Deep Learning for Fully Automated Radiographic Measurements of the Pelvis and Hip
Abstract
:1. Introduction
2. Materials and Methods
2.1. Manual Measurement
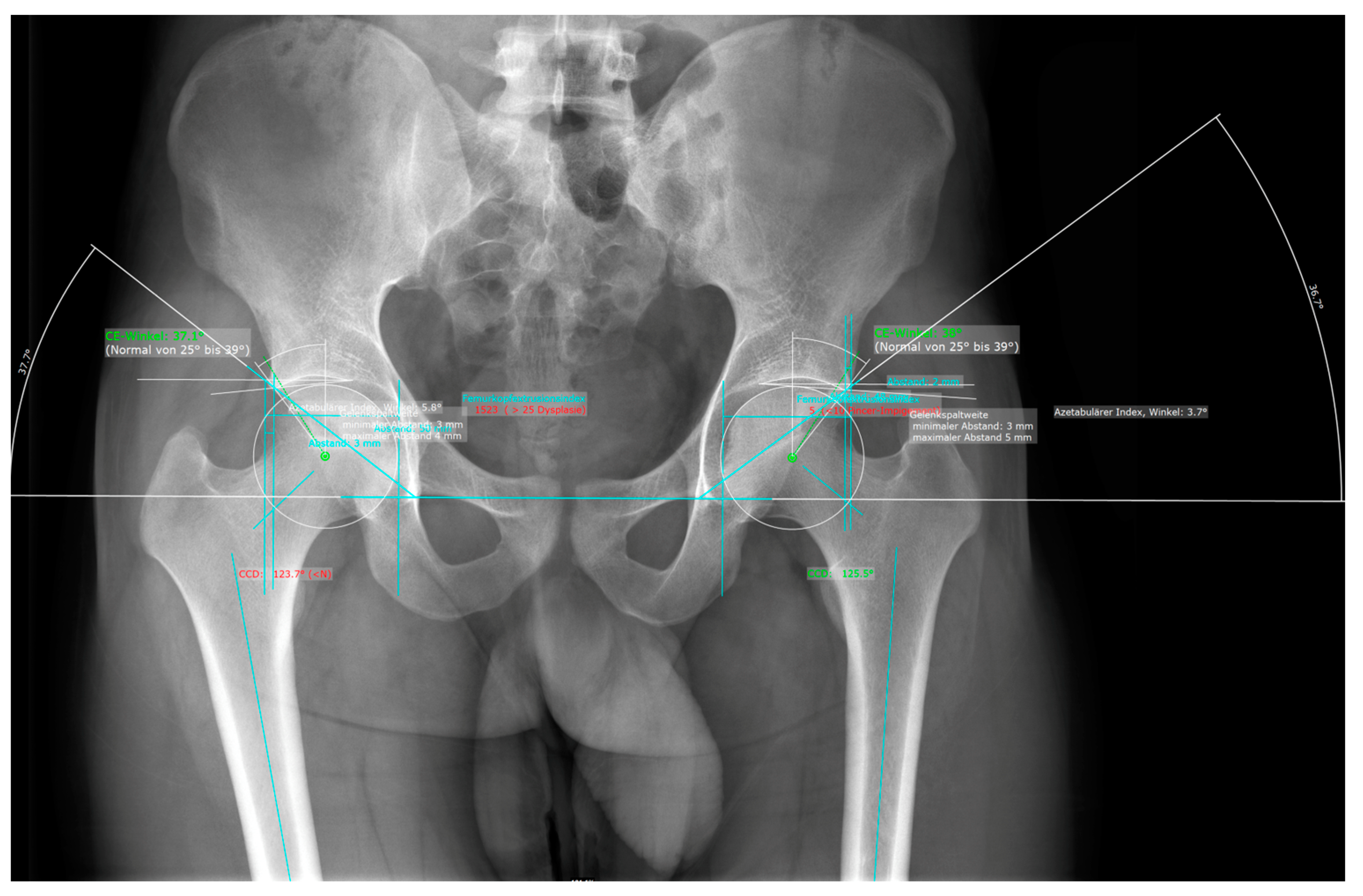
2.2. Automated Measurements Using AI Software
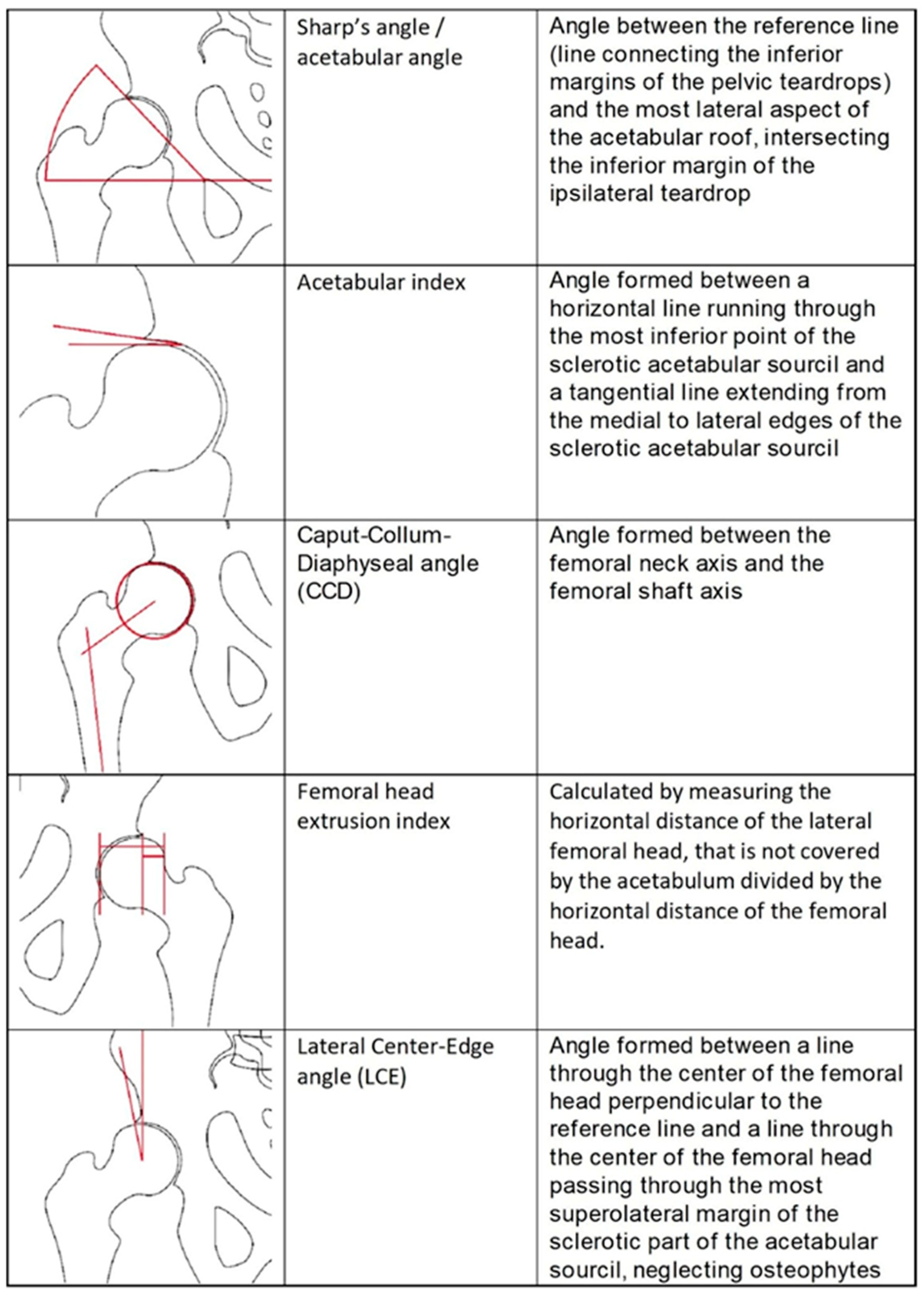
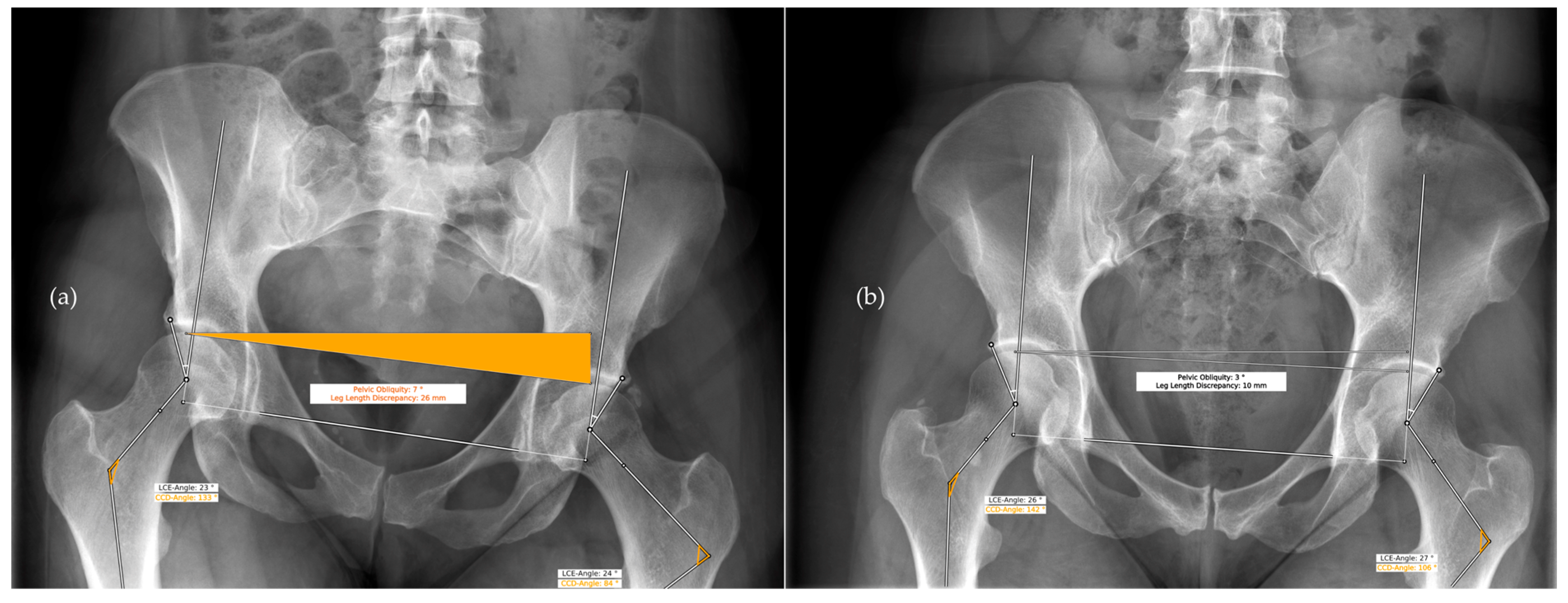
2.3. Measurements
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vuillemin, N.; Steppacher, S.D.; Meier, M.K.; Büchler, L. Therapieentscheidung bei Kombinationspathologien Dysplasie—FAI—Fehlrotation. Orthopädie 2022, 51, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Melugin, H.P.; Hale, R.F.; Zhou, J.; LaPrade, M.; Bernard, C.; Leland, D.; Levy, B.A.; Krych, A.J. Risk Factors for Long-term Hip Osteoarthritis in Patients with Femoroacetabular Impingement Without Surgical Intervention. Am. J. Sports Med. 2020, 48, 2881–2886. [Google Scholar] [CrossRef] [PubMed]
- Chiari, C.; Lutschounig, M.C.; Nöbauer-Huhmann, I.; Windhager, R. Femoroacetabular impingement syndrome in adolescents. Orthopade 2022, 51, 211–218. [Google Scholar] [CrossRef]
- Pascual-Garrido, C.; Li, D.J.; Grammatopoulos, G.; Yanik, E.L.; Clohisy, J.C.; ANCHOR Group. The Pattern of Acetabular Cartilage Wear Is Hip Morphology-dependent and Patient Demographic-dependent. Clin. Orthop. Relat. Res. 2019, 477, 1021–1033. [Google Scholar] [CrossRef] [PubMed]
- Ganz, R.; Parvizi, J.; Beck, M.; Leunig, M.; Nötzli, H.; Siebenrock, K.A. Femoroacetabular impingement: A cause for osteoarthritis of the hip. Clin. Orthop. Relat. Res. 2003, 417, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Bech, N.H.; Haverkamp, D. Impingement around the hip: Beyond cam and pincer. EFORT Open Rev. 2018, 3, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Gala, L.; Clohisy, J.C.; Beaulé, P.E. Hip Dysplasia in the Young Adult. J. Bone Jt. Surg. Am. 2016, 98, 63–73. [Google Scholar] [CrossRef]
- Gosvig, K.K.; Jacobsen, S.; Sonne-Holm, S.; Palm, H.; Troelsen, A. Prevalence of malformations of the hip joint and their relationship to sex, groin pain, and risk of osteoarthritis: A population-based survey. J. Bone Jt. Surg. Am. 2010, 92, 1162–1169. [Google Scholar] [CrossRef]
- Jacobsen, S.; Sonne-Holm, S. Hip dysplasia: A significant risk factor for the development of hip osteoarthritis. A cross-sectional survey. Rheumatology 2005, 44, 211–218. [Google Scholar] [CrossRef]
- Bache, C.E.; Clegg, J.; Herron, M. Risk factors for developmental dysplasia of the hip: Ultrasonographic findings in the neonatal period. J. Pediatr. Orthop. 2002, 11, 212–218. [Google Scholar]
- Schmaranzer, F.; Kheterpal, A.B.; Bredella, M.A. Best Practices: Hip Femoroacetabular Impingement. Am. J. Roentgenol. 2021, 216, 585–598. [Google Scholar] [CrossRef] [PubMed]
- Cadet, E.R.; Babatunde, O.M.; Gorroochurn, P.; Chan, A.K.; Stancato-Pasik, A.; Brown, M.; Johnson, S.; Kaiser, P.B.; Gardner, T.R.; Ayeni, O.R. Inter- and intra-observer agreement of femoroacetabular impingement (FAI) parameters comparing plain radiographs and advanced, 3D computed tomographic (CT)-generated hip models in a surgical patient cohort. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 2324–2331. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.; Graumann, O.; Overgaard, S.; Gerke, O.; Lundemann, M.; Haubro, M.H.; Varnum, C.; Bak, L.; Rasmussen, J.; Olsen, L.B.; et al. A Deep Learning Algorithm for Radiographic Measurements of the Hip in Adults-A Reliability and Agreement Study. Diagnostics 2022, 12, 2597. [Google Scholar] [CrossRef]
- Archer, H.; Reine, S.; Alshaikhsalama, A.; Wells, J.; Kohli, A.; Vazquez, L.; Hummer, A.; DiFranco, M.D.; Ljuhar, R.; Xi, Y.; et al. Artificial intelligence-generated hip radiological measurements are fast and adequate for reliable assessment of hip dysplasia: An external validation study. Bone Jt. Open 2022, 3, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Mast, N.H.; Impellizzeri, F.; Keller, S.; Leunig, M. Reliability and agreement of measures used in radiographic evaluation of the adult hip. Clin. Orthop. Relat. Res. 2011, 469, 188–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayeni, O.R.; Chan, K.; Whelan, D.B.; Gandhi, R.; Williams, D.; Harish, S.; Choudur, H.; Chiavaras, M.M.; Karlsson, J.; Bhandari, M. Diagnosing Femoroacetabular Impingement from Plain Radiographs: Do Radiologists and Orthopaedic Surgeons Differ? Orthop. J. Sports Med. 2014, 2, 232–259. [Google Scholar] [CrossRef] [Green Version]
- Sidey-Gibbons, J.A.M.; Sidey-Gibbons, C.J. Machine learning in medicine: A practical introduction. BMC Med. Res. Methodol. 2019, 19, 64. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Stotter, C.; Klestil, T.; Nehrer, S. Artificial Intelligence in Orthopedic Radiography Analysis: A Narrative Review. Diagnostics 2022, 12, 2235. [Google Scholar] [CrossRef]
- Murphy, E.A.; Ehrhardt, B.; Gregson, C.L.; von Arx, O.A.; Hartley, A.; Whitehouse, M.R.; Thomas, M.S.; Stenhouse, G.; Chesser, T.J.; Budd, C.J.; et al. Machine learning outperforms clinical experts in classification of hip fractures. Sci. Rep. 2022, 12, 2058. [Google Scholar] [CrossRef]
- Jang, S.J.; Kunze, K.N.; Vigdorchik, J.M.; Jerabek, S.A.; Mayman, D.J.; Sculco, P.K. John Charnley Award: Deep Learning Prediction of Hip Joint Center on Standard Pelvis Radiographs. J. Arthroplast. 2022, 37, S400–S407.e1. [Google Scholar] [CrossRef]
- Rouzrokh, P.; Wyles, C.C.; Philbrick, K.A.; Ramazanian, T.; Weston, A.D.; Cai, J.C.; Taunton, M.J.; Lewallen, D.G.; Berry, D.J.; Erickson, B.J.; et al. A Deep Learning Tool for Automated Radiographic Measurement of Acetabular Component Inclination and Version After Total Hip Arthroplasty. J. Arthroplast. 2021, 36, 2510–2517. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Ye, Q.; Ming, S.; Hu, X.; Jiang, Z.; Shen, Q.; He, L.; Gong, X. Feasibility of automatic measurements of hip joints based on pelvic radiography and a deep learning algorithm. Eur. J. Radiol. 2020, 132, 109303. [Google Scholar] [CrossRef] [PubMed]
- Rouzrokh, P.; Khosravi, B.; Johnson, Q.J.; Faghani, S.; Vera Garcia, D.V.; Erickson, B.J.; Maradit Kremers, H.; Taunton, M.J.; Wyles, C.C. Applying Deep Learning to Establish a Total Hip Arthroplasty Radiography Registry: A Stepwise Approach. J. Bone Jt. Surg. Am. 2022, 104, 1649–1658. [Google Scholar] [CrossRef]
- Nagendran, M.; Chen, Y.; Lovejoy, C.A.; Gordon, A.C.; Komorowski, M.; Harvey, H.; Topol, E.J.; Ioannidis, J.P.; Collins, G.S.; Maruthappu, M. Artificial intelligence versus clinicians: Systematic review of design, reporting standards, and claims of deep learning studies. BMJ 2020, 25, 368–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor-Phillips, S.; Stinton, C. Fatigue in radiology: A fertile area for future research. Br. J. Radiol. 2019, 92, 20190043. [Google Scholar] [CrossRef]
- Stec, N.; Arje, D.; Moody, A.R.; Krupinski, E.A.; Tyrrell, P.N. A Systematic Review of Fatigue in Radiology: Is It a Problem? Am. J. Roentgenol. 2018, 210, 799–806. [Google Scholar] [CrossRef]






| Reference | Purpose | Method | Results and Performance |
|---|---|---|---|
| Jang et al., 2022 [20] | Automated determination of hip joint center | U-Net used for identification of bony landmarks and pelvic height ratio. A total of 6344 ap hip radiographs used for training, and used 1252 for testing. Compared to manual segmentation. | Prediction within 5 mm of error: 80% with nonspecific, 83% sex-specific. And 91% with patient-specific pelvic height ratio. |
| Yang et al., 2020 [22] | Feasibility Study for automated measurement of the hip joint (determination of CE, Tönnis angle, Sharp angle, FHEI) | Identification of bony landmarks. A total of 1060 ap hip radiographs used for training, and 200 used for testing. Compared to three radiologists. | PCK: 87–100%, ICC: 0.8–0.93, r: 0.83–0.93, RMSE: 0.02–3.27, MAE: 0.02–1.79. |
| Archer et al., 2022 [14] | Detection of Hip dysplasia through lateral CE, CCD, pelvic obliquity, Tönnis angle, Sharp angle, femoral head coverage using HIPPO™ | HIPPO™ used for Identification of bony landmarks. 256 ap hip radiographs for testing. Compared to three medical students who underwent instructions form one senior radiologist. | ICC for lateral CE: 0.71–0.86, for CCD: 0.62–0.79, for pelvic obliquity: 0.83–0.98, for Tönnis angle: 0.82–0.90, for Sharp angle: 0.74–0.86, for femoral head coverage: 0.5–0.73. |
| Rouzrokh et al., 2021 [21] | Automated measurement of acetabular component and version after THA | 2 U-Net models for Segmentation of bilateral ischial tuberosity on 600 ap hip radiographs and acetabular component on 600 ap and cross-table lateral hip radiographs. Training, validation and testing split in 8:1:1 ratio. Compared to two orthopedic surgeons. | For ap and cross-table lateral radiograph models, respectively: egmentation: mean DSC 0.878 and 0.903, Acetabular component angles: mean absolute difference 1.35° and 1.39°. |
| Rouzrokh et al., 2022 [23] | Creating THA radiography registry using deep learning | Four DL algorithms used for determination of radiograph appearance on 846,988 hip and pelvis radiographs. Compared to human annotators on random test sample of 5000 radiographs. | 209,331 radiographs were excluded as misclassified. Accuracy: 99.9%, precision: 99.6%, recall: 99.5%, F1-score: 99.6%. Registry automatically annotated in <8 h |
| Jensen et al., 2022 [13] | Detection of hip dysplasia through lateral CE and AIA | RBHip™ trained on 2900 pelvic radiographs, tested on 71 pelvic radiographs. Comparison to ground truth: 5 clinicians. | Lateral CE: Bland–Altman LoA ranging from 0.37 to 9.56 and 3.56 to 10.1 for right and left hip, respectively. AIA: Bland-Altman LoA ranging from −0.58 to 2.06 and −1.09 to 1.28 for right and left hip, respectively. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stotter, C.; Klestil, T.; Röder, C.; Reuter, P.; Chen, K.; Emprechtinger, R.; Hummer, A.; Salzlechner, C.; DiFranco, M.; Nehrer, S. Deep Learning for Fully Automated Radiographic Measurements of the Pelvis and Hip. Diagnostics 2023, 13, 497. https://doi.org/10.3390/diagnostics13030497
Stotter C, Klestil T, Röder C, Reuter P, Chen K, Emprechtinger R, Hummer A, Salzlechner C, DiFranco M, Nehrer S. Deep Learning for Fully Automated Radiographic Measurements of the Pelvis and Hip. Diagnostics. 2023; 13(3):497. https://doi.org/10.3390/diagnostics13030497
Chicago/Turabian StyleStotter, Christoph, Thomas Klestil, Christoph Röder, Philippe Reuter, Kenneth Chen, Robert Emprechtinger, Allan Hummer, Christoph Salzlechner, Matthew DiFranco, and Stefan Nehrer. 2023. "Deep Learning for Fully Automated Radiographic Measurements of the Pelvis and Hip" Diagnostics 13, no. 3: 497. https://doi.org/10.3390/diagnostics13030497





