A Puzzling Mast Cell Trilogy: Anaphylaxis, MCAS, and Mastocytosis
Abstract
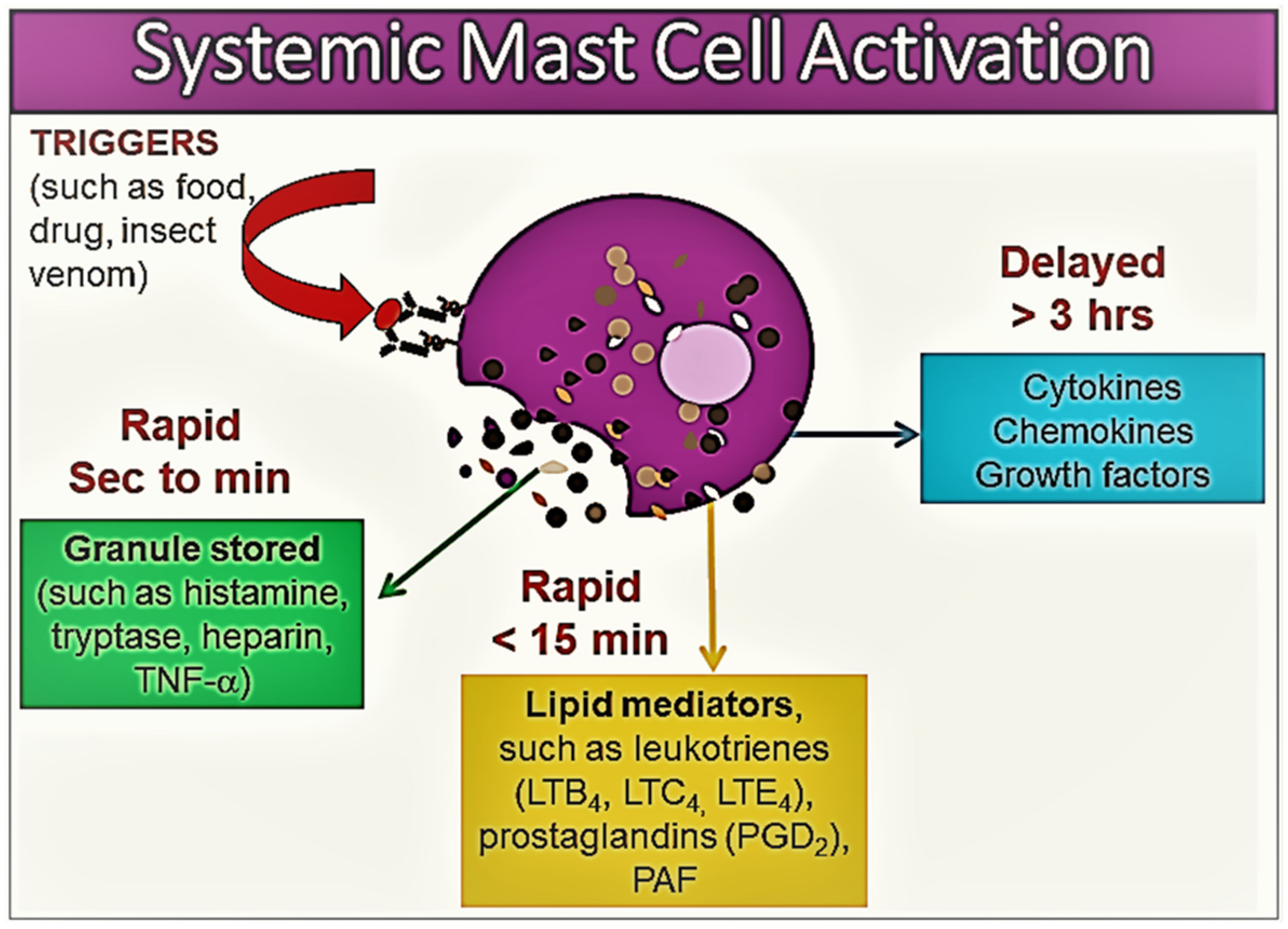
:1. Introduction
2. Mast Cells and Mast Cell Activation
3. Disorders Associated with Mast Cell Activation and Nomenclature
3.1. Mastocytosis
| SM | Diagnosis is confirmed if patient expresses one major criterion and one minor criterion or expresses three minor criteria in extracutaneous organ biopsy specimens |
| Major criterion Minor criteria | Multifocal aggregates of MCs (≥15 MCs per cluster) in biopsy sections
|
| MMAS | Diagnosis requires presence of one or two minor criteria of SM |
|
3.2. Monoclonal Mast Cell Activation Syndrome
3.3. Anaphylaxis
3.4. Mast Cell Activation Syndrome
4. Factors Determining the Severity of Mast Cell Activation and Mediator Release
4.1. Atopy
4.2. Hereditary Alpha-Tryptasemia
5. General Features of Anaphylaxis and MCAS in Mastocytosis
6. Management of Mast Cell Disorders
7. Concluding Remarks and Future Directions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Valent, P.; Akin, C.; Hartmann, K.; Nilsson, G.; Reiter, A.; Hermine, O.; Sotlar, K.; Sperr, W.R.; Escribano, L.; George, T.I.; et al. Mast cells as a unique hematopoietic lineage and cell system: From Paul Ehrlich’s visions to precision medicine concepts. Theranostics 2020, 10, 10743–10768. [Google Scholar] [CrossRef]
- Crivellato, E.; Ribatti, D.; Mallardi, F.; Beltrami, C.A. The mast cell: A multifunctional effector cell. Adv. Clin. Path. 2003, 7, 13–26. [Google Scholar]
- Galli, S.J.; Kalesnikoff, J.; Grimbaldeston, M.A.; Piliponsky, A.M.; Williams, C.M.; Tsai, M. Mast cells as “tunable” effector and immunoregulatory cells: Recent advances. Annu. Rev. Immunol. 2005, 23, 749–786. [Google Scholar] [CrossRef]
- Galli, S.J.; Borregaard, N.; Wynn, T.A. Phenotypic and functional plasticity of cells of innate immunity: Macrophages, mast cells and neutrophils. Nat. Immunol. 2011, 12, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- da Silva, E.Z.; Jamur, M.C.; Oliver, C. Mast cell function: A new vision of an old cell. J. Histochem. Cytochem. 2014, 62, 698–738. [Google Scholar] [CrossRef]
- Gilfillan, A.M.; Beaven, M.A. Regulation of mast cell responses in health and disease. Crit. Rev. Immunol. 2011, 31, 475–529. [Google Scholar] [CrossRef]
- Galli, S.J.; Tsai, M. Mast cells: Versatile regulators of inflammation, tissue remodeling, host defense and homeostasis. J. Dermatol. Sci. 2008, 49, 7–19. [Google Scholar] [CrossRef]
- Iwaki, S.; Tkaczyk, C.; Metcalfe, D.D.; Gilfillan, A.M. Roles of adaptor molecules in mast cell activation. Chem. Immunol. Allergy 2005, 87, 43–58. [Google Scholar]
- Galli, S.J.; Tsai, M. Mast cells in allergy and infection: Versatile effector and regulatory cells in innate and adaptive immunity. Eur. J. Immunol. 2010, 40, 1843–1851. [Google Scholar] [CrossRef] [PubMed]
- Castells, M. Mast cell mediators in allergic inflammation and mastocytosis. Immunol. Allergy Clin. N. Am. 2006, 26, 465–485. [Google Scholar] [CrossRef]
- Gulen, T.; Hagglund, H.; Dahlen, B.; Nilsson, G. Mastocytosis: The puzzling clinical spectrum and challenging diagnostic aspects of an enigmatic disease. J. Intern. Med. 2016, 279, 211–228. [Google Scholar] [CrossRef]
- Gülen, T.; Akin, C. Anaphylaxis and Mast Cell Disorders. Immunol. Allergy Clin. N. Am. 2022, 42, 45–63. [Google Scholar] [CrossRef] [PubMed]
- Kalesnikoff, J.; Galli, S.J. Anaphylaxis: Mechanisms of mast cell activation. Chem. Immunol. Allergy 2010, 95, 45–66. [Google Scholar]
- Schwartz, L.B. Diagnostic value of tryptase in anaphylaxis and mastocytosis. Immunol. Allergy Clin. N. Am. 2006, 26, 451–463. [Google Scholar] [CrossRef]
- Akin, C.; Valent, P.; Metcalfe, D.D. Mast cell activation syndrome: Proposed diagnostic criteria. J. Allergy Clin. Immunol. 2010, 126, 1099–1104. [Google Scholar] [CrossRef] [PubMed]
- Valent, P.; Akin, C.; Bonadonna, P.; Hartmann, K.; Brockow, K.; Niedoszytko, M.; Nedoszytko, B.; Siebenhaar, F.; Sperr, W.R.; Oude Elberink, J.N.G.; et al. Proposed diagnostic algorithm for patients with suspected mast cell activation syndrome. J. Allergy Clin. Immunol. Pract. 2019, 7, 1125–1133. [Google Scholar] [CrossRef] [PubMed]
- Gülen, T.; Akin, C.; Bonadonna, P.; Siebenhaar, F.; Broesby-Olsen, S.; Brockow, K.; Niedoszytko, M.; Nedoszytko, B.; Oude Elberink, H.N.G.; Butterfield, J.H.; et al. Selecting the right criteria and proper classification to diagnose mast cell activation syndromes: A critical review. J. Allergy Clin. Immunol. Pract. 2021, 9, 3918–3928. [Google Scholar] [CrossRef]
- Valent, P.; Akin, C.; Hartmann, K.; Alvarez-Twose, I.; Brockow, K.; Hermine, O.; Niedoszytko, M.; Schwaab, J.; Lyons, J.J.; Carter, M.C.; et al. Updated diagnostic criteria and classification of mast cell disorders: A consensus proposal. Hemasphere 2021, 5, e646. [Google Scholar] [CrossRef]
- Gulen, T.; Hagglund, H.; Dahlen, B.; Nilsson, G. High prevalence of anaphylaxis in patients with systemic mastocytosis—A single-centre experience. Clin. Exp. Allergy 2014, 44, 121–129. [Google Scholar] [CrossRef]
- Kelso, J.M. MRGPRX2 signaling and skin test results. J. Allergy Clin. Immunol. Pract. 2020, 8, 426. [Google Scholar] [CrossRef]
- Butterfield, J.H. Nontryptase Urinary and Hematologic Biomarkers of Mast Cell Expansion and Mast Cell Activation: Status 2022. J. Allergy Clin. Immunol. Pract. 2022, 10, 1974–1984. [Google Scholar] [CrossRef]
- Parente, R.; Giudice, V.; Cardamone, C.; Serio, B.; Selleri, C.; Triggiani, M. Secretory and Membrane-Associated Biomarkers of Mast Cell Activation and Proliferation. Int. J. Mol. Sci. 2023, 24, 7071. [Google Scholar] [CrossRef] [PubMed]
- Valent, P.; Hartmann, K.; Bonadonna, P.; Gülen, T.; Brockow, K.; Alvarez-Twose, I.; Hermine, O.; Niedoszytko, M.; Carter, M.C.; Hoermann, G.; et al. Global Classification of Mast Cell Activation Disorders: An ICD-10-CM–Adjusted Proposal of the ECNM-AIM Consortium. J. Allergy Clin. Immunol. Pract. 2022, 10, 1941–1950. [Google Scholar] [CrossRef]
- van Doormaal, J.J.; Arends, S.; Brunekreeft, K.L.; van der Wal, V.B.; Sietsma, J.; van Voorst Vader, P.C.; Oude Elberink, J.N.; Kluin-Nelemans, J.C.; van der Veer, E.; de Monchy, J.G. Prevalence of indolent systemic mastocytosis in a Dutch region. J. Allergy Clin. Immunol. 2013, 131, 1429–1431.e1. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.S.; Skovbo, S.; Vestergaard, H.; Kristensen, T.; Møller, M.; Bindslev-Jensen, C.; Fryzek, J.P.; Broesby-Olsen, S. Epidemiology of systemic mastocytosis in Denmark. Br. J. Haematol. 2014, 166, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Zanotti, R.; Bonifacio, M.; Isolan, C.; Tanasi, I.; Crosera, L.; Olivieri, F.; Orsolini, G.; Schena, D.; Bonadonna, P. A Multidisciplinary Diagnostic Approach Reveals a Higher Prevalence of Indolent Systemic Mastocytosis: 15-Years’ Experience of the GISM Network. Cancers 2021, 13, 6380. [Google Scholar] [CrossRef]
- Ungerstedt, J.; Ljung, C.; Klimkowska, M.; Gülen, T. Clinical Outcomes of Adults with Systemic Mastocytosis: A 15-Year Multidisciplinary Experience. Cancers 2022, 14, 3942. [Google Scholar] [CrossRef]
- Hägglund, H.; Sander, B.; Gülen, T.; Lindelöf, B.; Nilsson, G. Increased risk of malignant melanoma in patients with systemic mastocytosis? Acta Derm. Venereol 2014, 94, 583–584. [Google Scholar] [CrossRef]
- Gülen, T.; Möller Westerberg, C.; Lyberg, K.; Ekoff, M.; Kolmert, J.; Bood, J.; Öhd, J.; James, A.; Dahlén, S.E.; Nilsson, G.; et al. Assessment of in vivo mast cell reactivity in patients with systemic mastocytosis. Clin. Exp. Allergy 2017, 47, 909–917. [Google Scholar] [CrossRef]
- Gulen, T.; Hagglund, H.; Dahlen, S.E.; Sander, B.; Dahlén, B.; Nilsson, G. Flushing, fatigue, and recurrent anaphylaxis: A delayed diagnosis of mastocytosis. Lancet 2014, 383, 1608. [Google Scholar] [CrossRef]
- Akin, C.; Scott, L.M.; Kocabas, C.N.; Kushnir-Sukhov, N.; Brittain, E.; Noel, P.; Metcalfe, D.D. Demonstration of an aberrant mast-cell population with clonal markers in a subset of patients with “idiopathic” anaphylaxis. Blood 2007, 110, 2331–2333. [Google Scholar] [CrossRef] [PubMed]
- Sonneck, K.; Florian, S.; Mullauer, L.; Wimazal, F.; Födinger, M.; Sperr, W.R.; Valent, P. Diagnostic and subdiagnostic accumulation of mast cells in the bone marrow of patients with anaphylaxis: Monoclonal mast cell activation syndrome. Int. Arch. Allergy Immunol. 2007, 142, 158–164. [Google Scholar] [CrossRef]
- Bagos-Estevez, A.G.; Ledford, D.K. Anaphylaxis: Definition, Epidemiology, Diagnostic Challenges, Grading System. Immunol. Allergy Clin. N. Am. 2022, 42, 1–11. [Google Scholar] [CrossRef]
- Wood, R.A.; Camargo, C.A., Jr.; Lieberman, P.; Sampson, H.A.; Schwartz, L.B.; Zitt, M.; Collins, C.; Tringale, M.; Wilkinson, M.; Boyle, J.; et al. Anaphylaxis in America: The prevalence and characteristics of anaphylaxis in the United States. J. Allergy Clin. Immunol. 2014, 133, 461–467. [Google Scholar] [CrossRef]
- Decker, W.W.; Campbell, R.L.; Manivannan, V.; Luke, A.; St Sauver, J.L.; Weaver, A.; Bellolio, M.F.; Bergstralh, E.J.; Stead, L.G.; Li, J.T. The etiology and incidence of anaphylaxis in Rochester, Minnesota: A report from the Rochester Epidemiology Project. J. Allergy Clin. Immunol. 2008, 122, 1161–1165. [Google Scholar] [CrossRef]
- Worm, M. Epidemiology of anaphylaxis. Chem. Immunol. Allergy 2010, 95, 12–21. [Google Scholar]
- Panesar, S.S.; Javad, S.; de Silva, D.; Nwaru, B.I.; Hickstein, L.; Muraro, A.; Roberts, G.; Worm, M.; Bilò, M.B.; Cardona, V.; et al. The epidemiology of anaphylaxis in Europe: A systematic review. Allergy 2013, 68, 1353–1361. [Google Scholar] [CrossRef]
- Sheikh, A.; Hippisley-Cox, J.; Newton, J.; Fenty, J. Trends in national incidence, lifetime prevalence and adrenaline prescribing for anaphylaxis in England. J. R. Soc. Med. 2008, 101, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.J.; Jerschow, E.; Umasunthar, T.; Lin, R.; Campbell, D.E.; Boyle, R.J. Fatal anaphylaxis: Mortality rate and risk factors. J. Allergy Clin. Immunol. Pract. 2017, 5, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Grabenhenrich, L.B.; Dölle, S.; Moneret-Vautrin, A.; Köhli, A.; Lange, L.; Spindler, T.; Ruëff, F.; Nemat, K.; Maris, I.; Roumpedaki, E.; et al. Anaphylaxis in children and adolescents: The European Anaphylaxis Registry. J. Allergy Clin. Immunol. 2016, 137, 1128–1137.e1. [Google Scholar] [CrossRef]
- Worm, M.; Eckermann, O.; Dölle, S.; Aberer, W.; Beyer, K.; Hawranek, T.; Hompes, S.; Koehli, A.; Mahler, V.; Nemat, K.; et al. Triggers and treatment of anaphylaxis: An analysis of 4000 cases from Germany, Austria and Switzerland. Dtsch. Arztebl. Int. 2014, 111, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Sampson, H.A.; Munoz-Furlong, A.; Campbell, R.L.; Adkinson, N.F., Jr.; Bock, S.A.; Branum, A.; Brown, S.G.; Camargo, C.A., Jr.; Cydulka, R.; Galli, S.J.; et al. Second symposium on the definition and management of anaphylaxis: Summary report–second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. Ann. Emerg. Med. 2006, 47, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Campbell, R.L.; Hagan, J.B.; Manivannan, V.; Decker, W.W.; Kanthala, A.R.; Bellolio, M.F.; Smith, V.D.; Li, J.T. Evaluation of national institute of allergy and infectious diseases/food allergy and anaphylaxis network criteria for the diagnosis of anaphylaxis in emergency department patients. J. Allergy Clin. Immunol. 2012, 129, 748–752. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi Brauer, C.E.; Motosue, M.S.; Li, J.T.; Hagan, J.B.; Bellolio, M.F.; Lee, S.; Campbell, R.L. Prospective validation of the NIAID/FAAN criteria for emergency department diagnosis of anaphylaxis. J. Allergy Clin. Immunol. Pract. 2016, 4, 1220–1226. [Google Scholar] [CrossRef]
- Baalmann, D.V.; Hagan, J.B.; Li, J.T.; Hess, E.P.; Campbell, R.L. Appropriateness of epinephrine use in ED patients with anaphylaxis. Am. J. Emerg. Med. 2016, 34, 174–179. [Google Scholar] [CrossRef]
- Campbell, R.L.; Li, J.T.; Nicklas, R.A.; Sadosty, A.T.; Members of the Joint Task Force; Practice Parameter Workgroup. Emergency department diagnosis and treatment of anaphylaxis: A practice parameter. Ann. Allergy Asthma Immunol. 2014, 113, 599–608. [Google Scholar] [CrossRef]
- Dribin, T.E.; Sampson, H.A.; Camargo, C.A., Jr.; Brousseau, D.C.; Spergel, J.M.; Neuman, M.I.; Shaker, M.; Campbell, R.L.; Michelson, K.A.; Rudders, S.A.; et al. Persistent, refractory, and biphasic anaphylaxis: A multidisciplinary Delphi study. J. Allergy Clin. Immunol. 2020, 146, 1089–1096. [Google Scholar] [CrossRef]
- Dribin, T.E.; Schnadower, D.; Spergel, J.M.; Campbell, R.L.; Shaker, M.; Neuman, M.I.; Michelson, K.A.; Capucilli, P.S.; Camargo, C.A., Jr.; Brousseau, D.C.; et al. Severity grading system for acute allergic reactions: A multidisciplinary Delphi study. J. Allergy Clin. Immunol. 2021, 148, 173–181. [Google Scholar] [CrossRef]
- Valent, P.; Bonadonna, P.; Hartmann, K.; Broesby-Olsen, S.; Brockow, K.; Butterfield, J.H.; Triggiani, M.; Lyons, J.J.; Oude Elberink, J.N.G.; Arock, M.; et al. Why the 20% + 2 Tryptase Formula Is a Diagnostic Gold Standard for Severe Systemic Mast Cell Activation and Mast Cell Activation Syndrome. Int. Arch. Allergy Immunol. 2019, 180, 44–51. [Google Scholar] [CrossRef]
- Butterfield, J.H. Increased Excretion of Mast Cell Mediator Metabolites during Mast Cell Activation Syndrome. J. Allergy Clin. Immunol. Pract. 2023, 11, 2542–2546. [Google Scholar] [CrossRef]
- Gulen, T.; Akin, C. Pharmacotherapy of mast cell disorders. Curr. Opin. Allergy Clin. Immunol. 2017, 17, 295–303. [Google Scholar] [CrossRef]
- Gulen, T. Management of Mediator Symptoms, Allergy, and Anaphylaxis in Mastocytosis. Immunol. Allergy Clin. N. Am. 2023, 43, 681–698. [Google Scholar] [CrossRef]
- Gulen, T.; Akin, C. Idiopathic Anaphylaxis: A Perplexing Diagnostic Challenge for Allergists. Curr. Allergy Asthma Rep. 2021, 21, 11. [Google Scholar] [CrossRef] [PubMed]
- Peavy, R.D.; Metcalfe, D.D. Understanding the mechanisms of anaphylaxis. Curr. Opin. Allergy Clin. Immunol. 2008, 8, 310–315. [Google Scholar] [CrossRef]
- Metcalfe, D.D.; Peavy, R.D.; Gilfillan, A.M. Mechanisms of mast cell signaling in anaphylaxis. J. Allergy Clin. Immunol. 2009, 124, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Gilfillan, A.M.; Peavy, R.D.; Metcalfe, D.D. Amplification mechanisms for the enhancement of antigen-mediated mast cell activation. Immunol. Res. 2009, 43, 15–24. [Google Scholar] [CrossRef]
- Valent, P.; Akin, C.; Arock, M. Diagnosis and treatment of anaphylaxis in patients with mastocytosis. Curr. Treat. Options Allergy 2014, 1, 247–261. [Google Scholar] [CrossRef]
- Blumenthal, M.N. The role of genetics in the development of asthma and atopy. Curr. Opin. Allergy Clin. Immunol. 2005, 5, 141–145. [Google Scholar] [CrossRef]
- Rastogi, S.; Willmes, D.M.; Nassiri, M.; Babina, M.; Worm, M. PGE2 deficiency predisposes to anaphylaxis by causing mast cell hyperresponsiveness. J. Allergy Clin. Immunol. 2020, 146, 1387–1396.e13. [Google Scholar] [CrossRef]
- Lyons, J.J.; Sun, G.; Stone, K.D.; Nelson, C.; Wisch, L.; O’Brien, M.; Jones, N.; Lindsley, A.; Komarow, H.D.; Bai, Y.; et al. Mendelian inheritance of elevated serum tryptase associated with atopy and connective tissue abnormalities. J. Allergy Clin. Immunol. 2014, 133, 1471–1474. [Google Scholar] [CrossRef] [PubMed]
- Lyons, J.J.; Chovanec, J.; O’Connell, M.P.; Liu, Y.; Šelb, J.; Zanotti, R.; Bai, Y.; Kim, J.; Le, Q.T.; DiMaggio, T.; et al. Heritable risk for severe anaphylaxis associated with increased α-tryptase-encoding germline copy number at TPSAB1. J. Allergy Clin. Immunol. 2021, 147, 622–632. [Google Scholar] [CrossRef]
- Sprinzl, B.; Greiner, G.; Uyanik, G.; Arock, M.; Haferlach, T.; Sperr, W.R.; Valent, P.; Hoermann, G. Genetic regulation of tryptase production and clinical impact: Hereditary alpha tryptasemia, mastocytosis and beyond. Int. J. Mol. Sci. 2021, 22, 2458. [Google Scholar] [CrossRef]
- Sperr, W.R.; Czerwenka, K.; Mundigler, G.; Müller, M.R.; Semper, H.; Klappacher, G.; Glogar, H.D.; Lechner, K.; Valent, P. Specific activation of human mast cells by the ligand for c-kit: Comparison between lung, uterus and heart mast cells. Int. Arch. Allergy Immunol. 1993, 102, 170–175. [Google Scholar] [CrossRef]
- Valent, P. Risk factors and management of severe life-threatening anaphylaxis in patients with clonal mast cell disorders. Clin. Exp. Allergy 2014, 44, 914–920. [Google Scholar] [CrossRef]
- Valenta, R.; Karaulov, A.; Niederberger, V.; Gattinger, P.; van Hage, M.; Flicker, S.; Linhart, B.; Campana, R.; Focke-Tejkl, M.; Curin, M.; et al. Molecular aspects of allergens and allergy. Adv. Immunol. 2018, 138, 195–256. [Google Scholar] [PubMed]
- Reber, L.L.; Hernandez, J.D.; Galli, S.J. The pathophysiology of anaphylaxis. J. Allergy Clin. Immunol. 2017, 140, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Gulen, T.; Ljung, C.; Nilsson, G.; Akin, C. Risk factor analysis of anaphylactic reactions in patients with systemic mastocytosis. J. Allergy Clin. Immunol. Pract. 2017, 5, 1248–1255. [Google Scholar] [CrossRef] [PubMed]
- Brockow, K.; Jofer, C.; Behrendt, H.; Ring, J. Anaphylaxis in patients with mastocytosis: A study on history, clinical features and risk factors in 120 patients. Allergy 2008, 63, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Akin, C. Mast cell activation disorders. J. Allergy Clin. Immunol. Pract. 2014, 2, 252–257.e1. [Google Scholar] [CrossRef] [PubMed]
- Lyberg, K.; Ekoff, M.; Westerberg, C.M.; Engblom, C.; Dahlén, B.; Gülen, T.; Nilsson, G. Mast cells derived from systemic mastocytosis exhibit an increased responsiveness to hyperosmolarity. Allergy 2022, 77, 1909–1911. [Google Scholar] [CrossRef]
- Collington, S.J.; Williams, T.J.; Weller, C.L. Mechanisms underlying the localisation of mast cells in tissues. Trends Immunol. 2011, 32, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Warm, K.; Backman, H.; Lindberg, A.; Lundbäck, B.; Ronmark, E. Low incidence and high remission of allergic sensitization among adults. J. Allergy Clin. Immunol. 2012, 129, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Wüthrich, B.; Schmid-Grendelmeier, P.; Schindler, C.; Imboden, M.; Bircher, A.; Zemp, E.; Probst-Hensch, N. Prevalence of atopy and respiratory allergic diseases in the elderly SAPALDIA population. Int. Arch. Allergy Immunol. 2013, 162, 143–148. [Google Scholar] [CrossRef]
- Okui, T. Age-period-cohort analysis of asthma, allergic rhinitis, and atopic dermatitis prevalence in Japan. Environ. Anal. Health Toxicol. 2020, 35, e2020012. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez de Olano, D.; de la Hoz Caballer, B.; Nunez Lopez, R.; Sanchez Munoz, L.; Cuevas Agustin, M.; Dieguez, M.C.; Alvarez Twose, I.; Castells, M.C.; Escribano Mora, L. Prevalence of allergy and anaphylactic symptoms in 210 adult and pediatric patients with mastocytosis in Spain: A study of the Spanish network on mastocytosis (REMA). Clin. Exp. Allergy 2007, 37, 1547–1555. [Google Scholar] [CrossRef]
- Lyons, J.J.; Yu, X.; Hughes, J.D.; Le, Q.T.; Jamil, A.; Bai, Y.; Ho, N.; Zhao, M.; Liu, Y.; O’Connell, M.P.; et al. Elevated basal serum tryptase identifies a multisystem disorder associated with increased TPSAB1 copy number. Nat. Genet. 2016, 48, 1564–1569. [Google Scholar] [CrossRef]
- Robey, R.C.; Wilcock, A.; Bonin, H.; Beaman, G.; Myers, B.; Grattan, C.; Briggs, T.A.; Arkwright, P.D. Hereditary alpha-tryptasemia: UK prevalence and variability in disease expression. J. Allergy Clin. Immunol. Pract. 2020, 8, 3549–3556. [Google Scholar] [CrossRef]
- Chollet, M.B.; Akin, C. Hereditary alpha tryptasemia is not associated with specific clinical phenotypes. J. Allergy Clin. Immunol. 2022, 149, 728–735.e2. [Google Scholar] [CrossRef]
- Greiner, G.; Sprinzl, B.; Górska, A.; Ratzinger, F.; Gurbisz, M.; Witzeneder, N.; Schmetterer, K.G.; Gisslinger, B.; Uyanik, G.; Hadzijusufovic, E.; et al. Hereditary alpha tryptasemia is a valid genetic biomarker for severe mediator-related symptoms in mastocytosis. Blood 2021, 137, 238–247. [Google Scholar] [CrossRef]
- O’Connell, M.P.; Lyons, J.J. Hymenoptera venom-induced anaphylaxis and hereditary alpha-tryptasemia. Curr. Opin. Allergy Clin. Immunol. 2020, 20, 431–437. [Google Scholar] [CrossRef]
- Akin, C. Anaphylaxis and mast cell disease: What is the risk? Curr. Allergy Asthma Rep. 2010, 10, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Twose, I.; Gonzalez de Olano, D.; Sanchez-Munoz, L.; Matito, A.; Esteban-López, M.I.; Vega, A.; Mateo, M.B.; Alonso Díaz de Durana, M.D.; de la Hoz, B.; Del Pozo Gil, M.D.; et al. Clinical, biological, and molecular characteristics of clonal mast cell disorders presenting with systemic mast cell activation symptoms. J. Allergy Clin. Immunol. 2010, 125, 1269–1278.e2. [Google Scholar] [CrossRef] [PubMed]
- Bonadonna, P.; Perbellini, O.; Passalacqua, G.; Caruso, B.; Colarossi, S.; Dal Fior, D.; Castellani, L.; Bonetto, C.; Frattini, F.; Dama, A.; et al. Clonal mast cell disorders in patients with systemic reactions to Hymenoptera stings and increased serum tryptase levels. J. Allergy Clin. Immunol. 2009, 123, 680–686. [Google Scholar] [CrossRef]
- Jarkvist, J.; Brockow, K.; Gülen, T. Low frequency of IgE-mediated food hypersensitivity in mastocytosis. J. Allergy Clin. Immunol. Pract. 2020, 8, 3093–3101. [Google Scholar] [CrossRef]
- Roenneberg, S.; Bohner, A.; Brockow, K.; Arnold, A.; Darsow, U.; Eberlein, B.; Biedermann, T. alpha-Gal—A new clue for anaphylaxis in mastocytosis. J. Allergy Clin. Immunol. Pract. 2016, 4, 531–532. [Google Scholar] [CrossRef]
- Prieto-García, A.; Álvarez-Perea, A.; Matito, A.; Sánchez-Muñoz, L.; Morgado, J.M.; Escribano, L.; Álvarez-Twose, I. Systemic mastocytosis presenting as IgE-mediated food-induced anaphylaxis: A report of two cases. J. Allergy Clin. Immunol. Pract. 2015, 3, 456–458. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes, L.; Ring, J.; Brockow, K. Clonal mast cell activation syndrome with anaphylaxis to sulfites. Int. Arch. Allergy Immunol. 2013, 162, 94–96. [Google Scholar] [CrossRef]
- Bonadonna, P.; Pagani, M.; Aberer, W.; Bilò, M.B.; Brockow, K.; Oude Elberink, H.; Garvey, L.; Mosbech, H.; Romano, A.; Zanotti, R.; et al. Drug hypersensitivity in clonal mast cell disorders: ENDA/EAACI position paper. Allergy 2015, 70, 755–763. [Google Scholar] [CrossRef]
- Weingarten, T.N.; Volcheck, G.W.; Sprung, J. Anaphylactoid reaction to intravenous contrast in patient with systemic mastocytosis. Anaesth. Intensive Care 2009, 37, 646–649. [Google Scholar] [CrossRef]
- Renauld, V.; Goudet, V.; Mouton-Faivre, C.; Debaene, B.; Dewachter, P. Case report: Perioperative immediate hypersensitivity involves not only allergy but also mastocytosis. Can. J. Anaesth. 2011, 58, 456–459. [Google Scholar] [CrossRef]
- Seitz, C.S.; Brockow, K.; Hain, J.; Trautmann, A. Non-steroidal anti-inflammatory drug hypersensitivity: Association with elevated basal serum tryptase? Allergy Asthma Clin. Immunol. 2014, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Bonadonna, P.; Olivieri, F.; Jarkvist, J.; Nalin, F.; Zanotti, R.; Maclachlan, L.; Gülen, T. Non-steroidal anti-inflammatory drug-induced anaphylaxis infrequent in 388 patients with mastocytosis: A two-center retrospective cohort study. Front. Allergy 2022, 3, 1071807. [Google Scholar] [CrossRef] [PubMed]
- Rama, T.A.; Morgado, J.M.; Henriques, A.; Escribano, L.; Alvarez-Twose, I.; Sanchez-Muñoz, L.; Moreira, A.; Romão, J.; Órfão, A.; Matito, A. Mastocytosis presenting with mast cell-mediator release-associated symptoms elicited by cyclooxygenase inhibitors: Prevalence, clinical, and laboratory features. Clin. Transl. Allergy 2022, 12, e12132. [Google Scholar] [CrossRef]
- Jarkvist, J.; Gülen, T. Diagnostic Evaluation of Hypersensitivity Reactions to Antibiotics in a Large Cohort of Mastocytosis Patients. Diagnostics 2023, 13, 2241. [Google Scholar] [CrossRef] [PubMed]
- Gulen, T.; Hagglund, H.; Sander, B.; Dahlen, B.; Nilsson, G. The presence of mast cell clonality in patients with unexplained anaphylaxis. Clin. Exp. Allergy 2014, 44, 1179–1187. [Google Scholar] [CrossRef]
- Carter, M.C.; Desai, A.; Komarow, H.D.; Bai, Y.; Clayton, S.T.; Clark, A.S.; Ruiz-Esteves, K.N.; Long, L.M.; Cantave, D.; Wilson, T.M.; et al. A distinct biomolecular profile identifies monoclonal mast cell disorders in patients with idiopathic anaphylaxis. J. Allergy Clin. Immunol. 2018, 141, 180–188. [Google Scholar] [CrossRef] [PubMed]
- van Anrooij, B.; van der Veer, E.; de Monchy, J.G.; van der Heide, S.; Kluin-Nelemans, J.C.; van Voorst Vader, P.C.; van Doormaal, J.J.; Oude Elberink, J.N. Higher mast cell load decreases the risk of Hymenoptera venom-induced anaphylaxis in patients with mastocytosis. J. Allergy Clin. Immunol. 2013, 132, 125–130. [Google Scholar] [CrossRef]
- Rama, T.A.; Torrado, I.; Henriques, A.F.; Sánchez-Muñoz, L.; Jara-Acevedo, M.; Navarro-Navarro, P.; Caldas, C.; Mayado, A.; Muñoz-González, J.; García-Montero, A.; et al. Mast Cell Activation Syndromes: Comparison Between Two Scoring Models to Predict for Mast Cell Clonality. J. Allergy Clin. Immunol. Pract. 2023, 11, 908–919.e4. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, P.L. Recognition and first-line treatment of anaphylaxis. Am. J. Med. 2014, 127 (Suppl. S1), S6–S11. [Google Scholar] [CrossRef]
- Roberts, L.J., 2nd; Turk, J.W.; Oates, J.A. Shock syndrome associated with mastocytosis: Pharmacologic reversal of the acute episode and therapeutic prevention of recurrent attacks. Adv. Shock. Res. 1982, 8, 145–152. [Google Scholar]
- Turk, J.; Oates, J.A.; Roberts, L.J., 2nd. Intervention with epinephrine in hypotension associated with mastocytosis. J. Allergy Clin. Immunol. 1983, 71, 189–192. [Google Scholar] [CrossRef]
- Campbell, D.E. Anaphylaxis management: Time to re-evaluate the role of corticosteroids. J. Allergy Clin. Immunol. Pract. 2019, 7, 2239–2240. [Google Scholar] [CrossRef]
- Jarkvist, J.; Salehi, C.; Akin, C.; Gülen, T. Venom immunotherapy in patients with clonal mast cell disorders: IgG4 correlates with protection. Allergy 2020, 75, 169–177. [Google Scholar] [CrossRef]
- Broesby-Olsen, S.; Vestergaard, H.; Mortz, C.G.; Jensen, B.; Havelund, T.; Hermann, A.P.; Siebenhaar, F.; Møller, M.B.; Kristensen, T.K.; Bindslev-Jensen, C. Omalizumab prevents anaphylaxis and improves symptoms in systemic mastocytosis: Efficacy and safety observations. Allergy 2018, 73, 230–238. [Google Scholar] [CrossRef]
- Jendoubi, F.; Gaudenzio, N.; Gallini, A.; Negretto, M.; Paul, C.; Bulai Livideanu, C. Omalizumab in the treatment of adult patients with mastocytosis: A systematic review. Clin. Exp. Allergy 2020, 50, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Kluin-Nelemans, H.C.; Jansen, J.H.; Breukelman, H.; Wolthers, B.G.; Kluin, P.M.; Kroon, H.M.; Willemze, R. Response to interferon alfa-2b in a patient with systemic mastocytosis. N. Engl. J. Med. 1992, 326, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Tefferi, A.; Li, C.-Y.; Butterfield, J.H.; Hoagland, H.C. Treatment of systemic mast-cell disease with cladribine. N. Engl. J. Med. 2001, 344, 307–309. [Google Scholar] [CrossRef]
- Valent, P.; Sperr, W.R.; Akin, C. How I treat patients with advanced systemic mastocytosis. Blood 2010, 116, 5812–5817. [Google Scholar] [CrossRef]
- Gilreath, J.A.; Tchertanov, L.; Deininger, M.W. Novel approaches to treating advanced systemic mastocytosis. Clin. Pharmacol. 2019, 11, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Akin, C.; Arock, M.; Valent, P. Tyrosine kinase inhibitors for the treatment of indolent systemic mastocytosis: Are we there yet? J. Allergy Clin. Immunol. 2022, 149, 1912–1918. [Google Scholar] [CrossRef]
- Gotlib, J.; Kluin-Nelemans, H.C.; George, T.I.; Akin, C.; Sotlar, K.; Hermine, O.; Awan, F.T.; Hexner, E.; Mauro, M.J.; Sternberg, D.W.; et al. Efficacy and safety of midostaurin in advanced systemic mastocytosis. N. Engl. J. Med. 2016, 374, 2530–2541. [Google Scholar] [CrossRef]
- Akin, C.; Elberink, H.O.; Gotlib, J.; Sabato, V.; Hartmann, K.; Broesby-Olsen, S.; Castells, M.; Deininger, M.; Heaney, M.; George, T.; et al. PIONEER: A randomized, double-blind, placebo-controlled, phase 2 study of avapritinib in patients with indolent or smoldering systemic mastocytosis (SM) with symptoms inadequately controlled by standard therapy. J. Allergy Clin. Immunol. 2020, 145, AB336. [Google Scholar] [CrossRef]
- Hartmann, K.; Gotlib, J.; Akin, C.; Hermine, O.; Awan, F.T.; Hexner, E.; Mauro, M.J.; Menssen, H.D.; Redhu, S.; Knoll, S.; et al. Midostaurin improves quality of life and mediator-related symptoms in advanced systemic mastocytosis. J. Allergy Clin. Immunol. 2020, 146, 356–366.e4. [Google Scholar] [CrossRef] [PubMed]
- Konantz, M.; Williams, M.; Merkel, T.; Reiss, A.; Dirnhofer, S.; Meyer, S.C.; Valent, P.; George, T.I.; Tzankov, A.; Hartmann, K. Increased TIM-3 and galectin-9 serum levels in patients with advanced systemic mastocytosis. J. Allergy Clin. Immunol. 2023, 152, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Gulen, T.; Teufelberger, A.; Ekoff, M.; Westerberg, C.M.; Lyberg, K.; Dahlén, S.E.; Dahlén, B.; Nilsson, G. Distinct plasma biomarkers confirm the diagnosis of mastocytosis and identify increased risk of anaphylaxis. J. Allergy Clin. Immunol. 2021, 148, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Marcella, S.; Petraroli, A.; Canè, L.; Ferrara, A.L.; Poto, R.; Parente, R.; Palestra, F.; Cristinziano, L.; Modestino, L.; Galdiero, M.R.; et al. Thymic stromal lymphopoietin (TSLP) is a substrate for tryptase in patients with mastocytosis. Eur. J. Intern. Med. 2023. [Google Scholar] [CrossRef]



| Anaphylaxis Is Highly Likely When Any One of the Following Three Criteria is Fulfilled | ||
|---|---|---|
| Criterion 1 Acute onset of an illness (minutes to several hours) with involvement of the skin, mucosal tissue, or both (e.g., generalized hives, itching, or flushing, swollen lips-tongue-uvula) AND at least one of the following:
| Criterion 2 Two or more of the following that occur rapidly after exposure to a likely allergen for that patient (minutes to several hours):
| Criterion 3 * Hypotension after exposure to known allergen for that patient (minutes to several hours) |
| REMA Score | Karolinska Score | NICAS | ||||
|---|---|---|---|---|---|---|
| VARIABLES | Yes | No | Yes | No | Yes | No |
| Male gender | +1 | −1 | +1 | −1 | +1 | −1 |
| Clinical symptoms | ||||||
| Angioedema | n/a | n/a | n/a | n/a | n/a | +1 |
| Urticaria | n/a | n/a | n/a | n/a | +1 | n/a |
| Flushing | n/a | n/a | n/a | n/a | −1 | n/a |
| Urticaria/Pruritus/Angioedema | −2 | +1 | −2 | +1 | n/a | n/a |
| Syncope | +3 | 0 | +3 | 0 | +3 | 0 |
| Baseline tryptase | ||||||
| ≤11.4 ng/mL | n/a | n/a | −1 | n/a | −1 | n/a |
| >11.4 ng/mL | n/a | n/a | n/a | n/a | +1 | n/a |
| >20 ng/mL | n/a | n/a | +2 | n/a | n/a | n/a |
| <15 ng/mL | −1 | n/a | n/a | n/a | n/a | n/a |
| >25 ng/mL | +2 | n/a | n/a | n/a | n/a | n/a |
| KIT D816V mutation | n/a | n/a | n/a | n/a | +3 | −1 |
| Positive score * | ≥2 points | ≥2 points | ≥2 points | |||
| Outcome | High risk | High risk | High risk | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gülen, T. A Puzzling Mast Cell Trilogy: Anaphylaxis, MCAS, and Mastocytosis. Diagnostics 2023, 13, 3307. https://doi.org/10.3390/diagnostics13213307
Gülen T. A Puzzling Mast Cell Trilogy: Anaphylaxis, MCAS, and Mastocytosis. Diagnostics. 2023; 13(21):3307. https://doi.org/10.3390/diagnostics13213307
Chicago/Turabian StyleGülen, Theo. 2023. "A Puzzling Mast Cell Trilogy: Anaphylaxis, MCAS, and Mastocytosis" Diagnostics 13, no. 21: 3307. https://doi.org/10.3390/diagnostics13213307
APA StyleGülen, T. (2023). A Puzzling Mast Cell Trilogy: Anaphylaxis, MCAS, and Mastocytosis. Diagnostics, 13(21), 3307. https://doi.org/10.3390/diagnostics13213307






