More Than a Decade of GeneXpert® Mycobacterium tuberculosis/Rifampicin (Ultra) Testing in South Africa: Laboratory Insights from Twenty-Three Million Tests
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Setting
2.2. Study Design
2.3. Overview of the National GeneXpert Program for TB Molecular Testing
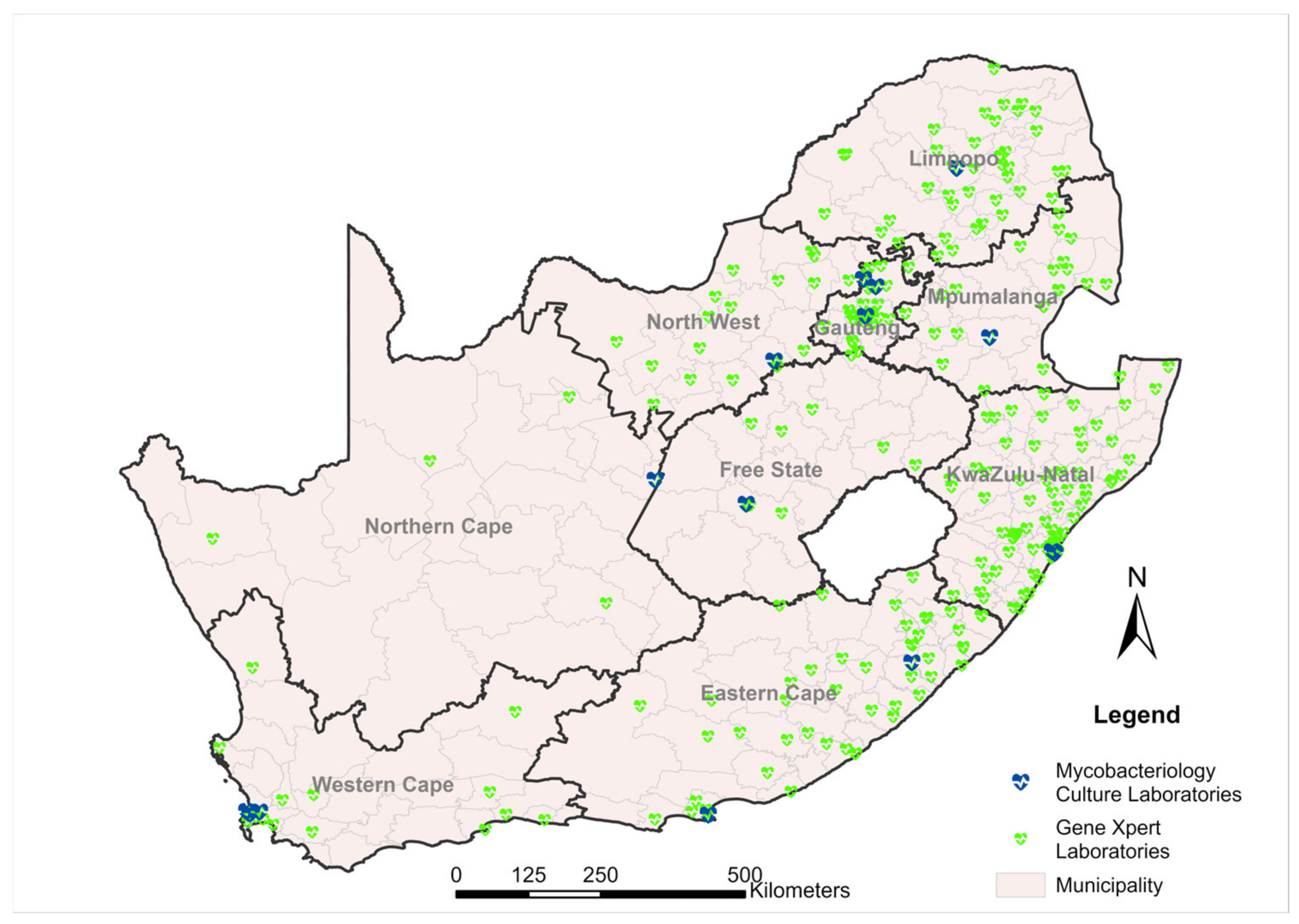
2.3.1. Phased Implementation of GeneXpert Platforms
2.3.2. Reporting of ‘Mycobacterium tuberculosis Complex Trace Detected’ Results
2.3.3. Specimen Collection Practice
2.3.4. Xpert® MTB/RIF and Xpert® MTB/RIF Ultra in the Context of the National Diagnostic Algorithm
2.4. Data Preparation
2.5. Statistical Analysis
3. Results
3.1. National Testing Overview
3.2. Provincial Overview
3.3. Testing by Specimen Origin
3.3.1. Pulmonary TB
3.3.2. Extra-Pulmonary TB
3.4. Demographic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organisation (WHO). Global Tuberculosis Report. Available online: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2022 (accessed on 10 May 2023).
- National Department of Health (NDOH); South African Medical Research Council (SAMRC); Human Sciences Research Council (HSRC); National Institute for Communicable Diseases (NICD); World Health Organization (WHO). The First National TB Prevalence Survey: South Africa. Available online: https://www.nicd.ac.za/wp-content/uploads/2021/02/TB-Prevalence-survey-report_A4_SA_TPS-Short_Feb-2021.pdf (accessed on 14 September 2021).
- World Health Organisation (WHO). Global Tuberculosis Report. Available online: https://apps.who.int/iris/bitstream/handle/10665/336069/9789240013131-eng.pdf (accessed on 13 September 2021).
- World Health Organisation (WHO). Global Tuberculosis Report. Available online: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2021 (accessed on 23 March 2021).
- Nicol, M.P.; Whitelaw, A.; Stevens, W. Using Xpert MTB/RIF. Curr. Respir. Med. Rev. 2013, 9, 187–192. [Google Scholar] [CrossRef]
- World Health Organisation (WHO). Automated Real-Time Nucleic Acid Amplification Technology for Rapid and Simultaneous Detection of Tuberculosis and Rifampicin Resistance: Xpert MTB/RIF System: Policy Statement. Available online: https://apps.who.int/iris/handle/10665/44586 (accessed on 31 March 2022).
- Stevens, W.S.; Scott, L.; Noble, L.; Gous, N.; Dheda, K. Impact of the GeneXpert MTB/RIF Technology on Tuberculosis Control. In Tuberculosis and the Tubercle Bacillus; Wiley: New York, NY, USA, 2017; pp. 389–410. [Google Scholar]
- Cox, H.S.; Mbhele, S.; Mohess, N.; Whitelaw, A.; Muller, O.; Zemanay, W.; Little, F.; Azevedo, V.; Simpson, J.; Boehme, C.C.; et al. Impact of Xpert MTB/RIF for TB Diagnosis in a Primary Care Clinic with High TB and HIV Prevalence in South Africa: A Pragmatic Randomised Trial. PLoS Med. 2014, 11, e1001760. [Google Scholar] [CrossRef]
- Erasmus, L.; Coetzee, G.; Stevens, W. Scale up of Xpert MTB/RIF from the national laboratory perspective: Issues and challenges. Int. J. Tuberc. Lung Dis. 2011, 15, S61. [Google Scholar]
- National Health Laboratory Service (NHLS). Annual Report 2019/20. Available online: https://www.nhls.ac.za/wp-content/uploads/2021/03/NHLS_AR_2020_25_Nov.pdf (accessed on 15 February 2022).
- National Health Laboratory Service (NHLS). Annual Report 2021/22. Available online: https://www.nhls.ac.za/wp-content/uploads/2022/10/NHLS_AR_2022_web_version.pdf (accessed on 31 July 2023).
- Stevens, W.S.; Cunningham, B.; Cassim, N.; Gous, N.; Scott, L.E. Cloud-Based Surveillance, Connectivity, and Distribution of the GeneXpert Analyzers for Diagnosis of Tuberculosis (TB) and Multiple-Drug-Resistant TB in South Africa. In Molecular Microbiology; Wiley: New York, NY, USA, 2016; pp. 707–718. [Google Scholar]
- National Department of Health (NDOH); National Health Laboratory Service (NHLS). Primary Health Care (PHC) Laboratory Handbook. Available online: https://knowledgehub.health.gov.za/system/files/elibdownloads/2023-04/PHC%252520Laboratory%252520Handbook%25252022%252520May%2525202018%252520Lo%252520Res.pdf (accessed on 14 September 2021).
- Cohen, L. The Use and Impacts of a Corporate Data Warehouse: The Case of the National Health Laboratory Service. Master’s Thesis, University of the Witwatersrand, Johannesburg, South Africa, 2018. Available online: https://wiredspace.wits.ac.za/server/api/core/bitstreams/dbd44d5e-6564-4634-aab3-0c6f17c2d6cf/content (accessed on 14 September 2021).
- Scott, L.E.; Beylis, N.; Nicol, M.; Nkuna, G.; Molapo, S.; Berrie, L.; Duse, A.; Stevens, W.S. Diagnostic accuracy of Xpert MTB/RIF for extrapulmonary tuberculosis specimens: Establishing a laboratory testing algorithm for South Africa. J. Clin. Microbiol. 2014, 52, 1818–1823. [Google Scholar] [CrossRef]
- World Health Organisation (WHO). Automated Real-Time Nucleic Acid Amplification Technology for Rapid and Simultaneous Detection of Tuberculosis and Rifampicin Resistance: Xpert MTB/RIF Assay for the Diagnosis of Pulmonary and Extrapulmonary TB in Adults and Children: Policy Update. Available online: https://www.who.int/publications/i/item/9789241506335 (accessed on 31 March 2022).
- World Health Organisation (WHO). WHO Meeting Report of a Technical Expert Consultation: Non-Inferiority Analysis of Xpert MTB/RIF Ultra Compared to Xpert MTB/RIF. Available online: https://apps.who.int/iris/bitstream/handle/10665/254792/WHO-HTM-TB-20?sequence=1 (accessed on 10 May 2023).
- Dorman, S.E.; Schumacher, S.G.; Alland, D.; Nabeta, P.; Armstrong, D.T.; King, B.; Hall, S.L.; Chakravorty, S.; Cirillo, D.M.; Tukvadze, N. Xpert MTB/RIF Ultra for detection of Mycobacterium tuberculosis and rifampicin resistance: A prospective multicentre diagnostic accuracy study. Lancet Infect. Dis. 2018, 18, 76–84. [Google Scholar] [CrossRef]
- World Health Organisation (WHO). WHO Consolidated Guidelines on Tuberculosis: Module 3: Diagnosis—Rapid Diagnostics for Tuberculosis Detection. Available online: https://www.ncbi.nlm.nih.gov/books/NBK572344/ (accessed on 10 May 2023).
- National Department of Health (NDOH). National Tuberculosis Management Guidelines. Available online: https://www.nicd.ac.za/assets/files/National%20TB%20management%20guidelines%202014.pdf (accessed on 14 September 2021).
- National Health Laboratory Service (NHLS). Annual Performance Plan: Fiscal Year 2023–2024. Available online: https://static.pmg.org.za/NHLS_APP_2023-24.pdf (accessed on 10 May 2023).
- Statistics South Africa (STATS SA). P0302—Mid-Year Population Estimates, 2022: Provincial Projection by Sex and Age (2002–2022). Available online: https://www.statssa.gov.za/?page_id=1854&PPN=P0302&SCH=73305 (accessed on 26 September 2023).
- Statistics South Africa (STATS SA). 2011 Census: Statistical Release. Available online: http://www.statssa.gov.za/census/census_2011/census_products/Census_2011_Statistical%20release.pdf (accessed on 31 July 2023).
- Andrews, J.R.; Cobelens, F.; Horsburgh, C.R.; Hatherill, M.; Basu, S.; Hermans, S.; Wood, R. Seasonal drivers of tuberculosis: Evidence from over 100 years of notifications in Cape Town. Int. J. Tuberc. Lung Dis. 2020, 24, 477–484. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, J.; Hou, S.; Lu, X.; Yang, C.; Pi, Q.; Zhang, M.; Liu, X.; Da, Q.; Zhou, L. Spatial-temporal analysis of pulmonary tuberculosis in Hubei Province, China, 2011–2021. PLoS ONE 2023, 18, e0281479. [Google Scholar] [CrossRef]
- World Health Organisation (WHO). Tuberculosis Profile: South Africa. Available online: https://worldhealthorg.shinyapps.io/tb_profiles/?_inputs_&entity_type=%22country%22&lan=%22EN%22&iso2=%22ZA%22 (accessed on 10 May 2023).
- National Institute for Communicable Diseases (NICD). Turning the Tide on TB. Available online: https://www.nicd.ac.za/turning-the-tide-on-tb/ (accessed on 14 February 2023).
- Ismail, N.A.; Mvusi, L.; Nanoo, A.; Dreyer, A.; Omar, S.V.; Babatunde, S.; Molebatsi, T.; van der Walt, M.; Adelekan, A.; Deyde, V.; et al. Prevalence of drug-resistant tuberculosis and imputed burden in South Africa: A national and sub-national cross-sectional survey. Lancet Infect. Dis. 2018, 18, 779–787. [Google Scholar] [CrossRef]
- McLaren, Z.M.; Brouwer, E.; Ederer, D.; Fischer, K.; Branson, N. Gender patterns of tuberculosis testing and disease in South Africa. Int. J. Tuberc. Lung Dis. 2015, 19, 104–110. [Google Scholar] [CrossRef]
- Bonadonna, L.V.; Saunders, M.J.; Zegarra, R.; Evans, C.; Alegria-Flores, K.; Guio, H. Why wait? The social determinants underlying tuberculosis diagnostic delay. PLoS ONE 2017, 12, e0185018. [Google Scholar] [CrossRef]
- Cucinotta, D.; Vanelli, M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020, 91, 157–160. [Google Scholar] [CrossRef]
- National Institute for Communicable Diseases (NICD). First Case of COVID-19 Announced—An Update. Available online: https://www.nicd.ac.za/first-case-of-covid-19-announced-an-update/ (accessed on 14 February 2022).
- Abdool Karim, S.S. The South African Response to the Pandemic. N. Engl. J. Med. 2020, 382, e95. [Google Scholar] [CrossRef]
- Hatefi, S.; Smith, F.; Abou-El-Hossein, K.; Alizargar, J. COVID-19 in South Africa: Lockdown strategy and its effects on public health and other contagious diseases. Public Health 2020, 185, 159–160. [Google Scholar] [CrossRef]
- Khan, M.S.; Rego, S.; Rajal, J.B.; Bond, V.; Fatima, R.K.; Isani, A.K.; Sutherland, J.; Kranzer, K. Mitigating the impact of COVID-19 on tuberculosis and HIV services: A cross-sectional survey of 669 health professionals in 64 low and middle-income countries. PLoS ONE 2021, 16, e0244936. [Google Scholar] [CrossRef]
- Theron, G.; Venter, R.; Calligaro, G.; Smith, L.; Limberis, J.; Meldau, R.; Chanda, D.; Esmail, A.; Peter, J.; Dheda, K. Xpert MTB/RIF Results in Patients with Previous Tuberculosis: Can We Distinguish True from False Positive Results? Clin. Infect. Dis. 2016, 62, 995–1001. [Google Scholar] [CrossRef]
- Chilukutu, L.; Mwanza, W.; Kerkhoff, A.D.; Somwe, P.; Kagujje, M.; Muyoyeta, M. Prevalence and interpretation of Xpert(®) Ultra trace results among presumptive TB patients. Public Health Action 2022, 12, 28–33. [Google Scholar] [CrossRef]
- De Vos, E.; Scott, L.; Voss De Lima, Y.; Warren, R.M.; Stevens, W.; Hayes, C.; da Silva, P.; Van Rie, A. Management of rifampicin-resistant TB: Programme indicators and care cascade analysis in South Africa. Int. J. Tuberc. Lung Dis. 2021, 25, 134–141. [Google Scholar] [CrossRef]
- Song, R.; Click, E.S.; McCarthy, K.D.; Heilig, C.M.; McHembere, W.; Smith, J.P.; Fajans, M.; Musau, S.K.; Okeyo, E.; Okumu, A.; et al. Sensitive and Feasible Specimen Collection and Testing Strategies for Diagnosing Tuberculosis in Young Children. JAMA Pediatr. 2021, 175, e206069. [Google Scholar] [CrossRef]
- Ahmad, M.; Ibrahim, W.H.; Sarafandi, S.A.; Shahzada, K.S.; Ahmed, S.; Haq, I.U.; Raza, T.; Hameed, M.A.; Thomas, M.; Swehli, H.A.I.; et al. Diagnostic value of bronchoalveolar lavage in the subset of patients with negative sputum/smear and mycobacterial culture and a suspicion of pulmonary tuberculosis. Int. J. Infect. Dis. 2019, 82, 96–101. [Google Scholar] [CrossRef]
- Kay, A.W.; González Fernández, L.; Takwoingi, Y.; Eisenhut, M.; Detjen, A.K.; Steingart, K.R.; Mandalakas, A.M. Xpert MTB/RIF and Xpert MTB/RIF Ultra assays for active tuberculosis and rifampicin resistance in children. Cochrane Database Syst. Rev. 2020, 8, CD013359. [Google Scholar] [CrossRef]
- Bahr, N.C.; Tugume, L.; Rajasingham, R.; Kiggundu, R.; Williams, D.A.; Morawski, B.; Alland, D.; Meya, D.B.; Rhein, J.; Boulware, D.R. Improved diagnostic sensitivity for tuberculous meningitis with Xpert(®) MTB/RIF of centrifuged CSF. Int. J. Tuberc. Lung Dis. 2015, 19, 1209–1215. [Google Scholar] [CrossRef]
- Bahr, N.C.; Marais, S.; Caws, M.; van Crevel, R.; Wilkinson, R.J.; Tyagi, J.S.; Thwaites, G.E.; Boulware, D.R. GeneXpert MTB/Rif to Diagnose Tuberculous Meningitis: Perhaps the First Test but not the Last. Clin. Infect. Dis. 2016, 62, 1133–1135. [Google Scholar] [CrossRef]
- Qiu, Y.R.; Chen, Y.Y.; Wu, X.R.; Li, Y.P.; Cao, X.J.; Yu, Z.Y.; Lin, M.; Li, Q.Y.; Chen, J.C.; Yin, X.; et al. Accuracy of Xpert MTB/RIF assay for the diagnosis of tuberculous pleural effusion. J. Clin. Lab. Anal. 2022, 36, e24185. [Google Scholar] [CrossRef]
- Friedrich, S.O.; von Groote-Bidlingmaier, F.; Diacon, A.H. Xpert MTB/RIF assay for diagnosis of pleural tuberculosis. J. Clin. Microbiol. 2011, 49, 4341–4342. [Google Scholar] [CrossRef]
- Murongazvombo, A.S.; Dlodlo, R.A.; Shewade, H.D.; Robertson, V.; Hirao, S.; Pikira, E.; Zhanero, C.; Taruvinga, R.K.; Andifasi, P.; Tshuma, C. Where, when, and how many tuberculosis patients are lost from presumption until treatment initiation? A step by step assessment in a rural district in Zimbabwe. Int. J. Infect. Dis. 2019, 78, 113–120. [Google Scholar] [CrossRef]
- Subbaraman, R.; Jhaveri, T.; Nathavitharana, R.R. Closing gaps in the tuberculosis care cascade: An action-oriented research agenda. J. Clin. Tuberc. Other Mycobact. Dis. 2020, 19, 100144. [Google Scholar] [CrossRef]
- Naidoo, P.; Theron, G.; Rangaka, M.X.; Chihota, V.N.; Vaughan, L.; Brey, Z.O.; Pillay, Y. The South African Tuberculosis Care Cascade: Estimated Losses and Methodological Challenges. J. Infect. Dis. 2017, 216, S702–S713. [Google Scholar] [CrossRef]
- Kendall, E.A.; Shrestha, S.; Dowdy, D.W. The Epidemiological Importance of Subclinical Tuberculosis. A Critical Reappraisal. Am. J. Respir. Crit. Care Med. 2021, 203, 168–174. [Google Scholar] [CrossRef]
- Times Live. Western Cape Health Authorities to Test Everyone at High Risk of TB. Available online: https://www.timeslive.co.za/news/south-africa/2023-03-24-western-cape-health-authorities-to-test-everyone-at-high-risk-of-tb/ (accessed on 14 May 2023).
- Spotlight NSP. New TB Testing Strategy Ups Diagnosis in Clinics by 17%. Available online: https://www.spotlightnsp.co.za/2021/03/23/new-tb-testing-strategy-ups-diagnosis-in-clinics-by-17/ (accessed on 14 May 2023).
- Martinson, N.A.; Nonyane, B.A.; Genade, L.P.; Berhanu, R.H.; Naidoo, P.; Brey, Z.; Kinghorn, A.; Nyathi, S.; Young, K.; Hausler, H. A cluster randomized trial of systematic targeted universal testing for tuberculosis in primary care clinics of South Africa (The TUTT Study). PLoS Med. 2023, 20, e1004237. [Google Scholar] [CrossRef]


| Year | Specimens Tested n = (%) | % Annual Change from Previous Year & | MTBC Detected n = (%) | MTBC Not Detected n = (%) | Unsuccessful Test |
|---|---|---|---|---|---|
| 2011 | 188,754 (0.8) | 29,947 (15.9) | 155,904 (82.6) | 2903 (1.5) | |
| 2012 | 636,241 (2.7) | 237.1% | 90,279 (14.2) | 535,694 (84.2) | 10,268 (1.6) |
| 2013 | 1,786,862 (7.5) | 180.8% | 208,645 (11.7) | 1,530,727 (85.7) | 47,490 (2.7) |
| 2014 | 2,384,710 (10.0) | 33.5% | 249,708 (10.5) | 2,075,309 (87.0) | 59,693 (2.5) |
| 2015 | 2,643,514 (11.1) | 10.9% | 245,393 (9.3) | 2,341,811 (88.6) | 56,310 (2.1) |
| 2016 | 2,416,517 (10.2) | −8.6% | 223,454 (9.2) | 2,144,461 (88.7) | 48,602 (2.0) |
| 2017 | 2,197,555 (9.3) | −9.1% | 208,924 (9.5) | 1,953,514 (88.9) | 33,475 (1.5) |
| 2018 | 2,199,299 (9.3) | 0.1% | 214,044 (9.7) | 1,910,477 (86.9) | 37,711 (1.7) |
| 2019 | 2,179,502 (9.2) | −0.9% | 197,138 (9.0) | 1,901,765 (87.3) | 43,753 (2.0) |
| 2020 | 1,690,520 (7.1) | −22.4% | 153,791 (9.1) | 1,478,496 (87.5) | 32,854 (1.9) |
| 2021 | 2,037,432 (8.6) | 20.5% | 163,641 (8.0) | 1,820,550 (89.4) | 26,987 (1.3) |
| 2022 | 2,534,050 (10.7) | 24.4% | 185,252 (7.3) | 2,285,341 (90.2) | 31,322 (1.2) |
| 2023 | 845,712 (3.6) | 58,261 (6.9) | 768,947 (90.9) | 8123 (1.0) | |
| Total | 23,740,668 (100.0) | 2,228,477 (9.4) | 20,902,996 (88.0) | 439,491 (1.9) |
| Year | MTBC Detected n = (%) | RIF Resistance Detected n = (%) | RIF Resistance Not Detected n = (%) | RIF Unsuccessful n = (%) |
|---|---|---|---|---|
| 2011 | 29,947 (15.9) | 2124 (7.1) | 27,422 (91.6) | 293 (1.0) |
| 2012 | 90,279 (14.2) | 6546 (7.3) | 81,810 (90.6) | 1285 (1.4) |
| 2013 | 208,645 (11.7) | 13,802 (6.6) | 188,383 (90.3) | 5380 (2.6) |
| 2014 | 249,708 (10.5) | 16,316 (6.5) | 226,941 (90.9) | 6101 (2.4) |
| 2015 | 245,393 (9.3) | 14,999 (6.1) | 226,898 (92.5) | 3296 (1.3) |
| 2016 | 223,454 (9.2) | 13,815 (6.2) | 207,105 (92.7) | 2395 (1.1) |
| 2017 | 208,924 (9.5) | 12,183 (5.8) | 194,384 (93.0) | 2278 (1.1) |
| 2018 | 214,044 (9.7) | 11,375 (5.3) | 198,321 (92.7) | 4282 (2.0) |
| 2019 | 197,138 (9.0) | 10,431 (5.3) | 180,550 (91.6) | 6132 (3.1) |
| 2020 | 153,791 (9.1) | 8129 (5.3) | 140,636 (91.4) | 5017 (3.3) |
| 2021 | 163,641 (8.0) | 8169 (5.0) | 150,773 (92.1) | 4677 (2.9) |
| 2022 | 185,252 (7.3) | 9375 (5.1) | 170,363 (92.0) | 5502 (3.0) |
| 2023 | 58,261 (6.9) | 3189 (5.5) | 53,257 (91.4) | 1814 (3.1) |
| Total | 2,228,477 (9.4) # | 130,453 (5.9) | 2,046,843 (91.8) | 48,452 (2.2) |
| Province | 2012 | 2022 | Change in MTBC Detected Rate (%) | Change in Population (%) | Change in MTBC Detected per 100,000 Population | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Specimens Tested n = (%) | MTBC Detected n = (%) | Population n = (%) | MTBC Detected per 100,000 Population | Specimens Tested n = (%) | MTBC Detected n = (%) | Population n = (%) | MTBC Detected per 100,000 Population | ||||
| EC | 104,574 (16.4) | 16,501 (15.8) | 6,594,537 (12.5) | 250 | 396,001 (15.6) | 43,778 (11.1) | 6,676,691 (11.0) | 656 | −4.7 | 1.2 | 406 |
| FS | 88,222 (13.9) | 11,501 (13.0) | 2,793,604 (5.3) | 412 | 73,544 (2.9) | 8119 (11.0) | 2,921,611 (4.8) | 278 | −2.0 | 4.6 | −134 |
| GP | 84,200 (13.2) | 11,137 (13.2) | 12,630,422 (24.0) | 88 | 377,698 (14.9) | 22,620 (6.0) | 16,098,571 (26.6) | 141 | −7.2 | 27.5 | 53 |
| KZN | 160,732 (25.3) | 22,032 (13.7) | 10,406,665 (19.7) | 212 | 955,702 (37.7) | 35,870 (3.8) | 11,538,325 (19.0) | 311 | −9.9 | 10.9 | 99 |
| LP | 35,417 (5.6) | 3937 (11.1) | 5,447,963 (10.3) | 72 | 119,198 (4.7) | 7074 (5.9) | 5,941,439 (9.8) | 119 | −5.2 | 9.1 | 47 |
| MP | 26,874 (4.2) | 4003 (14.9) | 4,114,293 (7.8) | 97 | 146,530 (5.8) | 7306 (5.0) | 4,720,497 (7.8) | 155 | −9.9 | 14.7 | 58 |
| NW | 37,456 (5.9) | 5398 (14.4) | 3,574,090 (6.8) | 151 | 101,860 (4.0) | 10,324 (10.1) | 4,186,984 (6.9) | 247 | −4.3 | 17.1 | 96 |
| NC | 25,292 (4.0) | 3800 (15.0) | 1,164,483 (2.2) | 326 | 73,137 (2.9) | 8345 (11.4) | 1,308,734 (2.2) | 638 | −3.6 | 12.4 | 312 |
| WC | 73,474 (11.5) | 11,970 (16.3) | 5,973,197 (11.3) | 200 | 290,380 (11.5) | 41,816 (14.4) | 7,212,142 (11.9) | 580 | −1.9 | 20.7 | 380 |
| Total | 636,241 (100.0) | 90,279 (14.2) | 52,699,253 (100.0) | 171 | 2,534,050 (100.0) | 185,252 (7.3) | 60,604,992 (100.0) | 306 | −6.9 | 15.0 | 135 |
| Province | 2012 | 2022 | Change in RIF Resistance Detected Rate (%) | Change in Population (%) | Change in RIF Resistance per 100,000 Population | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MTBC Detected n = (%) | RIF Resistance Detected n = (%) | Population n = (%) | RIF Resistance per 100,000 Population | MTBC Detected n = (%) | RIF Resistance Detected n = (%) | Population n = (%) | RIF Resistance per 100,000 Population | ||||
| EC | 16,501 (18.3) | 1118 (6.8) | 6,594,537 (12.5) | 17 | 43,778 (23.6) | 2311 (5.3) | 6,676,691 (11.0) | 35 | −1.5% | 1.2 | 18 |
| FS | 11,501 (12.7) | 734 (6.4) | 2,793,604 (5.3) | 26 | 8119 (4.4) | 303 (3.7) | 2,921,611 (4.8) | 10 | −2.7% | 4.6 | 16 |
| GP | 11,137 (12.3) | 773 (6.9) | 12,630,422 (24.0) | 6 | 22,620 (12.2) | 1024 (4.5) | 16,098,571 (26.6) | 6 | −2.4% | 27.5 | 0 |
| KZN | 22,032 (24.4) | 2040 (9.3) | 10,406,665 (19.7) | 20 | 35,870 (19.4) | 2349 (6.5) | 11,538,325 (19.0) | 20 | −2.7% | 10.9 | 0 |
| LP | 3937 (4.4) | 260 (6.6) | 5,447,963 (10.3) | 5 | 7074 (3.8) | 331 (4.7) | 5,941,439 (9.8) | 6 | −1.9% | 9.1 | 1 |
| MP | 4003 (4.4) | 397 (9.9) | 4,114,293 (7.8) | 10 | 7306 (3.9) | 403 (5.5) | 4,720,497 (7.8) | 9 | −4.4% | 14.7 | −1 |
| NW | 5398 (6.0) | 396 (7.3) | 3,574,090 (6.8) | 11 | 10,324 (5.6) | 376 (3.6) | 4,186,984 (6.9) | 9 | −3.7% | 17.1 | −2 |
| NC | 3800 (4.2) | 240 (6.3) | 1,164,483 (2.2) | 21 | 8345 (4.5) | 346 (4.1) | 1,308,734 (2.2) | 26 | −2.2% | 12.4 | 5 |
| WC | 11,970 (13.3) | 588 (4.9) | 5,973,197 (11.3) | 10 | 41,816 (22.6) | 1932 (4.6) | 7,212,142 (11.9) | 27 | −0.3% | 20.7 | 17 |
| Total | 90,279 (100.0) | 6546 (7.3) | 52,699,253 (100.0) | 12 | 185,252 (100.0) | 9375 (5.1) | 60,604,992 (100.0) | 16 | −2.2% | 15.0 | 4 |
| Assay | PTB Specimens Tested n = (%) | EPTB Specimens Tested n = (%) | Specimens of Unknown Origin Tested n = (%) | Total | MTBC Detected for PTB Specimens n = (%) | MTBC Detected for EPTB Specimens n = (%) | MTBC Detected for Specimens of Unknown Origin n = (%) |
|---|---|---|---|---|---|---|---|
| Xpert® MTB/RIF (from April 2011 to 2017/2018 transition) | 11,423,713 (92.8) | 179,345 (1.5) | 712,353 (5.8) | 12,315,411 (51.9) | 1,178,841 (10.3) | 17,122 (9.5) | 66,342 (9.3) |
| Xpert® MTB/RIF Ultra (from 2017/2018 transition to April 2023) | 10,528,081 (92.1) | 505,801 (4.4) | 391,375 (3.4) | 11,425,257 (48.1) | 881,333 (8,4) | 51,388 (10.2) | 33,451 (8.5) |
| Total | 21,951,794 (92.5) | 685,146 (2.9) | 1,103,728 (4.6) | 23,740,668 (100) | 2,060,174 (9.4) | 68,510 (10.0) | 99,793 (9.0) |
| PTB Specimen Type | Tested Specimens n = (%) | MTBC Detected n = (%) | MTBC Not Detected n = (%) | RIF Resistance Detected n = (%) | Unsuccessful Test n = (%) |
|---|---|---|---|---|---|
| Bronchial brushings | 872 (0.0) | 84 (9.6) | 769 (88.2) | 5 (6.0) | 11 (1.3) |
| BAL | 9924 (0.0) | 1133 (11.4) | 8507 (85.7) | 92 (8.1) | 73 (0.7) |
| Gastric aspirate | 191,198 (0.9) | 7702 (4.0) | 179,653 (94.0) | 486 (6.3) | 3123 (1.6) |
| Nasopharyngeal | 1166 (0.0) | 57 (4.9) | 1101 (94.4) | 3 (5.3) | 3 (0.3) |
| Sputum | 21,714,452 (98.9) | 2,048,812 (9.4) | 19,104,211 (88.0) | 119,273 (5.8) | 399,638 (1.8) |
| Tracheal aspirate | 34,182 (0.2) | 2386 (7.0) | 30,971 (90.6) | 130 (5.4) | 388 (1.1) |
| Total | 21,951,794 (100.0) | 2,060,174 (9.4) | 19,325,212 (88.0) | 119,989 (5.8) | 403,236 (1.8) |
| EPTB Specimen Type | Tested Specimens n = (%) | MTBC Detected n = (%) | MTBC Not Detected n = (%) | RIF Resistance Detected n = (%) | Unsuccessful Test n = (%) |
|---|---|---|---|---|---|
| Aspirate/FNA | 15,918 (2.3) | 5700 (35.8) | 9933 (62.4) | 447 (7.8) | 239 (1.5) |
| CSF | 369,251 (53.9) | 12,869 (3.5) | 351,014 (95.1) | 832 (6.5) | 5088 (1.4) |
| Fluid | 220,118 (32.1) | 32,398 (14.7) | 183,796 (83.5) | 1656 (5.1) | 3462 (1.6) |
| Pus/Abscess | 41,909 (6.1) | 11,910 (28.4) | 29,122 (69.5) | 1213 (10.2) | 844 (2.0) |
| Stool # | 458 (0.1) | 36 (7.9) | 415 (90.6) | 3 (8.3) | 7 (1.5) |
| Tissue | 24,014 (3.5) | 4044 (16.8) | 19,722 (82.1) | 243 (6.0) | 209 (0.9) |
| Urine # | 13,478 (2.0) | 1553 (11.5) | 11,589 (86.0) | 75 (4.8) | 332 (2.5) |
| Total | 685,146 (100.0) | 68,510 (10.0) | 605,591 (88.4) | 4469 (6.5) | 10,181 (1.5) |
| EPTB Specimen Type | MTBC Detection Rate by Anatomical Site in Extra-Pulmonary TB n = Specimens Tested (Detection Rate %) | |||||
|---|---|---|---|---|---|---|
| Abdominal # | Lymph Nodes @ | Pericardial | Pleural | Skeletal ^ | Unknown | |
| Aspirate/FNA | 165 (23.6) | 1377 (44.1) | 32 (21.9) | 30 (50.0) | 14,314 (35.2) | |
| Fluid | 2029 (11.4) | 118 (11.9) | 182 (37.9) | 6320 (21.7) | 2792 (4.4) | 208,677 (14.7) |
| Pus/Abscess | 57 (17.5) | 367 (41.4) | 1 (0.0) | 37 (43.2) | 189 (36.0) | 41,258 (28.3) |
| Tissue | 116 (19.8) | 189 (42.9) | 3 (33.3) | 46 (6.5) | 1153 (17.3) | 22,507 (16.6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, M.P.; Cassim, N.; Ndlovu, S.; Marokane, P.S.; Radebe, M.; Shapiro, A.; Scott, L.E.; Stevens, W.S. More Than a Decade of GeneXpert® Mycobacterium tuberculosis/Rifampicin (Ultra) Testing in South Africa: Laboratory Insights from Twenty-Three Million Tests. Diagnostics 2023, 13, 3253. https://doi.org/10.3390/diagnostics13203253
da Silva MP, Cassim N, Ndlovu S, Marokane PS, Radebe M, Shapiro A, Scott LE, Stevens WS. More Than a Decade of GeneXpert® Mycobacterium tuberculosis/Rifampicin (Ultra) Testing in South Africa: Laboratory Insights from Twenty-Three Million Tests. Diagnostics. 2023; 13(20):3253. https://doi.org/10.3390/diagnostics13203253
Chicago/Turabian Styleda Silva, Manuel Pedro, Naseem Cassim, Silence Ndlovu, Puleng Shiela Marokane, Mbuti Radebe, Anne Shapiro, Lesley Erica Scott, and Wendy Susan Stevens. 2023. "More Than a Decade of GeneXpert® Mycobacterium tuberculosis/Rifampicin (Ultra) Testing in South Africa: Laboratory Insights from Twenty-Three Million Tests" Diagnostics 13, no. 20: 3253. https://doi.org/10.3390/diagnostics13203253
APA Styleda Silva, M. P., Cassim, N., Ndlovu, S., Marokane, P. S., Radebe, M., Shapiro, A., Scott, L. E., & Stevens, W. S. (2023). More Than a Decade of GeneXpert® Mycobacterium tuberculosis/Rifampicin (Ultra) Testing in South Africa: Laboratory Insights from Twenty-Three Million Tests. Diagnostics, 13(20), 3253. https://doi.org/10.3390/diagnostics13203253






