Abstract
The term “Osmotic Demyelination Syndrome” (ODS) is synonymous with central pontine myelinolysis (CPM), denoting a condition characterised by brain damage, particularly affecting the white matter tracts of the pontine region. This damage arises due to the rapid correction of metabolic imbalances, primarily cases of hyponatremia. Noteworthy triggers encompass severe burns, liver transplantations, anorexia nervosa, hyperemesis gravidarum, and hyperglycaemia, all linked to the development of CPM. Clinical manifestations encompass a spectrum of signs and symptoms, including dysphagia, dysarthria, spastic quadriparesis, pseudobulbar paralysis, ataxia, lethargy, tremors, disorientation, catatonia, and, in severe instances, locked-in syndrome and coma. A recent case involving a 45-year-old woman illustrates these complexities. Upon admission to the Medicine Intensive Care Unit, she presented with symptoms indicative of diminished responsiveness and bilateral weakness in the upper and lower limbs. Of significance, the patient had a pre-existing medical history of hyperthyroidism. Extensive diagnostic investigations were undertaken, revealing compelling evidence of profound hyponatremia through blood analyses. Furthermore, magnetic resonance imaging (MRI) was performed, unveiling conspicuous areas of abnormal hyperintensity located in the central pons, intriguingly accompanied by spared peripheral regions. These radiological findings align with the characteristic pattern associated with osmotic demyelination syndrome, illuminating the underlying pathology.
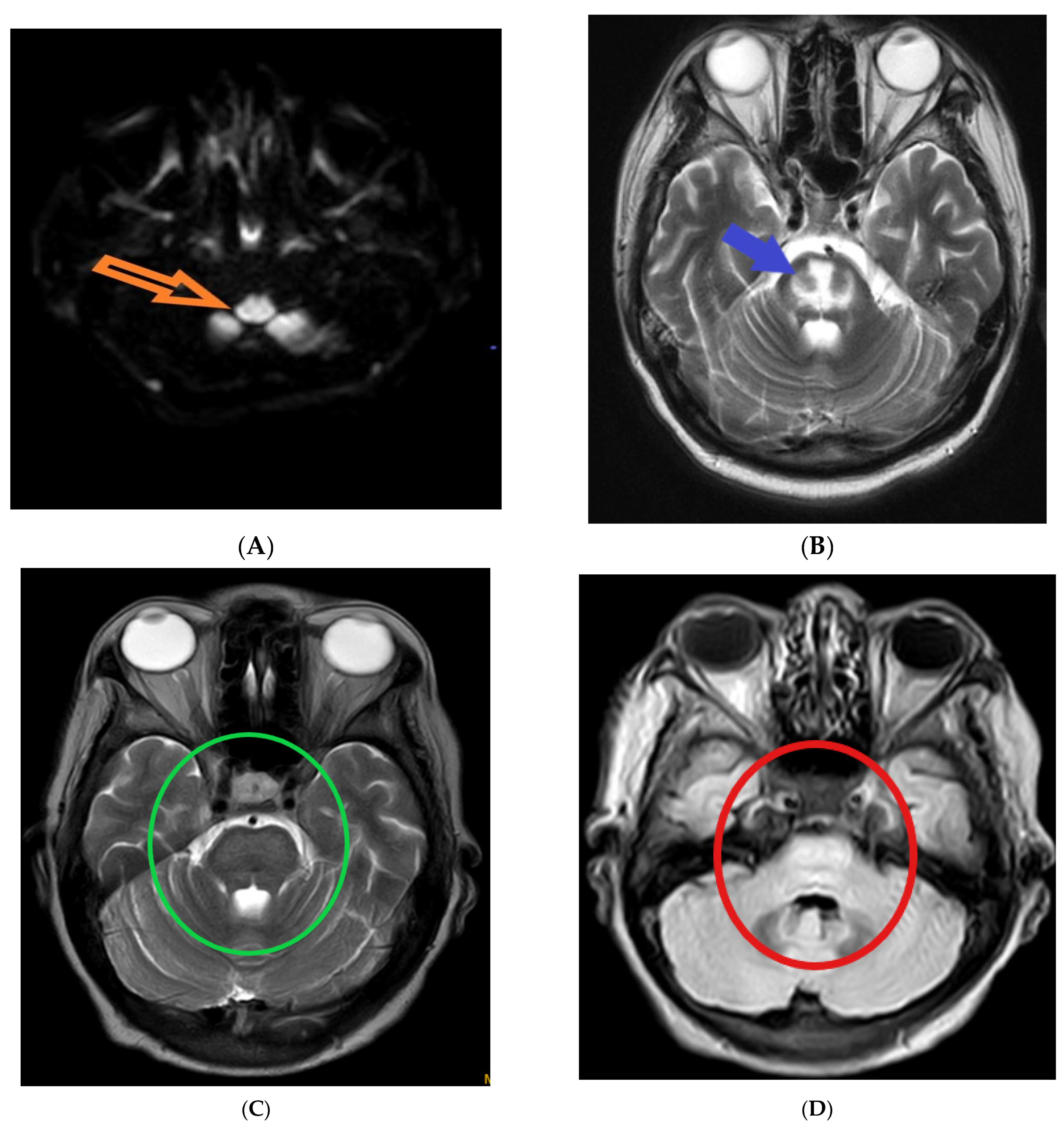
The following magnetic resonance imaging (MRI) images displaying different indicators observed in osmotic demyelination syndrome (Figure 1).
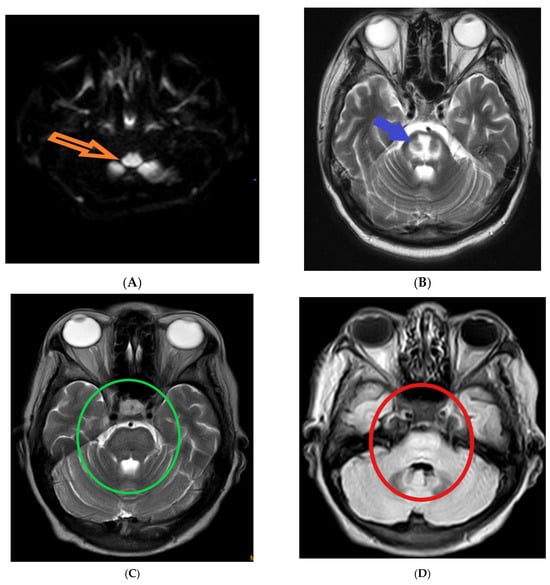
Figure 1.
Magnetic resonance imaging: (A) hyper-intensity of central pons in diffuse weighted image (orange arrow) [1]; (B) trident-shaped appearance (omega sign) of central pons in T2 weighted image (blue arrow) [2]. The characteristic “trident”-shaped appearance is attributed to the primary affectation of the transverse pontine fibres and the relatively limited involvement of the descending corticospinal tracts [3]; (C,D) piglet sign appearance of upper pons in T2 and FLAIR images (green and red circles, respectively) [4]. T2-weighted MR images exhibit a distinctive pattern known as the "piglet face" sign, initially described by Wagner et al. [5]. In this sign, the pons takes on a distinctive resemblance to the snout of a piglet, while the internal carotid arteries (ICAs) and the fourth ventricle together form the eyes and mouth, respectively, of the piglet-like configuration.
Author Contributions
P.K. was responsible for formulating, writing, and preparing the original draft of the manuscript. All authors conducted the review and editing process as well. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Written consent was obtained from the patient’s caregivers.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ruzek, K.A.; Campeau, N.G.; Miller, G.M. Early Diagnosis of Central Pontine Myelinolysis with Diffusion-Weighted Imaging. Am. J. Neuroradiol. 2004, 25, 210–213. [Google Scholar] [PubMed]
- Miller, G.M.; Baker, H.L.; Okazaki, H.; Whisnant, J.P. Central Pontine Myelinolysis and Its Imitators: MR Findings. Radiology 1988, 168, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Biotti, D.; Durupt, D. A Trident in the Brain, Central Pontine Myelinolysis. Pract. Neurol. 2009, 9, 231–232. [Google Scholar] [CrossRef] [PubMed]
- Beh, S.C. Temporal Evolution of the Trident and Piglet Signs of Osmotic Demyelination Syndrome. J. Neurol. Sci. 2017, 373, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.O.; Bös, K.; Jascenoka, J.; Jekauc, D.; Petermann, F. Peer Problems Mediate the Relationship between Developmental Coordination Disorder and Behavioral Problems in School-Aged Children. Res. Dev. Disabil. 2012, 33, 2072–2079. [Google Scholar] [CrossRef] [PubMed][Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).