Flow Sorting, Whole Genome Amplification and Next-Generation Sequencing as Combined Tools to Study Heterogeneous Acute Lymphoblastic Leukemia
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Sample Preparation for Clonality Detection via Next-Generation Sequencing
2.3. IdentiClone Clonality Detection Based on EuroClonality BIOMED-2
2.4. Cell Sorting via Flowcytometry
2.5. Whole Genome Amplification and DNA Purification
3. Results
3.1. Comparison between EuroClonality NGS and GeneScan for Clonality Assessment
3.2. Clonality Assessment by Using WGA for Low-Input DNA Samples
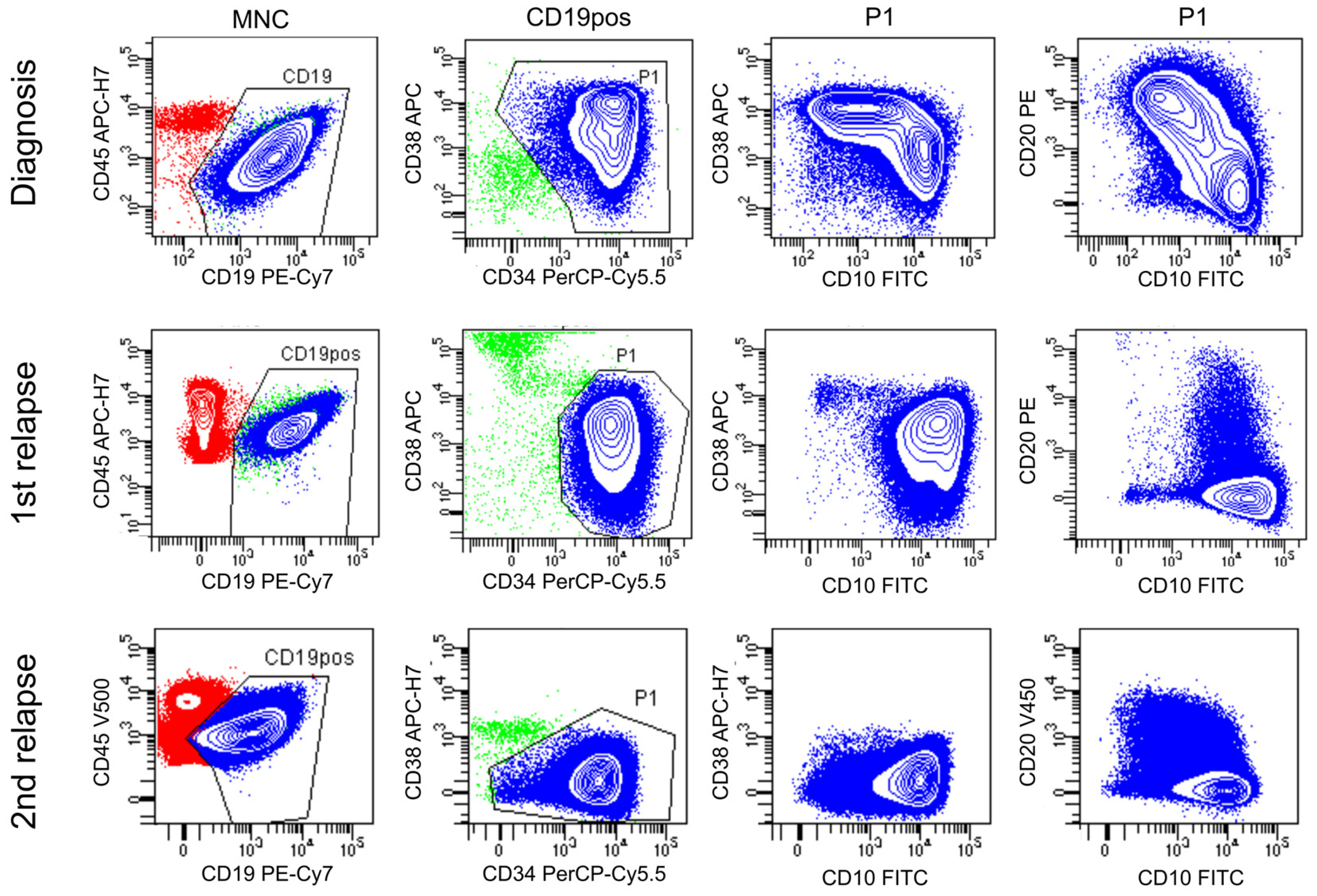
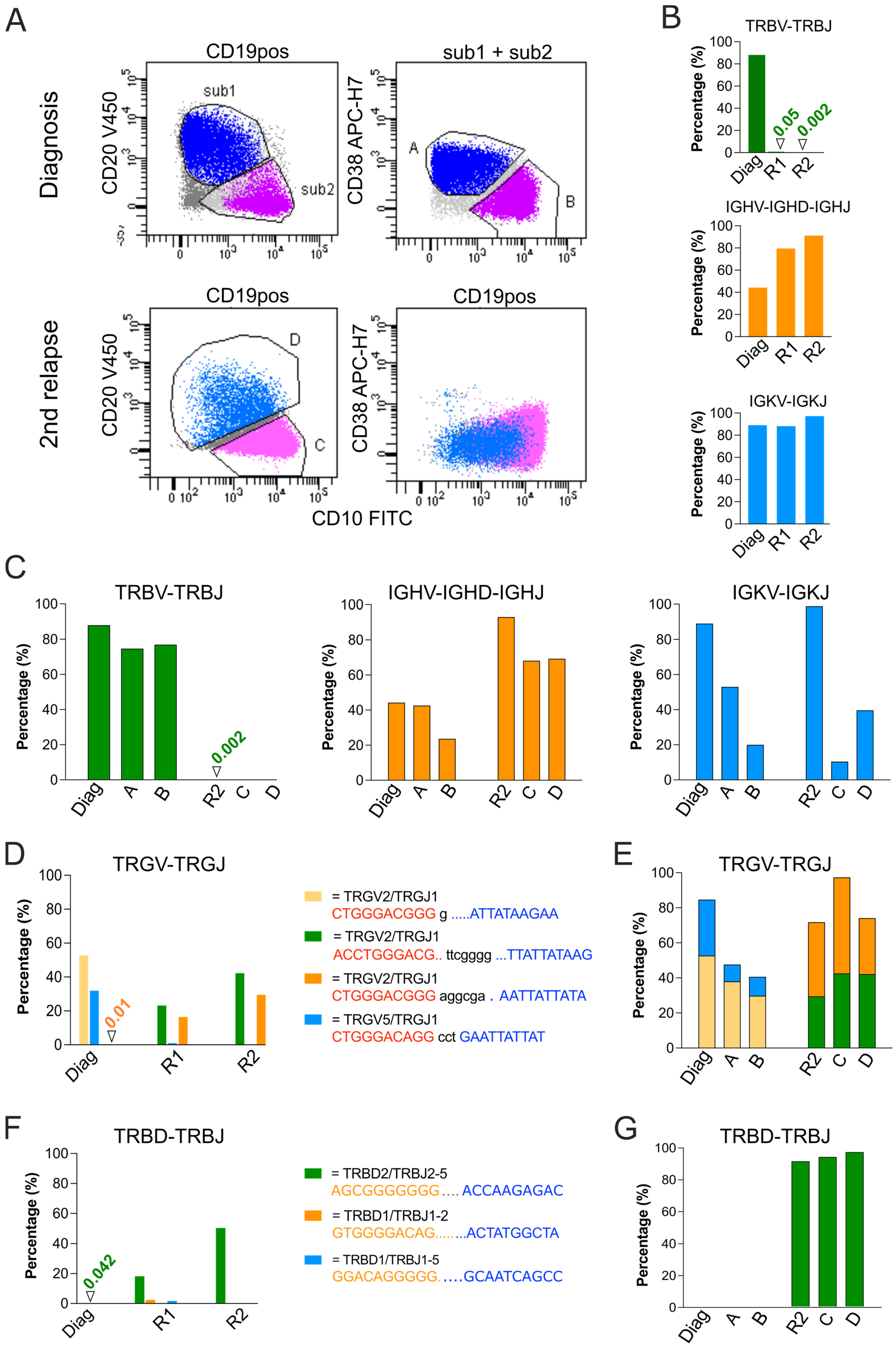
3.3. Analysis of Relapse in a BCP-ALL Patient Using Flow Sorting, WGA and NGS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Inaba, H.; Greaves, M.; Mullighan, C.G. Acute lymphoblastic leukaemia. Lancet 2013, 381, 1943–1955. [Google Scholar]
- van der Velden, V.H.; Hochhaus, A.; Cazzaniga, G.; Szczepanski, T.; Gabert, J.; van Dongen, J.J. Detection of minimal residual disease in hematologic malignancies by real-time quantitative PCR: Principles, approaches, and laboratory aspects. Leukemia 2003, 17, 1013–1034. [Google Scholar] [CrossRef]
- van der Velden, V.H.; van Dongen, J.J. MRD detection in acute lymphoblastic leukemia patients using Ig/TCR gene rearrangements as targets for real-time quantitative PCR. Methods Mol. Biol. 2009, 538, 115–150. [Google Scholar] [CrossRef] [PubMed]
- Øbro, N.F.; Marquart, H.V.; Madsen, H.O.; Ryder, L.P.; Andersen, M.K.; Lausen, B.; Albertsen, B.K.; Wehner, P.S.; Helgestad, J.; Schmiegelow, K. Immunophenotype-defined sub-populations are common at diagnosis in childhood B-cell precursor acute lymphoblastic leukemia. Leukemia 2011, 25, 1652–1657. [Google Scholar] [CrossRef]
- Modvig, S.; Wernersson, R.; Øbro, N.F.; Olsen, L.R.; Christensen, C.; Rosthøj, S.; Degn, M.; Jürgensen, G.W.; Madsen, H.O.; Albertsen, B.K.; et al. High CD34 surface expression in BCP-ALL predicts poor induction therapy response and is associated with altered expression of genes related to cell migration and adhesion. Mol. Oncol. 2022, 16, 2015–2030. [Google Scholar] [CrossRef]
- Szczepański, T. Why and how to quantify minimal residual disease in acute lymphoblastic leukemia? Leukemia 2007, 21, 622–626. [Google Scholar] [CrossRef]
- Szczepański, T.; Willemse, M.J.; Brinkhof, B.; van Wering, E.R.; van der Burg, M.; van Dongen, J.J. Comparative analysis of Ig and TCR gene rearrangements at diagnosis and at relapse of childhood precursor-B-ALL provides improved strategies for selection of stable PCR targets for monitoring of minimal residual disease. Blood 2002, 99, 2315–2323. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Modvig, S.; Jeyakumar, J.; Marquart, H.V.; Christensen, C. Integrins and the Metastasis-like Dissemination of Acute Lymphoblastic Leukemia to the Central Nervous System. Cancers 2023, 15, 2504. [Google Scholar] [CrossRef]
- Bartram, J.; Goulden, N.; Wright, G.; Adams, S.; Brooks, T.; Edwards, D.; Inglott, S.; Yousafzai, Y.; Hubank, M.; Halsey, C. High throughput sequencing in acute lymphoblastic leukemia reveals clonal architecture of central nervous system and bone marrow compartments. Haematologica 2018, 103, e110–e114. [Google Scholar] [CrossRef] [PubMed]
- van Dongen, J.J.; Langerak, A.W.; Brüggemann, M.; Evans, P.A.; Hummel, M.; Lavender, F.L.; Delabesse, E.; Davi, F.; Schuuring, E.; García-Sanz, R.; et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: Report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia 2003, 17, 2257–2317. [Google Scholar] [CrossRef]
- Di Lorenzo, B.; Ravens, S.; Silva-Santos, B. High-throughput analysis of the human thymic Vδ1+ T cell receptor repertoire. Sci. Data 2019, 6, 115. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, A.M.; Desmarais, C.; Livingston, R.J.; Andriesen, J.; Haussler, M.; Carlson, C.S.; Robins, H. Deep sequencing of the human TCRγ and TCRβ repertoires suggests that TCRβ rearranges after αβ and γδ T cell commitment. Sci. Transl. Med. 2011, 3, 90ra61. [Google Scholar] [CrossRef]
- Turchaninova, M.A.; Davydov, A.; Britanova, O.V.; Shugay, M.; Bikos, V.; Egorov, E.S.; Kirgizova, V.I.; Merzlyak, E.M.; Staroverov, D.B.; Bolotin, D.A.; et al. High-quality full-length immunoglobulin profiling with unique molecular barcoding. Nat. Protoc. 2016, 11, 1599–1616. [Google Scholar] [CrossRef]
- Jiang, Y.; Redmond, D.; Nie, K.; Eng, K.W.; Clozel, T.; Martin, P.; Tan, L.H.; Melnick, A.M.; Tam, W.; Elemento, O. Deep sequencing reveals clonal evolution patterns and mutation events associated with relapse in B-cell lymphomas. Genome Biol. 2014, 15, 432. [Google Scholar] [CrossRef]
- Nadeu, F.; Clot, G.; Delgado, J.; Martín-García, D.; Baumann, T.; Salaverria, I.; Beà, S.; Pinyol, M.; Jares, P.; Navarro, A.; et al. Clinical impact of the subclonal architecture and mutational complexity in chronic lymphocytic leukemia. Leukemia 2018, 32, 645–653. [Google Scholar] [CrossRef]
- Bashford-Rogers, R.J.; Nicolaou, K.A.; Bartram, J.; Goulden, N.J.; Loizou, L.; Koumas, L.; Chi, J.; Hubank, M.; Kellam, P.; Costeas, P.A.; et al. Eye on the B-ALL: B-cell receptor repertoires reveal persistence of numerous B-lymphoblastic leukemia subclones from diagnosis to relapse. Leukemia 2016, 30, 2312–2321. [Google Scholar] [CrossRef]
- Wu, D.; Sherwood, A.; Fromm, J.R.; Winter, S.S.; Dunsmore, K.P.; Loh, M.L.; Greisman, H.A.; Sabath, D.E.; Wood, B.L.; Robins, H. High-throughput sequencing detects minimal residual disease in acute T lymphoblastic leukemia. Sci. Transl. Med. 2012, 4, 134ra163. [Google Scholar] [CrossRef] [PubMed]
- Faham, M.; Zheng, J.; Moorhead, M.; Carlton, V.E.; Stow, P.; Coustan-Smith, E.; Pui, C.H.; Campana, D. Deep-sequencing approach for minimal residual disease detection in acute lymphoblastic leukemia. Blood 2012, 120, 5173–5180. [Google Scholar] [CrossRef]
- Salson, M.; Giraud, M.; Caillault, A.; Grardel, N.; Duployez, N.; Ferret, Y.; Duez, M.; Herbert, R.; Rocher, T.; Sebda, S.; et al. High-throughput sequencing in acute lymphoblastic leukemia: Follow-up of minimal residual disease and emergence of new clones. Leuk. Res. 2017, 53, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Theunissen, P.M.J.; de Bie, M.; van Zessen, D.; de Haas, V.; Stubbs, A.P.; van der Velden, V.H.J. Next-generation antigen receptor sequencing of paired diagnosis and relapse samples of B-cell acute lymphoblastic leukemia: Clonal evolution and implications for minimal residual disease target selection. Leuk. Res. 2019, 76, 98–104. [Google Scholar] [CrossRef]
- Brüggemann, M.; Kotrová, M.; Knecht, H.; Bartram, J.; Boudjogrha, M.; Bystry, V.; Fazio, G.; Froňková, E.; Giraud, M.; Grioni, A.; et al. Standardized next-generation sequencing of immunoglobulin and T-cell receptor gene recombinations for MRD marker identification in acute lymphoblastic leukaemia; a EuroClonality-NGS validation study. Leukemia 2019, 33, 2241–2253. [Google Scholar] [CrossRef]
- Knecht, H.; Reigl, T.; Kotrová, M.; Appelt, F.; Stewart, P.; Bystry, V.; Krejci, A.; Grioni, A.; Pal, K.; Stranska, K.; et al. Quality control and quantification in IG/TR next-generation sequencing marker identification: Protocols and bioinformatic functionalities by EuroClonality-NGS. Leukemia 2019, 33, 2254–2265. [Google Scholar] [CrossRef] [PubMed]
- Toft, N.; Birgens, H.; Abrahamsson, J.; Griškevičius, L.; Hallböök, H.; Heyman, M.; Klausen, T.W.; Jónsson, Ó.G.; Palk, K.; Pruunsild, K.; et al. Results of NOPHO ALL2008 treatment for patients aged 1–45 years with acute lymphoblastic leukemia. Leukemia 2018, 32, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Modvig, S.; Hallböök, H.; Madsen, H.O.; Siitonen, S.; Rosthøj, S.; Tierens, A.; Juvonen, V.; Osnes, L.T.N.; Vålerhaugen, H.; Hultdin, M.; et al. Value of flow cytometry for MRD-based relapse prediction in B-cell precursor ALL in a multicenter setting. Leukemia 2021, 35, 1894–1906. [Google Scholar] [CrossRef]
- Theunissen, P.; Mejstrikova, E.; Sedek, L.; van der Sluijs-Gelling, A.J.; Gaipa, G.; Bartels, M.; Sobral da Costa, E.; Kotrová, M.; Novakova, M.; Sonneveld, E.; et al. Standardized flow cytometry for highly sensitive MRD measurements in B-cell acute lymphoblastic leukemia. Blood 2017, 129, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Bystry, V.; Reigl, T.; Krejci, A.; Demko, M.; Hanakova, B.; Grioni, A.; Knecht, H.; Schlitt, M.; Dreger, P.; Sellner, L.; et al. ARResT/Interrogate: An interactive immunoprofiler for IG/TR NGS data. Bioinformatics 2017, 33, 435–437. [Google Scholar] [CrossRef]
- Szczepanski, T.; van der Velden, V.H.; Hoogeveen, P.G.; de Bie, M.; Jacobs, D.C.; van Wering, E.R.; van Dongen, J.J. Vdelta2-Jalpha rearrangements are frequent in precursor-B-acute lymphoblastic leukemia but rare in normal lymphoid cells. Blood 2004, 103, 3798–3804. [Google Scholar] [CrossRef]
- Scheijen, B.; Meijers, R.W.J.; Rijntjes, J.; van der Klift, M.Y.; Möbs, M.; Steinhilber, J.; Reigl, T.; van den Brand, M.; Kotrová, M.; Ritter, J.M.; et al. Next-generation sequencing of immunoglobulin gene rearrangements for clonality assessment: A technical feasibility study by EuroClonality-NGS. Leukemia 2019, 33, 2227–2240. [Google Scholar] [CrossRef]
- Kotrova, M.; Trka, J.; Kneba, M.; Brüggemann, M. Is Next-Generation Sequencing the way to go for Residual Disease Monitoring in Acute Lymphoblastic Leukemia? Mol. Diagn. Ther. 2017, 21, 481–492. [Google Scholar] [CrossRef]
- Paulsen, K.; Marincevic, M.; Cavelier, L.; Hollander, P.; Amini, R.M. LymphoTrack is Equally Sensitive as PCR GeneScan and Sanger Sequencing for Detection of Clonal Rearrangements in ALL Patients. Diagnostics 2022, 12, 1389. [Google Scholar] [CrossRef]
- van den Brand, M.; Rijntjes, J.; Möbs, M.; Steinhilber, J.; van der Klift, M.Y.; Heezen, K.C.; Kroeze, L.I.; Reigl, T.; Porc, J.; Darzentas, N.; et al. Next-Generation Sequencing-Based Clonality Assessment of Ig Gene Rearrangements: A Multicenter Validation Study by EuroClonality-NGS. J. Mol. Diagn. 2021, 23, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Darzentas, F.; Szczepanowski, M.; Kotrová, M.; Hartmann, A.; Beder, T.; Gökbuget, N.; Schwartz, S.; Bastian, L.; Baldus, C.D.; Pál, K.; et al. Insights into IGH clonal evolution in BCP-ALL: Frequency, mechanisms, associations, and diagnostic implications. Front. Immunol. 2023, 14, 1125017. [Google Scholar] [CrossRef] [PubMed]
- Modvig, S.; Madsen, H.O.; Siitonen, S.M.; Rosthøj, S.; Tierens, A.; Juvonen, V.; Osnes, L.T.N.; Vålerhaugen, H.; Hultdin, M.; Thörn, I.; et al. Minimal residual disease quantification by flow cytometry provides reliable risk stratification in T-cell acute lymphoblastic leukemia. Leukemia 2019, 33, 1324–1336. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.E.; Triboulet, M.; Zia, A.; Vuppalapaty, M.; Kidess-Sigal, E.; Coller, J.; Natu, V.S.; Shokoohi, V.; Che, J.; Renier, C.; et al. Workflow optimization of whole genome amplification and targeted panel sequencing for CTC mutation detection. NPJ Genom. Med. 2017, 2, 34. [Google Scholar] [CrossRef] [PubMed]
- Sabina, J.; Leamon, J.H. Bias in Whole Genome Amplification: Causes and Considerations. Methods Mol. Biol. 2015, 1347, 15–41. [Google Scholar] [CrossRef]
- Nuria Estévez-Gómez, T.P.; Guillaumet-Adkins, A.; Heyn, H.; Prado-López, S.; Posada, D. Comparison of single-cell whole-genome amplification strategies. bioRxiv 2018, 443754. [Google Scholar] [CrossRef]
- Huang, L.; Ma, F.; Chapman, A.; Lu, S.; Xie, X.S. Single-Cell Whole-Genome Amplification and Sequencing: Methodology and Applications. Annu. Rev. Genom. Hum. Genet. 2015, 16, 79–102. [Google Scholar] [CrossRef]
- Pinard, R.; de Winter, A.; Sarkis, G.J.; Gerstein, M.B.; Tartaro, K.R.; Plant, R.N.; Egholm, M.; Rothberg, J.M.; Leamon, J.H. Assessment of whole genome amplification-induced bias through high-throughput, massively parallel whole genome sequencing. BMC Genom. 2006, 7, 216. [Google Scholar] [CrossRef]
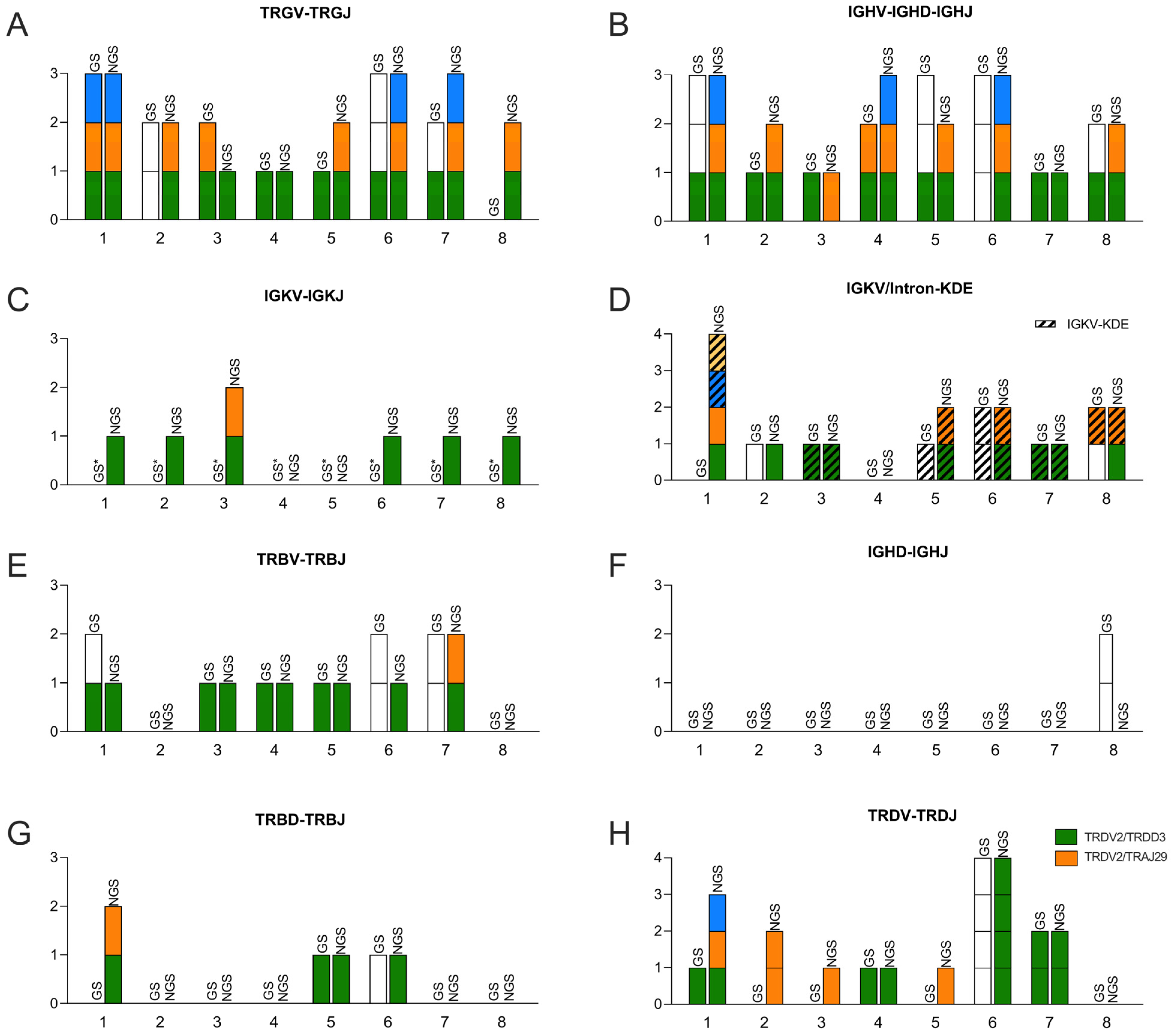
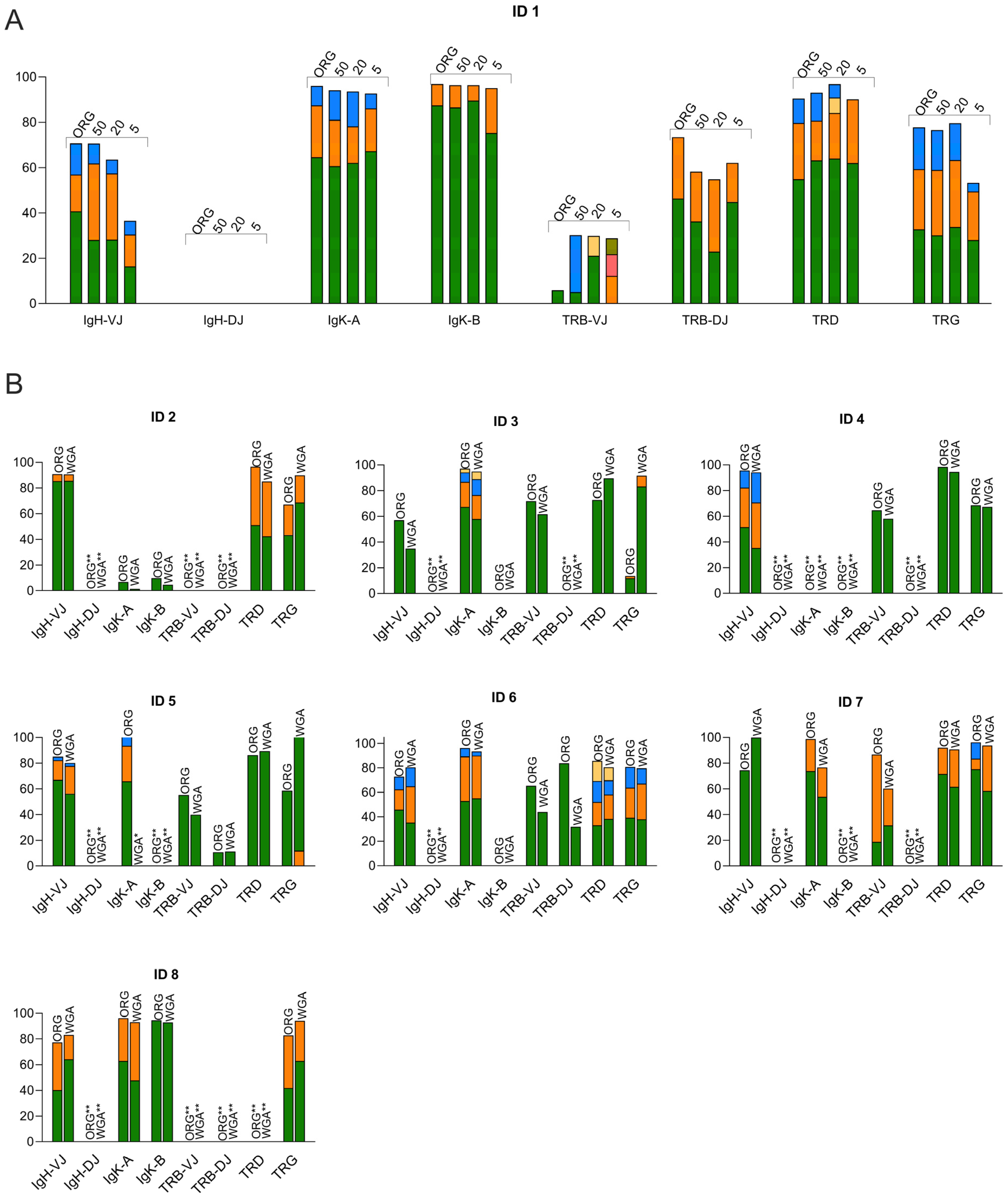
| IGH VDJ | IGH DJ | IGK VJ + IGK V/Intron-Kde | ||||||
|---|---|---|---|---|---|---|---|---|
| GS | NGS | GS | NGS | GS | NGS | NGS | ||
| ID 1 | 3 | 3 | P | - | P | 1 | 4 | |
| ID 2 | 1 | 2 | P | - | 1 | 1 | 1 | |
| ID 3 | 1 | 1 | P | - | 1 | 2 | 1 | |
| ID 4 | 2 | 3 | P | - | P | - | - | |
| ID 5 | 3 | 2 PB | P | - | 1 | - | 2 | |
| ID 6 | 3 | 3 PB | P | - | 2 | 1 | 2 | |
| ID 7 | 1 | 1 | P | - | 1 | 1 | 1 | |
| ID 8 | 2 | 2 | 2 | - | 2 | 1 | 2 | |
| TRB VJ | TRB DJ | TRG VJ | TRD VJ | |||||
| GS | NGS | GS | NGS | GS | NGS | GS | NGS | |
| ID 1 | 2 | 1 | P | 2 | 3 | 3 | 1 | 3 |
| ID 2 | P | - | P | - | 2 | 2 | P | 2 |
| ID 3 | 1 | 1 | P | - | 2 | 1 | P | 1 |
| ID 4 | 1 | 1 | P | - | 1 | 1 | 1 | 1 |
| ID 5 | 1 | 1 | 1 | 1 | 1 PB | 2 | P | 1 |
| ID 6 | 2 | 1 | 1 | 1 | 3 | 3 | 4 O | 4 |
| ID 7 | 2 | 2 | P | - | 2 | 3 | 2 | 2 |
| ID 8 | P | - | P | - | P | 2 | P | P |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fardoos, R.; Christensen, C.; Øbro, N.F.; Overgaard, U.M.; Als-Nielsen, B.; Madsen, H.O.; Marquart, H.V. Flow Sorting, Whole Genome Amplification and Next-Generation Sequencing as Combined Tools to Study Heterogeneous Acute Lymphoblastic Leukemia. Diagnostics 2023, 13, 3306. https://doi.org/10.3390/diagnostics13213306
Fardoos R, Christensen C, Øbro NF, Overgaard UM, Als-Nielsen B, Madsen HO, Marquart HV. Flow Sorting, Whole Genome Amplification and Next-Generation Sequencing as Combined Tools to Study Heterogeneous Acute Lymphoblastic Leukemia. Diagnostics. 2023; 13(21):3306. https://doi.org/10.3390/diagnostics13213306
Chicago/Turabian StyleFardoos, Rabiah, Claus Christensen, Nina Friesgaard Øbro, Ulrik Malthe Overgaard, Bodil Als-Nielsen, Hans Ole Madsen, and Hanne Vibeke Marquart. 2023. "Flow Sorting, Whole Genome Amplification and Next-Generation Sequencing as Combined Tools to Study Heterogeneous Acute Lymphoblastic Leukemia" Diagnostics 13, no. 21: 3306. https://doi.org/10.3390/diagnostics13213306
APA StyleFardoos, R., Christensen, C., Øbro, N. F., Overgaard, U. M., Als-Nielsen, B., Madsen, H. O., & Marquart, H. V. (2023). Flow Sorting, Whole Genome Amplification and Next-Generation Sequencing as Combined Tools to Study Heterogeneous Acute Lymphoblastic Leukemia. Diagnostics, 13(21), 3306. https://doi.org/10.3390/diagnostics13213306





