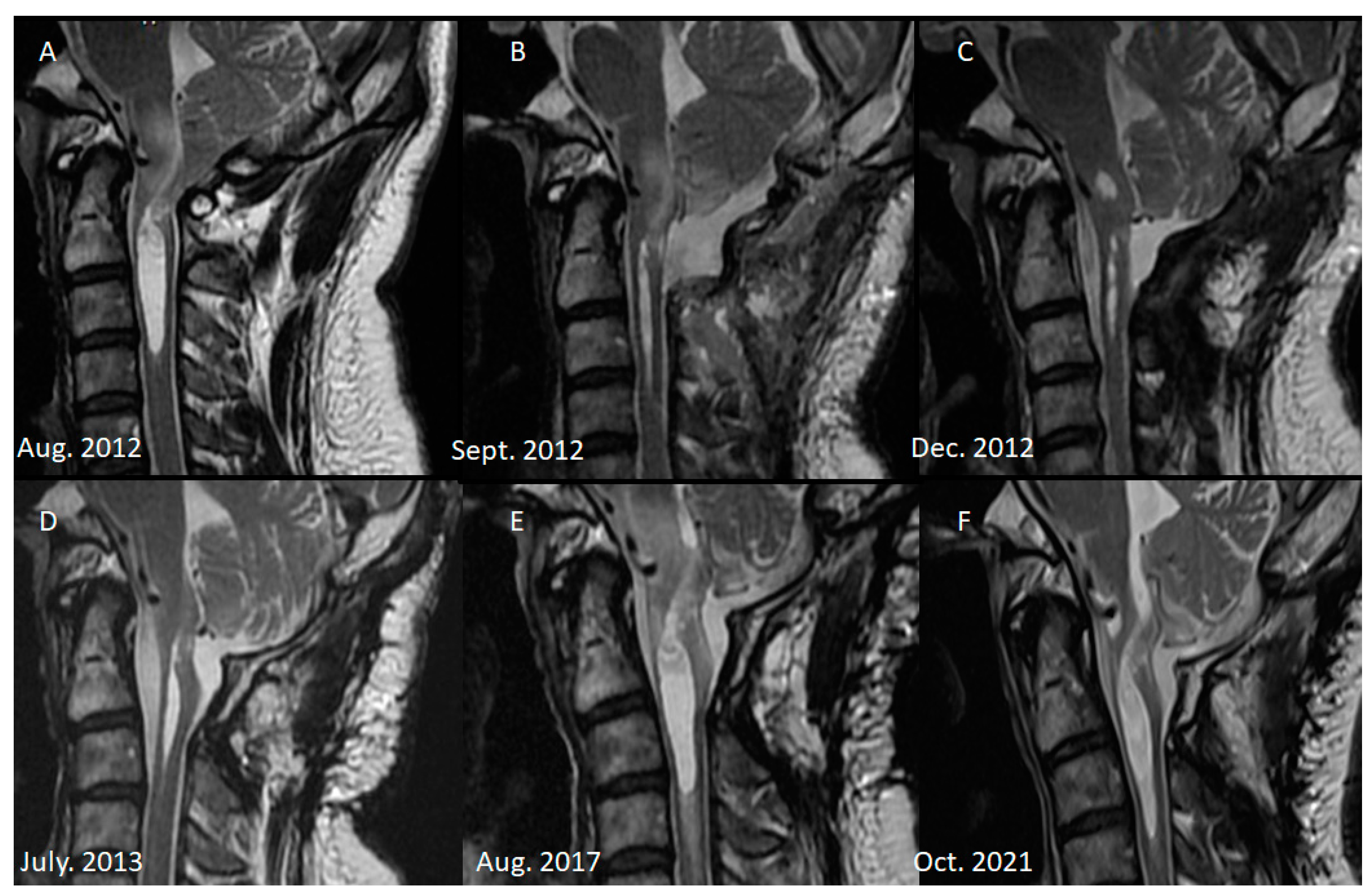
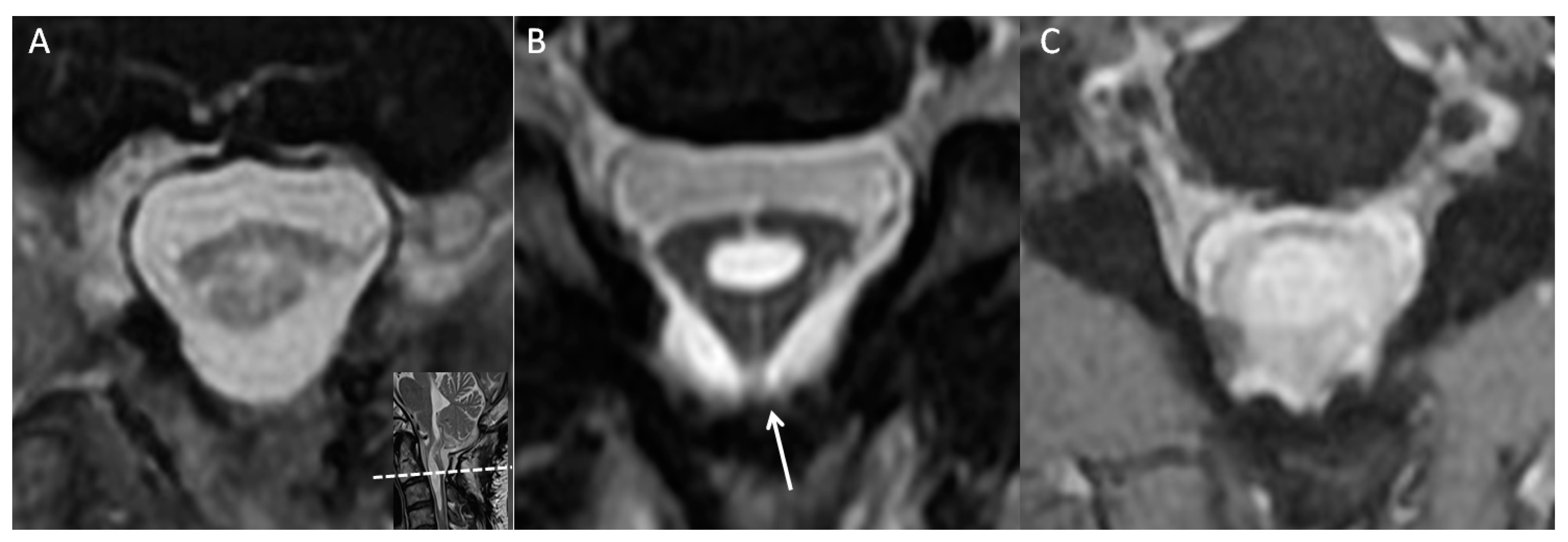
2. Case Illustration
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fitt, G.J.; Stevens, J.M. Postoperative arachnoiditis diagnosed by high resolution fast spin-echo MRI of the lumbar spine. Neuroradiology 1995, 37, 139–145. [Google Scholar] [CrossRef]
- Parenti, V.; Huda, F.; Richardson, P.K.; Brown, D.; Aulakh, M.; Taheri, M.R. Lumbar arachnoiditis: Does imaging associate with clinical features? Clin. Neurol. Neurosurg. 2020, 192, 105717. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.L.; Morris, J.M.; Wald, J.T.; Kotsenas, A.L. Imaging Appearance of Advanced Chronic Adhesive Arachnoiditis: A Retrospective Review. AJR Am. J. Roentgenol. 2017, 209, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Hirai, T.; Kato, T.; Kawabata, S.; Enomoto, M.; Tomizawa, S.; Yoshii, T.; Sakaki, K.; Shinomiya, K.; Okawa, A. Adhesive arachnoiditis with extensive syringomyelia and giant arachnoid cyst after spinal and epidural anesthesia: A case report. Spine 2012, 37, E195–E198. [Google Scholar] [CrossRef] [PubMed]
- Ishizaka, S.; Hayashi, K.; Otsuka, M.; Fukuda, S.; Tsunoda, K.; Ushijima, R.; Kitagawa, N.; Suyama, K.; Nagata, I. Syringomyelia and arachnoid cysts associated with spinal arachnoiditis following subarachnoid hemorrhage. Neurol. Med. Chir. 2012, 52, 686–690. [Google Scholar] [CrossRef]
- Pasoglou, V.; Janin, N.; Tebache, M.; Tegos, T.J.; Born, J.D.; Collignon, L. Familial adhesive arachnoiditis associated with syringomyelia. AJNR Am. J. Neuroradiol. 2014, 35, 1232–1236. [Google Scholar] [CrossRef]
- Maenhoudt, W.; Rasschaert, R.; Bontinck, H.; Pinson, H.; Van Roost, D.; Hallaert, G. Postarachnoiditis Anterior Spinal Arachnoid Cyst Formation with Compressive Myelopathy: Report of 2 Cases. World Neurosurg. 2018, 118, 59–62. [Google Scholar] [CrossRef]
- Todeschi, J.; Chibbaro, S.; Gubian, A.; Pop, R.; Proust, F.; Cebula, H. Spinal adhesive arachnoiditis following the rupture of an Adamkiewicz aneurysm: Literature review and a case illustration. Neurochirurgie 2018, 64, 177–182. [Google Scholar] [CrossRef]
- Jurga, S.; Szymańska-Adamcewicz, O.; Wierzchołowski, W.; Pilchowska-Ujma, E.; Urbaniak, Ł. Spinal adhesive arachnoiditis: Three case reports and review of literature. Acta Neurol. Belg. 2021, 121, 47–53. [Google Scholar] [CrossRef]
- Carlsward, C.; Darvish, B.; Tunelli, J.; Irestedt, L. Chronic adhesive arachnoiditis after repeat epidural blood patch. Int. J. Obstet. Anesth. 2015, 24, 280–283. [Google Scholar] [CrossRef]
- Killeen, T.; Kamat, A.; Walsh, D.; Parker, A.; Aliashkevich, A. Severe adhesive arachnoiditis resulting in progressive paraplegia following obstetric spinal anaesthesia: A case report and review. Anaesthesia 2012, 67, 1386–1394. [Google Scholar] [CrossRef]
- Belen, D.; Er, U.; Gurses, L.; Yigitkanli, K. Delayed pseudomyelomeningocele: A rare complication after foramen magnum decompression for Chiari malformation. Surg. Neurol. 2009, 71, 357–361; discussion 361. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Nemoto, Y.; Ohata, K.; Daikokuya, H.; Hakuba, A.; Tashiro, T.; Shakudo, M.; Nagai, K.; Nakayama, K.; Yamada, R. Syringomyelia associated with adhesive spinal arachnoiditis: MRI. Neuroradiology 2001, 43, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Kleindienst, A.; Engelhorn, T.; Roeckelein, V.; Buchfelder, M. Development of pre-syrinx state and syringomyelia following a minor injury: A case report. J. Med. Case Rep. 2020, 14, 223. [Google Scholar] [CrossRef] [PubMed]
- Safi, S.; Thabat, A.; Arshad, M.; Hanoun, M. Arachnoiditis—A challenge in diagnosis and success in outcome—Case report. Interdiscip. Neurosurg. 2021, 25, 101219. [Google Scholar] [CrossRef]
- Klekamp, J.; Iaconetta, G.; Batzdorf, U.; Samii, M. Syringomyelia associated with foramen magnum arachnoiditis. J. Neurosurg. 2002, 97, 317–322. [Google Scholar] [CrossRef]
- Wright, M.H.; Denney, L.C. A comprehensive review of spinal arachnoiditis. Orthop. Nurs. 2003, 22, 215–219; quiz 220–211. [Google Scholar] [CrossRef]
- Peng, H.; Conermann, T. Arachnoiditis. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2023. [Google Scholar]
- Koyanagi, I.; Chiba, Y.; Uemori, G.; Imamura, H.; Yoshino, M.; Aida, T. Pathophysiology and surgical treatment of spinal adhesive arachnoid pathology: Patient series. J. Neurosurg. Case Lessons 2021, 2, Case21426. [Google Scholar] [CrossRef]
- Shikata, J.; Yamamuro, T.; Iida, H.; Sugimoto, M. Surgical treatment for symptomatic spinal adhesive arachnoiditis. Spine 1989, 14, 870–875. [Google Scholar] [CrossRef]
- Silva, A.; Thanabalasundaram, G.; Wilkinson, B.; Tsermoulas, G.; Flint, G. Experience with revision craniovertebral decompression in adult patients with Chiari malformation type 1, with or without syringomyelia. Br. J. Neurosurg. 2022, 36, 750–755. [Google Scholar] [CrossRef]
- Klekamp, J. The pathophysiology of syringomyelia—Historical overview and current concept. Acta Neurochir. 2002, 144, 649–664. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, D. Human spinal arachnoid septa, trabeculae, and “rogue strands”. Am. J. Anat. 1991, 192, 498–509. [Google Scholar] [CrossRef] [PubMed]
- Benner, B.; Ehni, G. Spinal arachnoiditis. The postoperative variety in particular. Spine 1978, 3, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Burton, C.V. Lumbosacral arachnoiditis. Spine 1978, 3, 24–30. [Google Scholar] [CrossRef]
- Auld, A.W. Chronic spinal arachnoiditis. A postoperative syndrome that may signal its onset. Spine 1978, 3, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Quiles, M.; Marchisello, P.J.; Tsairis, P. Lumbar adhesive arachnoiditis. Etiologic and pathologic aspects. Spine 1978, 3, 45–50. [Google Scholar] [CrossRef]
- Matsui, H.; Tsuji, H.; Kanamori, M.; Kawaguchi, Y.; Yudoh, K.; Futatsuya, R. Laminectomy-induced arachnoradiculitis: A postoperative serial MRI study. Neuroradiology 1995, 37, 660–666. [Google Scholar] [CrossRef]
- Morisako, H.; Takami, T.; Yamagata, T.; Chokyu, I.; Tsuyuguchi, N.; Ohata, K. Focal adhesive arachnoiditis of the spinal cord: Imaging diagnosis and surgical resolution. J. Craniovertebr. Junction Spine 2010, 1, 100–106. [Google Scholar] [CrossRef]
- De Vlieger, J.; Dejaegher, J.; Van Calenbergh, F. Posterior fossa decompression for Chiari malformation type I: Clinical and radiological presentation, outcome and complications in a retrospective series of 105 procedures. Acta Neurol. Belg. 2019, 119, 245–252. [Google Scholar] [CrossRef]
- Yilmaz, A.; Kanat, A.; Musluman, A.M.; Colak, I.; Terzi, Y.; Kayacı, S.; Aydin, Y. When is duraplasty required in the surgical treatment of Chiari malformation type I based on tonsillar descending grading scale? World Neurosurg. 2011, 75, 307–313. [Google Scholar] [CrossRef]
- Xu, H.; Chu, L.; He, R.; Ge, C.; Lei, T. Posterior fossa decompression with and without duraplasty for the treatment of Chiari malformation type I-a systematic review and meta-analysis. Neurosurg. Rev. 2017, 40, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Abla, A.A.; Link, T.; Fusco, D.; Wilson, D.A.; Sonntag, V.K. Comparison of dural grafts in Chiari decompression surgery: Review of the literature. J. Craniovertebr. Junction Spine 2010, 1, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Shaffrey, C.I.; Spotnitz, W.D.; Shaffrey, M.E.; Jane, J.A. Neurosurgical Applications of Fibrin Glue: Augmentation of Dural Closure in 134 Patients. Neurosurgery 1990, 26, 207–210. [Google Scholar] [CrossRef]
- Green, A.L.; Arnaud, A.; Batiller, J.; Eljamel, S.; Gauld, J.; Jones, P.; Martin, D.; Mehdorn, M.; Ohman, J.; Weyns, F. A multicentre, prospective, randomized, controlled study to evaluate the use of a fibrin sealant as an adjunct to sutured dural repair. Br. J. Neurosurg. 2015, 29, 11–17. [Google Scholar] [CrossRef]
- Atkinson, J.B.; Gomperts, E.D.; Kang, R.; Lee, M.; Arensman, R.M.; Bartlett, R.H.; Rais-Bharami, K.; Breaux, C.W., Jr.; Cornish, J.D.; Haase, G.M.; et al. Prospective, randomized evaluation of the efficacy of fibrin sealant as a topical hemostatic agent at the cannulation site in neonates undergoing extracorporeal membrane oxygenation. Am. J. Surg. 1997, 173, 479–484. [Google Scholar] [CrossRef]
- Beierlein, W.; Scheule, A.M.; Dietrich, W.; Ziemer, G. Forty years of clinical aprotinin use: A review of 124 hypersensitivity reactions. Ann. Thorac. Surg. 2005, 79, 741–748. [Google Scholar] [CrossRef]
- Hayashi, K.; Nagano, J.; Hattori, S. Adhesive arachnoiditis after percutaneous fibrin glue treatment of a sacral meningeal cyst. J. Neurosurg. Spine 2014, 20, 763–766. [Google Scholar] [CrossRef]
- Cohen, A.R.; Aleksic, S.; Ransohoff, J. Inflammatory reaction to synthetic dural substitute. Case report. J. Neurosurg. 1989, 70, 633–635. [Google Scholar] [CrossRef]
- Kok, A.J.; Verhagen, W.I.; Bartels, R.H.; van Dijk, R.; Prick, M.J. Spinal arachnoiditis following subarachnoid haemorrhage: Report of two cases and review of the literature. Acta Neurochir. 2000, 142, 795–798; discussion 798–799. [Google Scholar] [CrossRef]
- Tumialán, L.M.; Cawley, C.M.; Barrow, D.L. Arachnoid cyst with associated arachnoiditis developing after subarachnoid hemorrhage. Case report. J. Neurosurg. 2005, 103, 1088–1091. [Google Scholar] [CrossRef]
- Kroin, J.S.; Buvanendran, A.; Cochran, E.; Tuman, K.J. Characterization of pain and pharmacologic responses in an animal model of lumbar adhesive arachnoiditis. Spine 2005, 30, 1828–1831. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.; Hemley, S.; Jones, N.; Cheng, S.; Bilston, L.; Stoodley, M. Fluid outflow in a large-animal model of posttraumatic syringomyelia. Neurosurgery 2012, 71, 474–480; discussion 480. [Google Scholar] [CrossRef] [PubMed]
- Saboori, P.; Sadegh, A. Histology and Morphology of the Brain Subarachnoid Trabeculae. Anat. Res. Int. 2015, 2015, 279814. [Google Scholar] [CrossRef] [PubMed]


| Computed Tomography or MRI Finding | Evidence Type | Hypothesis of Pathophysiology |
|---|---|---|
| Nerve root clumping, cauda equina adhesion | Localization sign | Formation of arachnoid scar in accordance with the surgery and wound healing [28] |
| “Delta cord” sign, disrupted cord contour | Localization sign | Fibrinous bands lead to adhesions between the cord of the thecal sac [11] |
| Arachnoid septations, arachnoid cysts | Localization sign | Arachnoid web formation by chronic inflammation of the arachnoid [3] |
| Syringomyelia, syrinx | Associated sign | Blocked CSF flow likely caused by adhesions or pressure gradient at the obstruction site [3] |
| Pseudomyelomeningocele | Associated sign | Continuous CSF pressure that forces CSF to come into contact with muscular layer [12] |
| Studies | Characteristics | Suspected Etiology | Location and Image Sign Type | Management | Outcomes |
|---|---|---|---|---|---|
| Killeen. 2012 [11] | 27 y/o, F | Noxious agent (chlorhexidine) or blood in the pia-arachnoid | Multilevel clumped nerve roots and disrupted cord contour (localization signs) | Surgery (laminectomy, adhesiolysis, and shunting) | Worsened: Unable to work and dependent |
| Ishizaka. 2012 [5] | 66 y/o, F | Subarachnoid hemorrhage | Deformity of cord and arachnoid cyst (localization signs) | Surgery (laminectomy) | Improved: Can walk unaided but with abdominal paresthesia |
| Hirai. 2012 [4] | 29 y/o, F | Insertion of epidural tube or puncture, intradural administration of bupivacaine, or epidural bleeding | Arachnoid cyst compressing the spinal cord, and convergence of cauda equine (localization signs) | Surgery (laminectomy and adhesiolysis) | Improved: Can walk without cane but with numbness, moderate lower limb weakness, and anuresis |
| Pasoglou. 2014 [6] | Case 1: 35 y/o, F | Rare inherited genetic anomaly (not confirmed) | No image shown | Surgery (laminectomy) | Short amelioration then worsening |
| Case 2: 50 y/o, M | same as above | No data | Surgery * | Death due to perioperative complications | |
| Case 3: no data | same as above | No data | No data | No data | |
| Case 4: 49 y/o, F | same as above | No image shown | Surgery * | Regression of syrinx; no results regarding functional outcomes | |
| Case 5: 45 y/o, F | same as above | No image shown | Surgery * | No results regarding functional outcomes | |
| Case 6: 49 y/o, M | same as above | Arachnoid septations and cysts (localization signs) | Surgery (laminectomy and adhesiolysis) | Regression of syrinx; no results regarding functional outcomes | |
| Carlswärd. 2015 [10] | 29 y/o, F | Epidural blood patch with subsequent inflammation | Lumbar clumped root (localization sign) | Conservative | Worsened and wheelchair dependent |
| Todeschi, 2017 [8] | 57 y/o, F | SAH of ruptured aneurysm from Adamkiewicz artery | Arachnoid cysts (localization sign) | Surgery (laminectomy and adhesiolysis) | Improved: Can walk with crutches |
| Maenhoudt. 2018 [7] | Case 1: 59 y/o, F | Meningitis | Arachnoid cysts (localization sign) | Surgery (laminectomy and cyst aspiration) | No improvement of neurological status |
| Case 2: 50 y/o, F | SAH of ruptured PICA aneurysm | Arachnoid cysts, septations, clumped cauda equine and syringomyelia (localization and associated signs) | Surgery (laminectomy and cyst aspiration) | Improved, including syrinx regression and gait and bladder function improvements | |
| Kleindienst. 2020 [14] | 59 y/o, M | Spinal injury | Arachnoid septations and syringomyelia (localization and associated signs) | Surgery (laminectomy and duroplasty) | Stabilized neurological function |
| Jurga. 2021 [9] | Case 1: 50 y/o, F | Post-spinal surgery or spinal block, | Spinal sac and spinal cord deformities (localization signs) | Conservative | Unimproved and wheelchair dependent |
| Case 2: 26 y/o, M | Unknown inflammatory disease of the spinal cord | Intrathecal cyst and distorted spinal cord (localization signs) | Surgery * | Unimproved and wheelchair dependent | |
| Case 3: no age record, F | Post-spinal surgery | Archnoid cysts (localization sign) | Conservative | Unimproved and wheelchair dependent | |
| Safi. 2021 [15] | 29 y/o, M | Staphylococcus cohnii infection | Syringomyelia and arachnoid septations (localization and associated signs) | Surgery (laminectomy, adhesiolysis, and shunting) | Improved: Fully independent and syrinx regression |
| Present case | 29 y/o, M | Repeated spinal surgery, CSF leakage, foreign material use (Fibrin glue and artificial dural graft) | Delta cord sign, cord tethering, and syringomyelia (localization and associated signs) | Surgery (laminectomy, adhesiolysis, and duroplasty) | Improved: Can walk with cane |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, Y.-T.; Chiang, Y.-H.; Lin, J.-H. Delta Cord as a Radiological Localization Sign of Postoperative Adhesive Arachnoiditis: A Case Report and Literature Review. Diagnostics 2023, 13, 2942. https://doi.org/10.3390/diagnostics13182942
Tu Y-T, Chiang Y-H, Lin J-H. Delta Cord as a Radiological Localization Sign of Postoperative Adhesive Arachnoiditis: A Case Report and Literature Review. Diagnostics. 2023; 13(18):2942. https://doi.org/10.3390/diagnostics13182942
Chicago/Turabian StyleTu, Yi-Ting, Yung-Hsiao Chiang, and Jiann-Her Lin. 2023. "Delta Cord as a Radiological Localization Sign of Postoperative Adhesive Arachnoiditis: A Case Report and Literature Review" Diagnostics 13, no. 18: 2942. https://doi.org/10.3390/diagnostics13182942
APA StyleTu, Y.-T., Chiang, Y.-H., & Lin, J.-H. (2023). Delta Cord as a Radiological Localization Sign of Postoperative Adhesive Arachnoiditis: A Case Report and Literature Review. Diagnostics, 13(18), 2942. https://doi.org/10.3390/diagnostics13182942






